?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, Calophyllum inophyllum (CIO) loaded Poly (ε-caprolactone) (PCL) electrospun fibre mats were produced for potential wound healing applications. Physiochemical evaluation and in vitro characterisation of produced mats were evaluated. Average fibre diameters of the mats were determined as 0.9 ± 0.3 μm, 1.2 ± 0.2 μm, 1.3 ± 0.2 μm, and 1.5 ± 0.1 μm for PCL, PCL/CIO−2.5, PCL/CIO−5 and PCL/CIO−7.5, respectively. The contact angle values of the fibre mats were decreased up to 30 ± 5 (°) compared to oil-free PCL fibre mat, indicating improved surface wettability. The incorporation of CIO into fibre mats led to a two-fold increase in the antibacterial activity, as compared to the fibre mats composed of PCL. In vitro cytotoxicity evaluation indicated that all the fibre mats had increased cell viability compared to the control. The findings suggest that CIO-loaded PCL electrospun fibre mats have potential for use in tissue engineering applications.
Introduction
Skin is known as the largest organ of our body and performs many necessary functions for the survival of the organism. Different types of wounds can occur as a result of deterioration in skin integrity by various internal and external factors. The organism responds to tissue injury with a natural physiological reaction known as the wound healing process [Citation1]. Since skin plays an important role as a protective barrier, disruption of its integrity can result in unwanted conditions such as bacterial infections. Another drawback of the natural wound healing process that the body itself overcomes the tissue damage, is the relatively slow cure rate [Citation2]. Thus, it is important to promote wound healing process by selecting and applying appropriate wound dressing to the damaged tissue. Wound dressing plays a pivotal role in wound management by providing required moisture and protecting the wound from external damage. An ideal wound dressing should follow many characteristics from controlling the moisture around wound, provide the transmission of gases, having mechanical protection and to being both biocompatible and non-toxic. Most importantly, ideal wound dressings must protect the wound sites from infections and microorganisms [Citation3]. Thus, it is important to develop wound dressings not only promotes healing process but also act as a barrier to different kinds of microorganism infections [Citation4]. To date, many compounds from antibiotics to natural agents were used to fight against bacterial infections at wound sites. But such problems as antibiotic resistance in addition to difficulties applying drugs to massive wound sites require new approaches to treat different kinds of wounds.
Natural products derived from plants, such as phytochemicals, have been used for centuries to develop drugs to promote wound healing. One such plant is the Calophyllum inophyllum Linn. (Calophyllaceae) tree, which is rich in polyphenols and can be found in Africa, Asia, and Pacific countries. Different parts of this tree have been used in traditional medicine to treat a variety of diseases [Citation5]. C. inophyllum oil was used in traditional medicine to treat skin-related rheumatic diseases and especially skin burns [Citation6]. Research has shown that C. inophyllum oil contains diverse bioactive compounds, including coumarins, xanthones, flavonoids, steroids, and triterpenoids [Citation7]. In addition, C. inophyllum oil has demonstrated antioxidant, antimicrobial, antiviral, anti-inflammatory, and antiproliferative activities [Citation8]. Although the wound healing and antibacterial properties of C. inophyllum oil have been reported in various studies, there is no study in the literature regarding the integration of this oil into wound dressing materials for use.
Electrospinning is a fibre fabrication method that uses electrostatic forces. This method has gained attention in recent years because it allows control over porosity, morphology, and composition using simple equipment [Citation9]. The fibres produced by electrospinning can have two key features: fine pores and a high contact surface, which are crucial for wound dressing materials [Citation10]. Additionally, electrospun fibres can meet the needs of tissue repair by providing oxygen permeation and protection for the injury site [Citation11]. Due to its many benefits, various polymers have been successfully electrospun into fine fibres for the production of bioactive wound dressing materials. Poly(ε-caprolactone) (PCL) is one of the most extensively studied polymers in electrospinning for wound dressings. PCL fibres possess excellent biocompatibility, biodegradability, and mechanical properties, making them suitable for applications in wound healing [Citation12]. These fibres can provide a physical barrier against external contaminants while maintaining a moist environment favourable for wound healing. Additionally, PCL fibres can be loaded with bioactive agents such as growth factors or antimicrobial agents to enhance their therapeutic properties [Citation13] Another commonly utilised polymer is polyvinyl alcohol (PVA). PVA fibres offer good biocompatibility and can be easily blended with other polymers to improve mechanical strength and biodegradability. PVA-based fibres have demonstrated promising results in promoting wound healing, including enhanced cell adhesion, proliferation, and extracellular matrix deposition [Citation14].
Furthermore, electrospun fibres based on chitosan, a biopolymer derived from crustacean shells, have gained attention for their antibacterial properties and ability to promote wound healing. Chitosan fibres possess antimicrobial activity against various pathogens and can accelerate the wound healing process through enhanced cell attachment, migration, and angiogenesis [Citation15]. These examples highlight the wide range of polymers that have been successfully electrospun into fine fibres for the production of bioactive wound dressing materials. The versatility and tunability of the electrospinning technique allow researchers to tailor the properties of the fibres to meet the specific requirements of wound healing, thereby offering promising prospects for advanced wound dressing materials.
Among the various types of electrospun fibres, polyester fibres are the most biocompatible, easily processed, and non-toxic to cells. PCL is a synthetic and biodegradable polyester with a low melting point around 60°C [Citation16]. PCL is an FDA-approved polymer commonly used in wound dressing and drug release applications, and its biodegradability and biocompatibility are significant advantages. Although PCL is not soluble in water, it can be completely dissolved in various organic solvents. Therefore, it can be electrospun into fibres in different organic solvents such as dimethylformamide, dichloromethane, chloroform, and methanol, or a combination of these solvents [Citation17]. PCL has some limitations, including a long degradation cycle and poor hydrophilicity. However, it can be easily modified to improve its limitations and expand its area of use. These features make PCL an outstanding candidate for tissue engineering and electrospun wound dressing applications.
Electrospinning of PCL has been widely reported with varying solvent systems and polymer concentrations, as these parameters play significant roles in fibre formation and properties. In the study by Adhikari et al., electrospinning of honey or betel with PCL was explored in an optimised 12 w/v% PCL solution. In their procedure, the electrospinning parameters included a flow rate of 1 ml/h, a voltage of 20 kV, and a working distance of 12 cm. The resulting fibre diameter was reported to range from 300 to 500 nm [Citation18]. Fawal et al. used a different solvent system and loaded PCL with oregano oil [Citation19]. They prepared a gelatin (15 wt%) and PCL (15 wt%) solution in 1,1,1,3,3,3, -hexafluoro−2-isopropanol solvent and incorporated oregano oil at 10 wt% of the PCL. Their electrospinning conditions were a flow rate of 1 ml/h, a voltage of 15 kV, and a working distance of 17 cm and fibre diameter was about 377 nm [Citation19]. These studies reveal that a variety of electrospinning conditions and formulation compositions can be used to produce PCL fibres with desirable properties for wound healing applications. The ability to adjust the electrospinning parameters, such as polymer concentration, solvent system, flow rate, voltage, and working distance, enables researchers to tailor the properties of the electrospun PCL fibres according to the specific requirements of the wound healing application.
In this study, the aim is to produce a novel wound dressing with improved wound healing and anti-bacterial properties by co-electrospinning C. inophyllum oil with PCL. To achieve this, different concentrations of C. inophyllum oil (CIO) were loaded onto PCL electrospun mats. The produced fibre mats were then characterised using physicochemical and morphological analyses, including measurements of fibre diameter and functional groups. Additionally, anti-bacterial activity and in vitro cell viability were assessed in appropriate cell lines. To the best of our knowledge, this is the first study that produces and investigates CIO-loaded PCL electrospun fibre mats for use in wound healing applications.
Materials and methods
Materials
PCL with average molecular weight of 80.000 Da was obtained from Sigma-Aldrich. Dichloromethane (DCM) and N,N dimethyl formamide (DMF) were purchased from Merck. %100 pure Calophyllum inophyllum oil was provided from EuropeVital Herbal and Aromatic Oil, Turkey. The microorganism strains of Staphylococcus aureus (ATCC25923) and Escherichia coli (ATCC25922) were used. The normal human dermal fibroblasts (NHDF) cells were acquired from the American Type Culture Collection (ATCC, Manassas, VA). DMEM/F12, dimethyl sulphoxide (DMSO), foetal bovine serum (FBS), penicillin streptomycin and 3-(4,5-Dimethyl−2-thiazolyl)− 2,5-diphenyl−2 H-tetrazolium bromide (MTT) were provided from Sigma-Aldrich.
Electrospinning process
Electrospinning solution for PCL fibre mats were prepared by dissolving PCL (10%, w/v) in DCM:DMF (80:20 (v/v)) under stirring conditions. Calophyllum inophyllum oil-loaded PCL solutions were prepared by slowly adding different concentrations (at 2.5, 5% and 7.5% (v/v)) of CIO into the prepared polymer solution separately. Then the obtained solution was vigorously stirred for 1 h at room temperature until it was completely dissolved. Electrospinning process was carried out under constant conditions with 24 ◦C temperature and 25% relative humidity. The homogeneous solution was loaded into a plastic syringe with a 20 G needle and fed at 0.5 ml/h. The distance between the needle tip and the rotated collector was set up to 13 cm, and + 14 kV voltage was applied. The electrospun fibres were collected on a roller collector (diameter 80 mm, rotating at a speed of 800 ± 50 rpm), which was affixed to a sliding table. This roller was wrapped in aluminium foil with a thickness of 0.05 mm, providing a substrate for fibre deposition. Produced membranes were stored in the dark at + 4°C until use.
Characterization of electrospun fibre mats
Surface morphology
The surface morphology of the electrospun fibre mats was analysed by scanning electron microscopy (SEM) (Zeiss Evo LS 10). All samples were coated with gold prior to the SEM analysis. The average fibre diameters of PCL fibre mats with or without loaded CIO were determined by direct measurements from the SEM images using ImageJ software. The average fibre diameter and fibre distribution were calculated by selecting and measuring 30 random different points from the micrographs of each sample.
Fourier Transform Infrared Spectroscopy (FT-IR)
The Fourier transform infrared (FTIR) spectrophotometer (Perkin Elmer Spectrum One) was used to determine the chemical composition of PCL, CIO and CIO loaded PCL fibre mat. Measurements were taken in absorption mode in the wavelength range of 4000–500 cm−1, at a resolution of 4 cm−1.
Water contact angle measurement
The wettability of produced fibre mats was determined using automatic contact angle metre (OCA100, Dataphysics). In brief, fibre mats were placed on a glass slide and 5 μl of de-ionised water was dropped onto the surface of the samples. Each sample was tested three times with different locations of the same mat to obtain the average water contact angle value.
Swelling measurement of electrospun fiber mats
A Pseudo Extra Cellular Fluid (PECF) solution was prepared to simulate wound fluid. Sodium chloride (NaCl), potassium chloride (KCl), sodium bicarbonate (NaHCO3), and dipotassium phosphate (K2HPO4) were dissolved in distiled water, and the pH of the solution was adjusted to 8. Membranes were cut to 1 × 1.5 cm2 and weighed initially. These membranes were then placed in petri dishes containing PECF and incubated at 37°C for 24 h. After incubation, the excess PECF solution was removed from the petri dish, and the membranes were weighed again. The percentage of absorption was calculated using the following equation. Where W0 is the initial dry weight of the membranes, and W is the weight when wet.
In vitro drug release
In vitro release kinetics of CIO from PCL fibre mats were determined by UV–Vis spectrophotometry [Citation20]. First, a calibration curve was constructed for CIO to use as a reference in concentration calculation. For this, varying concentrations of CIO from 0 to 10 μL/mL were prepared and a calibration curve was drawn at 278 nm (maximum absorption of CIO). CIO loaded PCL fibre mats (5 × 5 cm2) were placed into PBS (pH = 7.4) under stirring conditions at 100 rpm at 37°C in a shaking incubator. Then, 2 ml of release medium was collected at certain time points between 0 and 120 hours to determine the released CIO amount. At the same time, an equal volume of fresh PBS (pH = 7.4) was added to the medium to maintain the sinking condition. The concentration of released CIO was determined by measuring the absorbance with a UV-Vis spectrophotometer at 278 nm and comparing it with the constructed standard calibration curve. Each measurement was performed in triplicate.
Antibacterial activity
The antibacterial efficacy of the CIO loaded fibre mats was tested on S. aureus (Gram-positive) and E. coli (Gram-negative) bacteria using broth dilution method. Each bacteria strain was cultivated in Luria-Bertani (LB) broth medium at 37°C for 24 h. Then, the optical density (OD) of the bacterial suspensions was measured at 600 nm and set to 0.01. CIO loaded fibre mats were cut into the dimensions of 1 × 1 cm2 and sterilised under UV irradiation for 20 min. Then, sterilised samples were placed into LB broth medium containing 30 μL of bacteria suspension and incubated at 37°C for 3, 6, 12 and 24 h. The optical density of the samples was measured at each time point at 600 nm and relative bacterial viability was calculated by following formula. All experiments were conducted in triplicates.
Cell culture
Prior to all cell culture experiments, pre-cut (1×1 cm2) CIO-loaded fibre mats were sterilised by UV irradiation for 20 min and membranes were placed into 24-well cell culture plates. The normal human dermal fibroblast (NHDF) cells were grown in 75 cm2 cell culture flasks containing 10 ml of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 at 37°C. After reaching confluency, cells NHDF cells were detached by trypsinisation and counted by haemocytometer. Then, cells were seeded onto membranes that were in 24-well plates at a density of 10,000 cells per/well and incubated in a humidified incubator containing 5% CO2 at 37°C for 24 h.
In vitro cytotoxicity
3-[4,5-dimethylthiazol−2-yl]−2,5 diphenyl tetrazolium bromide (MTT) assay was used to determine in vitro NHDF cell viability. Briefly, NHDF cells were cultivated in 24-well plates at a density of 5000 cell/well and cells were incubated for 24 h to reach confluency. Prior to experiment, produced fibre mats were sterilised by UV irradiation and mats were placed in CellCrown inserts in a 24 well plate. Following 48 h incubation of cells incubation medium was removed and 50 µg/ml MTT solution added on each well then plates were incubated at 37°C with 5% CO2 for 3 h. Then, 100 µl DMSO was added each well after removal of MTT solution. Cell viability was measured by recording the absorbance at 570 nm with a spectrophotometric microplate reader. Cells without mats were used as control and cell viability (%) was determined with the equation below:
In vitro scratch assay
In vitro wound healing activity of PCL/CIO mats were conducted by in vitro scratch assay. Initially, NHDF cells were seeded at a density of 50,000 cells per well in a 24-well plate and incubated at 37°C in a 5% CO2 atmosphere for 24 h. Following incubation, a linear scratch was generated in the centre of the NHDF monolayer using a sterile 1000 µL pipette tip. Next, the mats were secured on CellCrown 24 inserts and positioned in the 24-well plate without making contact with the surface. The wound closure progress was monitored at various time points (0 h, 4 h, 6 h, and 24 h) and captured using a light microscope. Image J software was employed for image analysis. Untreated cells were used as control cells and the wound closure rate was determined using the following equation:
The initial wound area is represented by A0, while the wound area at the designated time is represented by At.
Antioxidant capacity
The evaluation of the antioxidant capacity of PCL/CIO fibre mats was conducted using an oxidative stress model. L929 fibroblast cells (20,000 cells/well) were cultured in DMEM medium supplemented with 10% FBS, 1% L-glutamine, and 1% streptomycin, under conditions of 37°C and 5% CO2. The cells were seeded into 24-well plates, with 10,000 cells per well, and allowed to incubate for 48 h. The Total Oxidant Status (TOS) and Total Antioxidant Status (TAS) of the fibre mats were evaluated for their antioxidant capacity by measuring them using oxidative stress-induced L929 cells and following the recommended protocol provided by the manufacturer of the Rel Assay kit. All analyses were conducted in triplicate.
Immunotoxicity analysis
THP−1 cells were seeded into 24-well plates at a density of 10.000 cells per well. Subsequently, the cells were stimulated for differentiation using 20 mM PMA in RPMI cell culture medium containing 10% FBS and 1% L-glutamine. The three-day process of monocyte differentiation into macrophage cells was monitored via an inverted microscope. On the third day of PMA stimulation, PCL/CIO mats were placed into culture dishes and cells were incubated for 48 h at 37°C in a 5% CO2 atmosphere. Following incubation, the media were collected, centrifuged at 5000 rpm for 15 min, and analysed for cytokine levels. The assays, performed in triplicate, followed the manufacturer’s guidelines using the Thermo Fisher IL−1 beta Human ELISA Kit for interleukin−1β.
Results and discussion
Surface morphology of electrospun fiber mats
The surface morphology and size distribution of electrospun PCL and PCL/CIO fibre mats were determined by analysing SEM micrographs. As shown in , the use of 10%w/v PCL in the electrospinning process of the mats resulted in regular fibre production. SEM analysis revealed that all fibre mats were produced as uniform, homogeneously distributed straight fibres (). Furthermore, increasing concentrations of CIO did not significantly alter the morphology of fibres, and uniform, bead-free fibre structure was maintained in the production of all PCL/CIO mats.
Figure 1. The surface morphology and fiber diameter distribution of poly(ε-caprolactone) (a,b), PCL/Calophyllum inophyllum oil (CIO)−2.5 (c,d), PCL/CIO−5 (e,f) and PCL/CIO−7.5 (g,h) fiber mats, respectively.
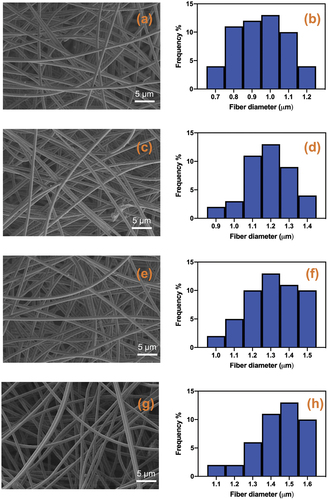
The average fibre diameter distribution of PCL, PCL/CIO−2.5, PCL/CIO−5 and PCL/CIO−7.5 is shown in , respectively. The average fibre diameters of the mats were determined as 0.9 ± 0.3 μm, 1.2 ± 0.2 μm, 1.3 ± 0.2 μm, and 1.5 ± 0.1 μm for PCL, PCL/CIO−2.5, PCL/CIO−5 and PCL/CIO−7.5, respectively (). Addition of CIO to the polymer solution in increasing concentrations during the electrospinning process resulted in a slight increase in fibre diameter.
Table 1. Sample name, average fibre diameter (μm) and contact angle values of electrospun fibre mats.
Essential oils extracted from different parts of plants have recently gained great attention due to their therapeutic benefits. A wide variety of essential oils have been electrospun for biomedical applications, especially for the production of wound dressings [Citation21]. Despite its broad biological activity in wound healing, no study has been reported on the production of Calophyllum inophyllum (CIO) electrospun PCL fibre wound dressings to date. This is the first study to our knowledge that produces and characterises CIO-incorporated PCL electrospun fibre mats for wound healing applications.
During the electrospinning process for fabrication of fibre mats, fibre morphology and fibre diameter could differ depending on many parameters such as flow rate, solution viscosity and polymer concentration in the polymer solution [Citation22]. For example, Wang and Nie demonstrated that enhancing a polymer surface with open-pore topography markedly increased fibre deposition [Citation23]. In order to produce smooth, homogeneous and bead-free fibres these parameters should be taken into account alongside humidity and temperature [Citation24]. Addition of essential oils in different concentrations to the polymer solution could also affect the solution viscosity and resulting fibre diameter and morphology can vary [Citation25,Citation26]. Our results indicate that CIO incorporation to the PCL did not change the fibre morphology. On the other hand, addition of CIO oil to the polymer solution slightly increased fibre diameter compared to the oil-free PCL fibres. The slight increase in the fibre diameter of PCL/CIO fibre mats after addition of increasing concentrations of CIO to the polymer solution could be attributed to the increase in the solution viscosity. A similar study reported that, the addition of clover essential oil to the PCL-Gelatin for the aim of producing nanofiber wound dressing mats, resulted in increased fibre diameter formation [Citation27]. Another study similarly reported that encapsulating fish oil has increased the average fibre diameter of PVA nanofibers [Citation28]. Another factor that can affect the fibre diameter in the electrospinning process is the electrical conductivity of the solution [Citation29]. Large fibre diameters in the produced mats may be the result of the low electrical conductivity of the solution, which affects the elongation of the jet by electrical forces [Citation27,Citation30]. The addition of CIO oil to the polymer solution could reduce the solution’s electrical conductivity and result in an increase in fibre diameter of PCL/CIO fibre mats.
CIO is a natural compound that possesses different physicochemical properties compared to the base polymer, PCL. CIO may have altered the solution properties, such as viscosity and surface tension, when incorporated into the PCL solution. These changes in solution properties can affect the jetting behaviour during the electrospinning process. Additionally, CIO may have affected the chain entanglement and polymer-solvent interactions within the PCL solution. The presence of CIO molecules could disrupt the polymer-chain entanglement and weaken the intermolecular forces between the PCL chains, leading to decreased polymer-chain elongation and an increase in fibre diameter during electrospinning. Also, CIO may have influenced the solution conductivity indirectly, such as potentially altering the charge distribution within the solution. This alteration in charge distribution might affect the electrostatic forces that govern the stretching and elongation of the polymer jet during electrospinning. As a result, changes in charge distribution can influence the fibre diameter [Citation31].
Consequently, based on our results, it is proposed that the increased fibre diameter observed after the addition of CIO to the electrospinning process can be attributed to changes in solution properties, disruption of polymer-chain entanglement, and potential alterations in charge distribution. However, further research is necessary to fully elucidate the specific mechanisms involved and to quantify the impact of electrical conductivity on fibre diameter.
FourIer-transform infrared spectroscopy analysis of electrospun fiber mats
FT-IR spectra of pure PCL, CIO and electrospun PCL/CIO−7.5 fibre mat was determined by Fourier-transform infrared spectroscopy (FTIR) and shown in .
Figure 2. FT-IR spectrums of pure poly(ε-caprolactone) (PCL), Calophyllum inophyllum essential oil loaded PCL fiber mat (PCL/CIO) and Calophyllum inophyllum (CIO) essential oil.
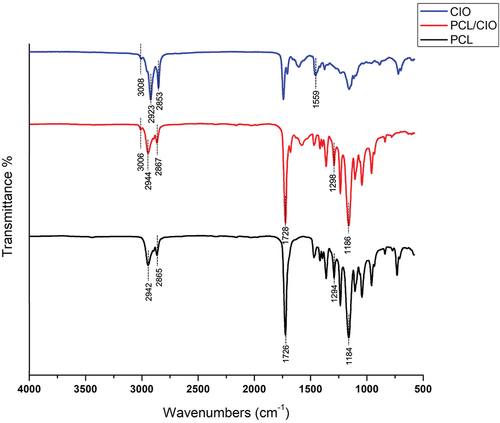
The PCL spectrum exhibited characteristic peaks at 2942 and 2865 cm−1 corresponding to asymmetric and symmetric vibrations of the CH2 groups. The peak at 1726 cm−1 was assigned to the stretching of the C=O groups. Additionally, peaks at 1294 cm−1 and 1184 cm−1 were attributed to the asymmetric and symmetric stretching of the C – O–C groups, respectively. A characteristic peak at 1243 cm−1 was assigned to the CH3 vibrations, and 1045 cm−1 belongs to C – O stretching and C – H bending [Citation32]. In the FT-IR spectrum of CIO two main peaks were observed at 1559 cm−1 and 1742 cm−1 corresponding to stretching of C=O groups. Also, bands at 2923 cm−1 and 2853 cm−1 were attributed to N-H stretching in CIO [Citation33]. FT-IR spectrum of PCL/CIO−7.5 showed bands related to stretching of the C=O groups in CIO at 1541 cm−1, 1559 cm−1 and 1742 cm−1 confirming successful incorporation of oil into PCL fibres.
The FT-IR analysis provides valuable information about the chemical composition and functional groups present in the electrospun fibre mats. The characteristic peaks observed in the PCL spectrum are consistent with previous studies on electrospun PCL fibres [Citation34]. The peaks observed in the CIO spectrum can be attributed to the carbonyl stretching vibrations of the C=O groups, which are commonly observed in essential oils [Citation35]. The presence of N-H stretching bands in CIO also confirms the successful incorporation of the oil into the PCL fibres. The appearance of C=O stretching bands in the FT-IR spectrum of PCL/CIO−7.5 further confirms the incorporation of CIO into the PCL fibres. Overall, the FT-IR analysis supports the successful incorporation of CIO into the PCL fibres and provides insight into the chemical composition of the electrospun fibre mats.
Wettability of electrospun fiber mats
Sessile drop method was used to assess the surface wettability of electrospun fibre mats. The contact angle values of PCL, PCL/CIO−2.5, PCL/CIO−5 and PCL/CIO−7.5 fibre mats were shown in . Contact angle values were determined as 55 ± 7°, 35 ± 4°, 32 ± 5°, 30 ± 5° for PCL, PCL/CIO−2.5, PCL/CIO−5 and PCL/CIO−7.5 fibre mats, respectively. The average contact angle value for the PCL electrospun fibre mat was determined as 55 ± 7°. On the other hand, the addition of CIO to the PCL solution resulted in a slight decrease in the contact angle values of the produced fibre mats compared to the oil-free PCL fibre mat. However, there was no significant difference observed among the contact angle values of the PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5 fibre mats.
The role of surface wettability in determining the effectiveness of a wound dressing is well established. The hydrophilicity or hydrophobicity of the surface of a wound dressing can direct cellular processes such as cell attachment, adhesion, and migration, which are crucial for wound healing. In order to assess the surface wettability of electrospun fibre mats, contact angle measurements were performed. Our results showed that addition of the CIO decreased the contact angle value compared to oil-free PCL fibre mats. According to Unalan et al., addition of peppermint oil to the PCL solution contact angle of electrospun fibre mats were found to be decreased in parallel with our findings [Citation36]. Another study showed that incorporation of different essential oil extracts into PCL fibres resulted in decreased contact angle measurement [Citation37]. Also, a study by Agnes Mary and Giri Dev determined that aloe vera addition to PCL polymer solution decreased the contact angle of produced fibre mats [Citation38].
Swelling degree of electrospun fiber Mats
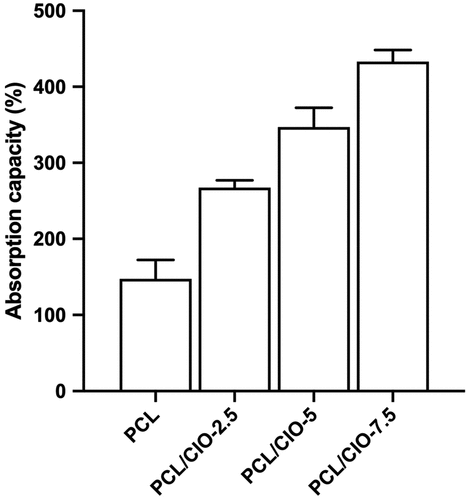
For a wound dressing material to excel in quality, it should be capable of maintaining an optimal moisture environment that encourages skin regeneration [Citation3]. The absorption capacity of the PCL fibre mats loaded with CIO was evaluated in PECF solution. The PCL/CIO−2.5, PCL/CIO−5 and PCL/CIO−7.5 fibre mats exhibited PECF absorption values of 267.5%, 347.25%, and 433.3%, respectively. As illustrated in , PCL/CIO fibre membranes outperformed pure PCL fibres in terms of PECF absorption, showcasing their superior exudate absorption capacity. Given the hydrophobic nature of PCL, the addition of alcoholic extracts of Calophyllum inophyllum essential oil enhances the hydrophilicity of the polymeric mat, thereby improving the mat’s absorption characteristics. In alignment with our findings, Ardekani et al. demonstrated that Zataria oil incorporated PVA electrospun nanofibers exhibited considerable water absorption capacity, ranging between 400% and 900%, suggesting a significant potential for these nanofibers in applications that require substantial moisture retention [Citation39]. Preventing the build-up of wound exudates is crucial for healing festering wounds since excessive secretion can impede the cell proliferation process. A vast array of wound dressings with differing liquid absorption features exist in the literature. Regardless of their varied absorption capacities, each absorbent has potential applications based on the wound type and amount of secretion. Moreover, it is essential to strike a balance in ensuring the wound does not become overly dry.
In vitro drug release
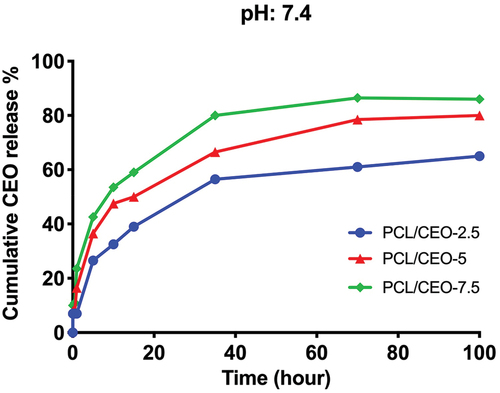
Sustained drug delivery, characterised by a controlled and extended release profile, represents a critical and preferred factor in wound healing applications due to its potential benefits in reducing the frequency of drug administration and mitigating the side effects caused by drug overload, which could otherwise compromise the efficacy and safety of the therapy [Citation40]. In order to determine CIO release from produced PCL fibre membranes, drug release profiles were investigated (). It is known that, PCL polymer has very low biodegradation due to its low hydrophilicity. Therefore, hydrophobic nature of PCL resulted in a burst and short initial release of CIO from the polymer [Citation41]. The rapid and intense initial release phase of the drug delivery system may be attributed to the localisation of drug molecules near the surface of the hydrophobic poly(ε-caprolactone) (PCL) fibres. This could be the result of entrapped excess CIO near the surface of the fibres. The PCL matrix hinders the penetration of aqueous media, thereby delaying the diffusion of the drug into the release environment. This phenomenon is further amplified by the low solubility of the drug, which necessitates its diffusion through the amorphous regions of the matrix, leading to a prolonged lag time and a reduced burst release effect. During the 5th hour of in vitro release, CIO burst released from PCL/CIO fibres, 25.5 ± 3.2% for PCL/CIO−2.5, 33.4 ± 4.4% for PCL/CIO−5 and 41.1 ± 3.1% for PCL/CIO−7.5, respectively. Following the burst release phase, a sustained and controlled CIO release was observed in all PCL/CIO fibre mats.
Antibacterial activity of electrospun fiber mats
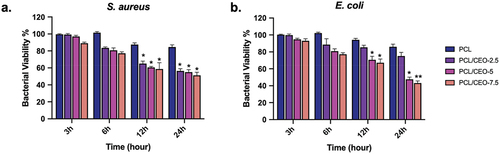
Antibacterial activity of fibre mats comprising PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5 were investigated on S. aureus (Gram-positive) and E. coli (Gram-negative) bacteria by using broth dilution method. The bacterial viability was detected and is illustrated in . The antibacterial activity of fibre mats was improved by the addition of CIO compared to pure PCL. The highest inhibition effect was observed against S. aureus at 12 h, while E. coli was inhibited for up to 24 h. However, no significant differences were found among the PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5 fibre mats. Furthermore, after 24 h of incubation, lower E. coli bacterial viability was observed in PCL/CIO−5 and PCL/CIO−7.5 than in the control and PCL (). Meanwhile, bacterial viability of S. aureus was reduced in PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5 fibre mats compared to the control at each incubation time. These results indicate the potential of PCL/CIO fibre mats as antibacterial agents in various applications such as wound dressings and tissue engineering scaffolds.
Figure 5. Anti-bacterial activity of poly(ε-caprolactone) (PCL)/CIO fiber mats against Staphylococcus aureus (a) and Escherichia coli (b). *p<0.05, **p<0.01 compared to the control.
Calophyllum inophyllum essential oil (CIO) has been shown to possess significant antibacterial activity against a range of Gram-positive and Gram-negative bacteria. For instance, in a study by [Citation42], CIO exhibited potent antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) and E. coli, with inhibition zones ranging from 7 to 14 mm for S. aureus and 13 to 14 mm for E. coli, respectively. Similarly, in another study by [Citation43], CIO displayed considerable activity against Gram+ bacteria by direct inhibition of mitotic growth and another potent effect against Gram-bacteria due to increased release of β-defensin 2 peptide by macrophages. The antibacterial activity of CIO has been attributed to its complex mixture of chemical compounds, such as xanthones, coumarins, and flavonoids, which exert their bactericidal effects through various mechanisms, including disruption of cell membrane integrity, inhibition of DNA synthesis, and interference with metabolic processes [Citation43].
Electrospun polycaprolactone (PCL) wound dressings loaded with essential oils have been the subject of numerous studies in recent years, owing to their promising potential as natural and effective wound healing agents with antibacterial properties. For example, Phaiju et al., synthesised PCL wound dressings containing essential oils from cinnamon, which exhibited excellent antibacterial activity against S. aureus, E. coli, Salmonella typhimurium, and Pseudomonas aeruginosa [Citation44]. Similarly, Mouro et al., developed PCL/PVA/Chitosan fibre mats loaded with eugenol, and significant antibacterial activity was observed against S. aureus (inhibition ratios of 92.43% and 83.08%) and P. aeruginosa (inhibition ratios of 94.68% and 87.85%), indicating the efficacy of the treatment [Citation45]. A study by Chen et al., showed that PCL-tannic acid nanofibrous bio-composite scaffolds exhibited antibacterial properties against S. aureus, with a narrow inhibitory zone of 0.8 ± 0.3 mm [Citation46]. In corroboration with our findings, another study showed the electrospun poly(butylene carbonate) (PBC)/poly(lactic acid) (PLA)/chitosan (CS) blend nanofibre membranes exhibited potent antimicrobial activity against Escherichia coli and Staphylococcus aureus in in vitro assessments [Citation13]. The antibacterial activity of these essential oil-loaded PCL wound dressings has been attributed to the sustained release of the active compounds from the wound dressing into the wound bed, which disrupts the bacterial cell membrane and inhibits bacterial growth [Citation47]. Furthermore, these electrospun PCL wound dressings offer several advantages over conventional wound dressings, such as biocompatibility, flexibility, and ease of application, making them promising candidates for wound healing applications. Overall, the results of these studies highlight the potential of electrospun PCL wound dressings loaded with essential oils as a novel approach to wound healing, particularly in the context of antibacterial activity.
In vitro Cytotoxicity evaluation of electrospun fiber mats
To evaluate the biocompatibility of PCL, PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5 fibre mats, the viability of NHDF cells was assessed after 48 h of incubation, as shown in . Results showed that all of the fibre mats had increased cell viability compared to the control, with no significant difference between the different groups. These findings suggest that CIO-loaded PCL electrospun fibre mats are not cytotoxic to NHDF cells, indicating their potential for use in tissue engineering and regenerative medicine applications.
Figure 6. In vitro cytotoxicity of poly(ε-caprolactone) (PCL) electrospun fiber mats loaded with different concentrations of Calophyllum inophyllum oil (CIO) (PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5) on NHDF cells.
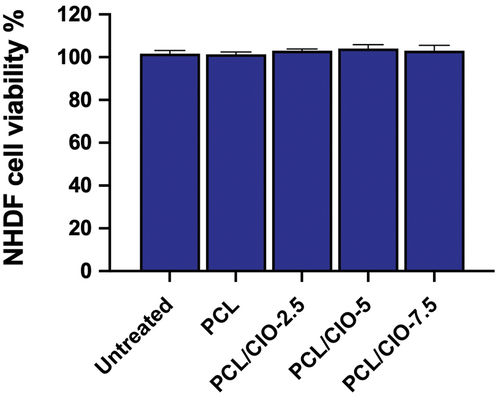
The in vitro cytotoxicity of PCL electrospun fibre mats loaded with different concentrations of Calophyllum inophyllum oil (CIO) on NHDF cells was investigated in this study. The results of the MTT assay indicated that all of the PCL/CIO fibre mats had no cytotoxic effects on NHDF cells after 48 hours of incubation. This suggests that these PCL/CIO fibre mats are biocompatible and have the potential to be used in tissue engineering and regenerative medicine applications.
Several studies have reported on the biocompatibility of PCL and its composites with different materials. For example, a study by [Citation48] evaluated the in vitro cytotoxicity of PCL/Chitosan nanofibrous scaffolds loaded with Nigella sativa extract on L929 fibroblast cells and found that the scaffolds had no significant cytotoxic effects. Similarly, in another study [Citation49], investigated the cytotoxicity of a novel tea tree oil-integrated PCL/soy protein electrospun mats on NIH3T3 fibroblast cells and reported that the mats had good biocompatibility. These studies are consistent with the results of our study and support the biocompatibility of PCL and its composites with different materials.
The absence of cytotoxicity of the PCL/CIO fibre mats observed in this study may be attributed to several factors. One possible explanation is that the incorporation of CIO into the PCL fibres did not interfere with the biocompatibility of the PCL matrix. In addition, the PCL fibre mats have a highly porous structure, which allows for the exchange of nutrients and waste products between the cells and the surrounding environment, enabling cell growth and proliferation.
Wound healing activity evaluation of electrospun fiber mats
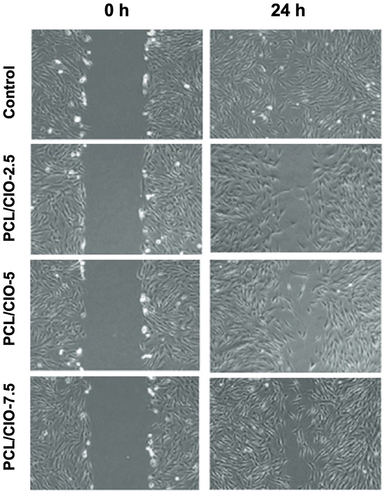
The study evaluated the impact of Calophyllum inophyllum (CIO)-loaded polycaprolactone (PCL) electrospun fibre mats on the wound healing activity of NHDF cells using the in vitro wound healing (scratch) assay. present the cell migration into the wound areas and the wound closure rate, respectively. After 6 h of treatment, the control group, PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5 fibre mats exhibited 31 ± 1%, 15 ± 2%, 21 ± 2%, and 25 ± 1% of cells in the wound area, respectively (). However, after 24 h, the control group showed complete closure, while PCL/CIO−2.5, PCL/CIO−5, and PCL/CIO−7.5 fibre mats exhibited 55 ± 1%, 75 ± 1%, and 88 ± 2% of closure, respectively. The findings suggest that increasing the concentration of CIO increased NHDF cell migration and proliferation in a dose-dependent manner. CIO has been found to possess significant wound healing properties. Studies have shown that CIO promotes wound healing by increasing the proliferation and migration of fibroblasts, which are the cells responsible for producing collagen, the main component of connective tissue [Citation43]. Additionally, CIO exhibits anti-inflammatory properties, which can help to reduce swelling and inflammation at the site of a wound [Citation6].
Antioxidant capacity of electrospun fiber mats
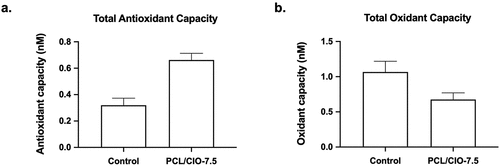
(a) and (b) present the total antioxidant and oxidant capacities, respectively, of PCL/CIO−7.5 fibre membranes on the L929 cell line. The results showed that the total antioxidant level of the cells cultured on PCL membranes was not significantly different from the control group (P > 0.05). However, the total antioxidant level of the PCL/CIO−7.5 membrane increased significantly compared to the control (P < 0.05). On the other hand, the total oxidant level of PCL membranes compared to the control group exhibited a statistically significant increase in the cells. However, CIO incorporation decreased the total oxidant level significantly (P < 0.05).
Figure 8. Total antioxidant (a) and oxidant capacities of poly(ε-caprolactone) (PCL)/CIO fiber mats. *p<0.05 compared to the control.
Oxidative stress occurs when oxidant molecules, such as reactive oxygen and nitrogen products, overwhelm the antioxidant system of the body. This can lead to various problems such as neurodegenerative diseases, cardiovascular diseases, cancer, inflammation, and ageing. Reactive oxygen species (ROS) have been found to play a role in regulating the functions of leukocytes and macrophages during the inflammation process. When biomaterials or their degradation products induce the release of oxidants by immune system cells, chronic infection, loss of biomaterial, or loss of function in the implanted region may occur.
The antioxidant activity of natural products has been extensively studied for their potential use in wound healing applications. In particular, plant-derivedvn compounds such as polyphenols have been shown to have significant antioxidant activity [Citation50]. Calophyllum inophyllum (CIO) oil, which was used in the present study, is rich in polyphenols and has demonstrated antioxidant activity in various studies [Citation8]. Previous studies have shown that wound dressings with antioxidant properties can provide beneficial effects by reducing oxidative stress and promoting the wound healing process [Citation6]. The incorporation of antioxidant compounds such as polyphenols into wound dressings has been shown to promote the healing of chronic wounds [Citation36]. The sustained and controlled release of antioxidants from wound dressings is important for their efficacy. Burst release of antioxidants can cause an imbalance in the antioxidant/oxidant ratio, which can have a negative impact on wound healing. Therefore, controlling the release of antioxidants from wound dressings is essential for their effectiveness [Citation11]. In addition, the biocompatibility of wound dressings is crucial to avoid adverse effects on the surrounding tissues and cells. Polycaprolactone (PCL) is a biocompatible and biodegradable polymer commonly used in wound dressing applications due to its favourable properties [Citation16]. The incorporation of CIO into PCL electrospun fibre mats can provide a promising wound dressing material with enhanced antioxidant activity. The sustained and controlled release of CIO from the mats could promote the wound healing process, while the biocompatibility of PCL makes it suitable for tissue engineering and regenerative medicine applications.
Viability of macrophage cells
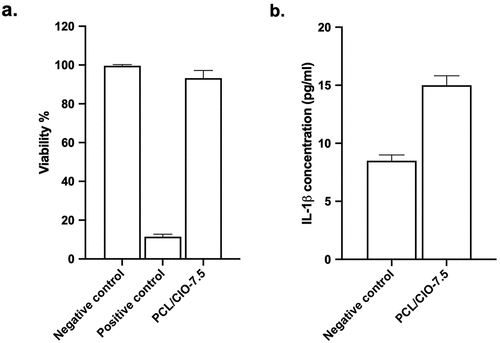
The viability of macrophage cells on PCL/CIO−7.5 fibre mats was evaluated, and the results showed a cell viability of 97.42 ± 2.78%, as depicted in (a). There was no significant difference observed between the negative control and PCL/CIO−7.5 (P > 0.05). These findings indicate that the PCL/CIO−7.5 fibre mats are not cytotoxic for macrophages, and therefore have the potential to be used in biomedical applications without adverse effects on cell viability. It is important to note that the biocompatibility of wound dressing materials is a crucial aspect to ensure they do not cause any harm to surrounding tissues and cells.
Interleukin−1β release assay
The levels of interleukin−1β in the media treated with PCL/CIO−7.5 and the control group were measured, and the results were presented in (b). The interleukin−1β concentration of the negative control and PCL/CIO−7.5 fibre mats were found to be 8.27 ± 0.20 pg/mL and 16.10 ± 0.15 pg/mL, respectively (P < 0.01 compared to the control). The results suggest that the PCL/CIO−7.5 fibre mats increased the secretion of interleukin−1β level as 7.83 pg/mL (p < 0.01 compared to the control).
Interleukin−1β is a proinflammatory cytokine that plays a crucial role in the regulation of the immune response during the wound healing process. It is known to activate macrophages, stimulate angiogenesis, and promote tissue repair [51]. In our study, we observed a significant increase in IL−1β levels following the application of a polycaprolactone (PCL) based wound dressing. This finding suggests that the PCL wound dressing may contribute to the modulation of the inflammatory response during the wound healing process. The enhanced IL−1β expression could be attributed to the biocompatibility and the porous structure of the PCL dressing, which may facilitate infiltration of immune cells, such as neutrophils and macrophages, into the wound site. As a result, these cells may release higher amounts of IL−1β, thereby promoting inflammation, angiogenesis, cell proliferation, matrix remodelling, and the overall wound healing process. While the increased IL−1β levels may be beneficial for promoting acute healing response, it is essential to consider the potential risk of prolonged inflammation and its negative impact on wound healing [51]. Further investigation is warranted to determine the optimal PCL dressing properties that provide a balance between promoting wound healing and minimising the risk of chronic inflammation. Additionally, studies comparing PCL-based wound dressings with other biomaterials would provide valuable insights into the therapeutic potential of PCL dressings in the context of wound management.
Conclusion
In this research, polycaprolactone (PCL) electrospun fibre mats loaded with Calophyllum inophyllum (CIO) essential oil were successfully fabricated and evaluated for potential use in wound healing applications. The morphological integrity of the fibres was maintained with the incorporation of increasing concentrations of CIO, and a slight increase in fibre diameter was observed. An important outcome of this study was the decrease in contact angle values of the CIO-loaded PCL fibre mats compared to the oil-free PCL fibre mats. This observation indicates improved wettability of the fibre mats, enhancing their potential application as antibacterial wound dressings or tissue engineering scaffolds.
Moreover, a controlled and sustained release of CIO was demonstrated by the PCL/CIO fibre mats, indicating their suitability for use in drug delivery applications. Compatibility with NHDF cells was also shown by these mats, suggesting their potential use in tissue engineering and regenerative medicine applications.
Based on the findings of this study, it was observed that CIO-loaded PCL electrospun fibre mats exhibited strong antibacterial properties and enhanced wound healing capabilities. Their suitability for managing a range of wounds, particularly those prone to bacterial infections such as open wounds, burns, and surgical wounds, is highlighted by these findings.
The sustained drug release profile and significant liquid absorption capacity of these fibre mats indicate that they could efficiently manage exuding wounds by maintaining a moist wound environment, which is critical for effective healing.
In terms of severity, the fibre mats could be particularly useful for treating moderate-to-severe wounds. However, further preclinical and clinical studies would be necessary to define precisely the wound types and severities for which these fibres would be most beneficial.
In conclusion, the potential of PCL/CIO electrospun fibre mats for use in wound healing and other biomedical applications has been demonstrated in this study. The need for further investigation and development in this field is underscored by these findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Rodrigues M, Kosaric N, Bonham CA, et al. WouNd healing: a cellular perspective. Physiol Rev. 2019;99. 665–14. doi:10.1152/physrev.00067.2017
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219. DOI:10.1177/0022034509359125.
- Ghomi ER, Khalili S, Khorasani SN, et al. WOund dressings: current advances and future directions. J Appl Polym Sci. 2019;136(27):47738. DOI:10.1002/APP.47738.
- Naeimi M, Noohi N. Fabrication and characterisation of antibacterial porous alginate/aloe vera structures containing chitosan nanoparticles for wound dressing applications. Mater Technol. 2021;37(8):822–828. DOI:10.1080/10667857.2021.1898713.
- Dweck AC, Meadows T. Tamanu (Calophyllum inophyllum) - the African, Asian, Polynesian and Pacific Panacea. Int J Cosmet Sci. 2002;24(6):341–348. DOI:10.1046/J.1467-2494.2002.00160.X.
- Nguyen VL, Truong CT, Nguyen BCQ, et al. Anti-inflammatory and wound healing activities of Calophyllolide isolated from Calophyllum Inophyllum Linn. PLoS ONE. 2017;12(10):e0185674. DOI:10.1371/JOURNAL.PONE.0185674.
- Poljšak N, Kreft S, Kočevar Glavač N. Vegetable butters and oils in skin wound healing: scientific evidence for new opportunities in dermatology. Phytother Res. 2020;34. 254–269. doi:10.1002/PTR.6524
- Erdemir A. Anti-proliferative and apoptosis inducing activity of Calophyllum Inophyllum L. oil extracts on C6 Glioma cell line. Biotech Studies. 2021;30(1):0–0. DOI:10.38042/BIOST.2021.30.01.01.
- Li D, Guo T, Zhong F, et al. Mechanically-enhanced fibre Topography via Electrospinning on a Poly(ε-Caprolactone) film for tendon tissue-engineering application. Materials Technology. 2021;37(9):1031–1039. DOI:10.1080/10667857.2021.1915664.
- Xue J, Wu T, Dai Y, et al. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev. 2019;119(8):5298. DOI:10.1021/ACS.CHEMREV.8B00593.
- Liu X, Xu H, Zhang M, et al. Electrospun medicated nanofibers for wound healing: review. Membranes 2021. 2021;11(10):770. DOI:10.3390/membranes11100770.
- Mostafavi P, Naeimi M. Investigation of Vitamin D-Loaded silk fibroin electrospun scaffolds for bone tissue engineering applications. Materials Technology. 2021;37(10):1329–1337. DOI:10.1080/10667857.2021.1940426.
- Gu X, Cao R, Li Y, et al. Three-component antibacterial membrane of poly(Butylene Carbonate), poly(Lactic Acid) and Chitosan prepared by electrospinning. Mater Technol. 2019;34(8):463–470. DOI:10.1080/10667857.2019.1576822.
- Aadil KR, Nathani A, Sharma CS, et al. Fabrication of biocompatible alginate-poly(Vinyl Alcohol) NanofIbers scaffolds for tissue engineering applications. Mater Technol. 2018;33. 507–512. doi:10.1080/10667857.2018.1473234
- Karimi Tar A, Karbasi S, Naghashzargar E, et al. Biodegradation and cellular evaluation of aligned and random poly (3-hydroxybutyrate)/chitosan electrospun scaffold for nerve tissue engineering applications. Materials Technology. 2019;35(2):92–101. DOI:10.1080/10667857.2019.1658170.
- Nanni G, Heredia-Guerrero JA, Paul UC, et al. POly(furfuryl alcohol)-polycaprolactone blends. Polymers. 2019;11(6):1069. DOI:10.3390/polym11061069.
- Altun E, Ahmed J, Onur Aydogdu M, et al. The effect of solvent and pressure on polycaprolactone solutions for particle and fibre formation. Eur Polym J. 2022;173:111300. DOI:10.1016/j.eurpolymj.2022.111300
- Adhikari J, Ghosh M, Das P, et al. PolycaprolActone assisted electrospinning of honey/betel with chitosan for tissue engineering. Mater Today Proc. 2022;57:307–315. DOI:10.1016/j.matpr.2022.03.096
- El Fawal G, Hong H, Mo X, et al. Fabrication of Scaffold based on Gelatin and Polycaprolactone (PCL) for wound dressing application. J Drug Deliv Sci Technol. 2021;63:102501. DOI:10.1016/J.JDDST.2021.102501
- Meteoglu I, Erdemir A. Genistein and Temozolomide-loaded Polymeric Nanoparticles: a Synergistic approach for improved anti-tumor efficacy against Glioblastoma. Process Biochem. 2021;110. 9–18. doi:10.1016/J.PROCBIO.2021.07.015
- Partheniadis I, Stathakis G, Tsalavouti D, et al. Essential oil—loaded nanofibers for pharmaceutical and biomedical applications: a systematic mini-review. Pharmaceutics. 2022;14(9):1799. DOI:10.3390/PHARMACEUTICS14091799.
- Haider A, Haider S, Kang IK. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arabian J Chem. 2018;11. 1165–1188. doi:10.1016/J.ARABJC.2015.11.015
- Amna R, Ali K, Malik MI, et al. A brief review of electrospinning of polymer nanofibers: history and main applications. JNMES. 2020;23(3):151–163. DOI:10.14447/JNMES.V23I3.A01.
- Rather AH, Wani TU, Khan RS, et al. ProsPects of polymeric nanofibers loaded with essential oils for biomedical and food-packaging applications. Int J Mol Sci. 2021;22(8):4017. DOI:10.3390/IJMS22084017.
- Mele E. Electrospinning of Essential Oils. Polymers. 2020;12(4):908. DOI:10.3390/POLYM12040908.
- Unalan I, Endlein SJ, Slavik B, et al. Evaluation of electrospun poly(ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics. 2019;11(11):570. DOI:10.3390/PHARMACEUTICS11110570.
- García-Moreno PJ, Guadix A, Guadix EM, et al. PhysIcal and oxidative stability of fish oil-in-water emulsions stabilized with fish protein hydrolysates. Food Chem. 2016;203:124–135. DOI:10.1016/J.FOODCHEM.2016.02.073
- Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. DOI:10.1016/J.BIOMATERIALS.2008.01.011.
- Garg K, Bowlin GL. Electrospinning jets and nanofibrous structures. Biomicrofluidics. 2011;5(1):013403. DOI:10.1063/1.3567097.
- Ura DP, Rosell-Llompart J, Zaszczyńska A, et al. THe role of electrical polarity in electrospinning and on the mechanical and structural properties of as-spun fibers. Materials. 2020;13(18):4169. DOI:10.3390/MA13184169
- Zeng W, Cheng NM, Liang X, et al. Wei electrospun polycaprolactone nanofibrous membranes loaded with baicalin for antibacterial wound dressing. Scientific Reports 2022 12:1 1–11, doi:10.1038/s41598-022-13141-0.
- Hafshah H, Prajitno DH, Roesyadi A. HydrotalCite catalyst for hydrocracking calophyllum inophyllum oil to biofuel: a comparative study with and without nickel impregnation. Bull Chem React Eng Catal. 2017;12(2):273–280. DOI:10.9767/BCREC.12.2.776.273-280.
- Mollaghadimi B. Preparation and characterisation of polycaprolactone–fibroin nanofibrous scaffolds containing Allicin. IET Nanobiotechnol. 2022;16(7–8):239–249. DOI:10.1049/NBT2.12092.
- Göksen G, Fabra MJ, Ekiz HI, et al. Phytochemical-loaded electrospun nanofibers as novel active edible films: characterization and antibacterial efficiency in cheese slices. Food Control. 2020;112:107133. DOI:10.1016/J.FOODCONT.2020.107133
- Unalan I, Slavik B, Buettner A, et al. PhysiCal and antibacterial properties of peppermint essential oil loaded poly (ε-Caprolactone) (PCL) electrospun fiber mats for wound healing. Front Bioeng Biotechnol. 2019;7. 346. doi:10.3389/fbioe.2019.00346
- Jin G, Prabhakaran MP, Kai D, et al. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials. 2013;34(3):724–734. DOI:10.1016/J.BIOMATERIALS.2012.10.026.
- Agnes Mary S, Giri Dev VR. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J Tex Inst. 2015;106(8):886–895. DOI:10.1080/00405000.2014.951247.
- Ardekani NT, Khorram M, Zomorodian K, et al. EvaluatIon of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with zataria multiflora essential oil as potential wound dressing. Int j biol macromol. 2019;125:743–750. DOI:10.1016/J.IJBIOMAC.2018.12.085
- Castillo-Henríquez L, Castro-Alpízar J, Lopretti-Correa M, et al. Exploration of bioengineered scaffolds composed of thermo-responsive polymers for drug delivery in wound healing. Int J Mol Sci. 2021;22. 1408. doi:10.3390/IJMS22031408
- Dias JR, Sousa A, Augusto A, et al. Electrospun Polycaprolactone (PCL) Degradation. An In Vitro And In Vivo Study Polymers (Basel). 2022;14(16):3397. DOI:10.3390/POLYM14163397.
- Sonmez T, Ardic B, Sevindik HG, et al. AntibacteRial effect of hypericum perforatum and calophyllum inophyllum against some bacteria causing infections in cesarean and episiotomy wounds corresponding author. Int J Caring Sci. 2021;14:1953–1960.
- Léguillier T, Lecsö-Bornet M, Lémus C, et al. The wound healing and antibacterial activity of five ethnomedical calophyllum inophyllum oils: an alternative therapeutic strategy to treat infected wounds. PLoS ONE. 2015;10(9):e0138602. DOI:10.1371/JOURNAL.PONE.0138602
- Phaiju S, Mulmi P, Shahi DK, et al. AntibActerial cinnamon essential oil incorporated poly(ɛ−caprolactone) nanofibrous mats: new platform for biomedical application. J Inst Sci Tech. 2020;25(2):9–16. DOI:10.3126/JIST.V25I2.33724.
- Mouro C, Simões M, Gouveia IC, et al. Emulsion electrospun fiber mats of PCL/PVA/Chitosan and eugenol for wound dressing applications. Adv Polym Technol. 2019;2019:1–11. doi: 10.1155/2019/9859506
- Chen X, Zhang Q, Wang Y, et al. FabriCation and characterization of electrospun poly(Caprolactone)/Tannic Acid scaffold as an antibacterial wound dressing. Polymers. 2023;15(3):593. DOI:10.3390/POLYM15030593/S1.
- Gheorghita D, Grosu E, Robu A, et al. EsseNtial oils as antimicrobial active substances in wound dressings. Materials 2022. 2022;15(19):6923. DOI:10.3390/MA15196923.
- Kahdim QS, Abdelmoula N, Al-Karagoly H, et al. Fabrication of a polycaprolactone/Chitosan nanofibrous scaffold loaded with nigella sativa extract for biomedical applications. Biotech. 2023;12(1):19. DOI:10.3390/BIOTECH12010019.
- Doustdar F, Ramezani S, Ghorbani M, et al. Optimization and characterization of a novel tea tree oil-integrated poly (ε-Caprolactone)/Soy protein isolate electrospun mat as a wound care system. Int J Pharm. 2022;627:122218. DOI:10.1016/J.IJPHARM.2022.122218
- Michalak M. Plant-derived antioxidants: significance in skin health and the ageing process. Int J Mol Sci. 2022;23. 585. doi:10.3390/IJMS23020585
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720. DOI:10.1182/BLOOD-2010-07-273417.