ABSTRACT
Bio-artificial organs, commonly referred to as bionic organs, are artificial structures that perform the same functions as natural organs. In regenerative medicine, damaged organs are repaired using biological components including growth factors and stem cells. The advancement of tissue engineering, which aims to harness the inherent regenerative potential of human body organs to rebuild normal biological function, has benefited greatly from the use of biomaterials. The rapidly expanding field of regenerative medicine has brought the usage of biomaterials and their functions in the production of new tissue. Organs-on-chips are seen as a concept performer in tissue engineering with significant potential for future ‘clinical trials on a chip’ and a step towards developing customized medicine. With the advancement of 3D printing technology, the manufacturing constraints of biomedical devices have been solved using cutting-edge biomimetic structures. This review covers the areas on bionic organs such as organ on a chip with tissue engineering, bio grafting, 3D bio-printing and the stem cell techniques that are employed in regenerative medicine.
Introduction
The human body is made up of intricate structures and material systems, current manufacturing techniques, which are constrained in their ability to choose and fabricate materials, are unable to produce biomedical devices that have intricate bio-inspired architectures and structures, which hinders the development of biomedical devices for a variety of applications. Due to their exceptional fabrication abilities, 3D printing techniques are widely used in many industries, including biomedical engineering, automotive, aviation, telecommunications, civil engineering, and sustainable energy [Citation1,Citation2]. By layer-by-layer depositing materials, such as metals, polymers, and ceramics, three-dimensional (3D) printing, sometimes referred to as additive manufacturing, is a technique that can create items with complicated architecture. Technology for 3D printing in the medical areas is a promising method for individualised care [Citation3]. One of the most alluring applications of bioprinting and bio fabrication is the development of biological 3D tissue models, such as those for drug screening, disease models, tissues or organ on a microchip, healthcare sensors, and biological actuators. To aid surgeons in their work and to better educate patients about their illnesses, 3D copies of a patient’s damaged organ could be created. Scaffolds and implants are created using 3D printing processes for regenerative medicine. Stem cells for example, are routinely expanded ex vivo as part of the current tissue engineering approach before being transplanted into injured sites. Stem cells have considerable promise in tissue engineering and reconstructive therapeutics because of their distinct regeneration capacity and immune modulatory qualities. Tissue engineering depends heavily on biomaterials, but this does not mean that conventional, artificial biomaterials must be employed [Citation4]. By using stem cells sown on artificial structures to replace damaged tissues, tissue engineering aims to regain the normal regulation of cell activity. The 3D surroundings inside the stem cell niche have an impact on stem cell therapy self-renewal and differentiation [Citation5]. The scaffold approach has been abandoned, and attempts to create 3D tissue using cells and growth regulators are now regularly documented. Bio fabrication is the term used to describe this approach, which includes organ printing and bioprinting [Citation1].
The creation of novel biomaterials-bound techniques that better imitate tissue and organ architecture is largely responsible for the recent successes in tissue engineering [Citation6]. Tissue engineering can be approached in a number of ways, including scaffold- and biomaterial-based procedures, the use of decellularized organic substances, scaffold-free techniques, and the inclusion of cellular components. The factors around a cell or a group of cells that have a direct or indirect impact on cell activity via physicochemical, biochemical, or other processes make up the cell microenvironment. Given that stem cells are presently the most technically possible source that can supply the significant number of cells required for engineering clinically relevant portions of tissue, the microenvironment of stem cells is an especially significant subject matter in regenerative medicine and tissue engineering [Citation7]. The potential and capacity to rejuvenate and rebuild injured organs and tissues is the foundation of the potential of regenerative medicine. With the potential to even treat some congenital defects, regenerative medicine has demonstrated encouraging outcomes for the restoration and regeneration of a number of tissues and organs, such skin, heart, kidneys, and liver. The components used in regenerative medicine strategies must be capable of replacing the damaged tissue/organ and operate as the original tissue/organ or be able to promote the restoration of the original tissue tissue in order for them to be effective. These components are typically mixtures of scaffolds, biomaterials, growth factors, and stem cells. In the past ten years, the Food and Drug Administration as well as the European Medicines Agency have approved a number of 3D-bioprinted structures and stem cell treatments. These treatments and goods include biologics, medical equipment, and biopharmaceuticals [Citation8]. In this review, we will focus on different aspects of tissue engineering applications with particular focus on bionic organs, 3D bioprinting, tissue grating, stem cells and regenerative medicine.
Bionic organs
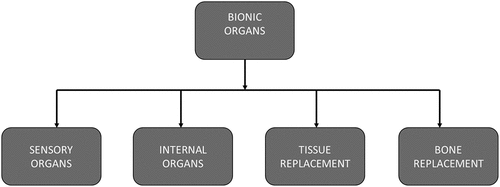
An organ is a unique part of the animal or plant that carries out special functions. These organs can become damaged and should be replaced with a prosthetic device or artificial organ [Citation9]. These replacement devices are often made of natural-based or synthetic-based materials and should be compatible with the blood and tissues. The bionic organs resemble the functioning of the original defective organ. These bionic organs are broadly divided into four types as depicted in [Citation10].
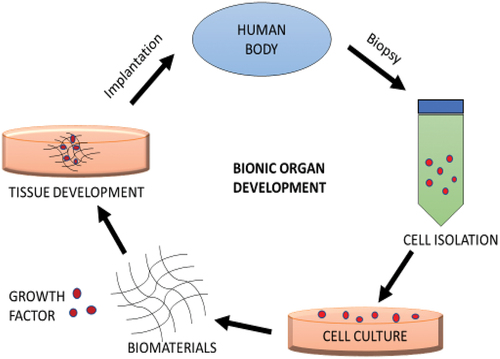
The bionic or artificial organ serves as a substitute for a biological organ in the human body. An organ is described as a specialised structure such as a brain, heart, and lungs in a human or animal which is made up of distinct tissues and cells and is specialised for its particular functions [Citation11]. The bionic organ development technology originated as a curative therapy for many difficult chronic illnesses with the use of stem cell technology along with growth factors and biomaterials. Although it is still in the development phase, regenerative medicine and tissue engineering can reduce the challenges associated with bionic organ development and implantations () [Citation12].
Classification of bionic organs
Sensory organ
For the replacement of the outer portions of the ear, polymeric biomaterials like silicons can be used. Additionally, some study has included inserting electrodes into the cochlea and connecting them to an outside microphone [Citation13]. People who are deaf have been given considerable hearing restoration because of using these gadgets. Generally, plastic is used for coating the cables and electrical components of the device. Several people wear contact lenses, which are the most useful application of polymeric materials. Hydrogel polymeric materials are used to make these contact lenses [Citation14,Citation15]. Additionally, some research is being done to directly stimulate the visual part of the brain using electrodes coupled to a camera, giving the illusion of vision to the blind.
Internal organs
The heart, liver, kidney, pancreas, gastrointestinal tract and lungs are among the artificial internal organs. These prosthetic replacements frequently perform specific tasks that are hard to replicate with artificial materials [Citation16]. Over one million pacemaker devices are implanted every year to control heart rate and these devices have biomaterial coatings to shield the electrical components from the bloodstream. To cure Stokes-Adam’s illness, pacemaker devices have been used on animals for experimental studies since 1932 and people since 1952 for treating heart blocks. Even though the heart has four chambers, the arterial or ventricular valves are always replaced [Citation17]. These valve replacements come in a wide range of designs that make use of both synthetic-based and natural-based biomaterials.
Tissue replacement
The skin is the greatest organ in the body. Although several people are seriously injured each year from skin damage that requires rapid treatment to avoid microbial contamination, there is currently no substance that can replace the functioning of skin [Citation18]. The effective solution to this issue has made use of a composite material with an outside layer made of silicone rubber and an interior membrane made of collagen-glycosaminoglycan. Other composite materials include collagen, polypeptides, dextran and hydrogels. These materials effectively mimic the skin’s barrier functions, but none of the materials have been able to mimic the other functions of the skin [Citation19]. The human body is mainly composed of connective tissues, soft tissues and fatty tissues. This kind of tissue is used extensively in the field of reconstructive or plastic surgery. At least 30,000 hernia repairs, 100,000 breast augmentation and 100,000 face plastic operations are performed annually [Citation20]. An appropriate soft tissue material must be physically suitable over the long term and should not have any negative effects on the patient and only a small range of materials can satisfy the required needs. Although polymers and fabrics may contain the right amount of soft texture, the ingrowth of fibrous tissue rapidly makes these materials unsuitable since the implantation is becoming more stiff or hard.
Joint/bone replacement
To treat an injury or chronic disease-related condition, it is essential to replace a damaged joint with a prosthetic device. Several joint replacement implants are performed each year with a poly-dimethylsiloxane polymer [Citation21]. Silicone polymer has also taken the place of several applications, including hand bone replacement. A metal ball comes into contact with a plastic surface consisting of high-molecular-weight polyethylene biomaterial in other types of joint replacement such as the knee and hip. Most of the time, a cement-like material made of polymethyl methacrylate that is polymerised in place is used to secure these plastic and metal components to the body [Citation22]. Designing an effective prosthesis arm is more challenging than designing a hand. It is more challenging to develop lower limb implants than upper limb implants. This is because the main use of the lower limbs is walking, in contrast to numerous upper limb usage which cannot be accomplished with one limb [Citation23]. Although there is still more work to be done in this field, advanced electrical stimulation-actuated devices offer great potential for mimicking the original arm functions [Citation24].
Organs-On-Chip (OOC) development
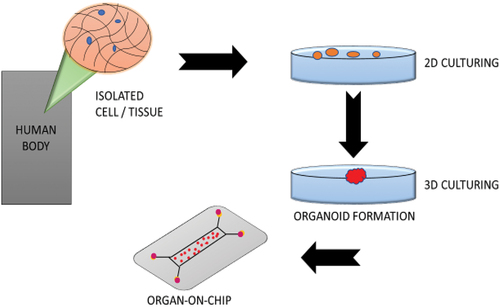
The Organs-on-chip (OOC) that emerged in 2010 is regarded as an extremely promising prospect for studying the mechanisms behind drug screening and organ functioning platforms with future developments to be utilised for targeted therapy and clinical diagnostics () [Citation25]. Donald Ingber was the first to establish the idea of OOC. A microfluidic culture known as an OOC has chambers that are continually stimulated with live cells inside of them to mimic the motions, physiological reactions and biomechanics of tissue or organ. The first lung-on-a-chip model was created by Dr. Ingber and his colleagues at the Wyss Institute. It is a microfluidic chip that simulates breathing, highly resistant lungs made of human passages, vasculature, and inflammatory responses [Citation26].
Other early instances of OOC, such as lymph node-on-a-chip, blood vessels-on-a-chip and intestine-on-a-chip have been introduced. Then more advanced devices that link many organ systems were introduced. OOCs are models produced by integrating advanced chip technology, which may offer these new models. In comparison to other methods mentioned, this can modify human reactions such that they can be effectively mimicked in the laboratory [Citation27]. In the chip tissue models, microfluidic technologies provide unique and improved controllability over cultural and cellular conditions. The idea of OOC refers to the emerging autonomous and active tissue and organs system with a structure that aims to mimic in vivo organ conditions [Citation28]. Another critical step for the modification and analysis of cellular activity is the integration of simulation and sensor technology on-chip and their interface with cell cultures. The various organ development using the chip and its function has been mentioned in [Citation29].
Table 1. Various Organs-On-Chip and its functions.
Challenges in bionic organ development
Bioartificial tissue design is not simple despite the fact it is created using a variety of component tools, including scaffolds, bioreactors, growth and differentiation agents, cells and so on [Citation43]. None of these parts make up a device with separate functions, hence neither does their combination in an in vitro method for bionic organ synthesis result in a product with a modular structure. Biofilm development is one of the challenges of bionic organ development which is detailed in .
Table 2. Challenges in the development of bionic organs.
Biofilm development in implants
A bionic organ or a prosthetic device develops bacteria growth and becomes the cause of device-related infections known as biofilm development [Citation52,Citation53]. The definition of biofilm was suggested by Donlan and Costerton as a microbially formed motile organism containing cells that are physically attached to surfaces or their interfaces, incorporated in matrix substances they have developed and displaying irregular growth patterns and expression of genes [Citation54]. This new generation of environmental diseases is especially dangerous to vulnerable people who might not have recovered previously. They make use of the biofilm approach that has worked so effectively for them in their natural surroundings. Some human illnesses are not linked to devices but are driven by bacteria that form a biofilm [Citation55]. It is debatable if biofilms are the true aetiological agents in these situations because the conventional Koch’s postulate cannot be used. Heart infections are caused by staphylococci 25%, fungi and gram-negative bacteria caused 30% and Streptococci produced 56% of infections [Citation56]. They enter the bloodstream through the gastrointestinal tract.
Biofilm in medical devices
Over the past 20 years, a great deal of study has been done on the biofilms that develop on various medical implants. According to Donlan and Costerton, a characterisation of the biofilm’s attachment on certain devices, including artificial heart valves, urinary bladder, central venous catheters, intrauterine devices and contact lenses [Citation57]. For some devices like contact lenses research has also clarified the sensitivity of different materials to microbial attachment and biofilm development. The implants that are mainly harmed by infections are dental implants, neurological implants, ophthalmological implants and orthopaedic fracture fixation implants [Citation58]. Along with adhering to hygiene standards while implanting medical devices, another emerging approach is the development of novel materials that could be resistant to microbial adhesion and colonisation [Citation59]. The best options to prevent colonisation and contamination would be medical devices constructed of a material that is generally colonisation-resistant or antiadhesive.
Dawn of tissue engineering in bionic organs
The tissue engineering approach is evolving rapidly to meet the challenges and main issues, taking into consideration the advancements in tissue engineering using animal modelling, scaffold preparation techniques, and 3D bioprinting [Citation60]. The tissue engineering field has great potential for overcoming a variety of obstacles with inventive advancements, and will soon bring numerous advancements that can effectively be applied to humans [Citation61]. There are new opportunities for research in several scientific fields, such as genetics, human biology, pharmacokinetics, and others by involving stem cells and tissue engineering for the regeneration of bionic organs [Citation62]. The fundamental of tissue engineering is to transform the cells that can begin and maintain the regeneration process, maybe using growth hormones or genetic material, so that they can produce new, functioning tissue of the requirement [Citation63]. This can be done with scaffold biomaterials to direct the structural form of the new tissue, and it can happen on an individual basis at the location of the tissue or organ damage in a single patient or on a larger scale in a fermentation broth by which the newly generated tissue is implanted into the patient body [Citation64].
Biomaterials for bionic organ development
The primary function of biomaterials in tissue engineering is to regulate the activity of the regenerated tissue also providing temporary mechanical support and mass transfer to promote cell attachment, multiplication, and division. Considering that extracellular matrix (ECM) biomaterials contain the intrinsic signals vital for interacting with and controlling niche cells [Citation65,Citation66]. Biomaterials which are frequently referred to as scaffolds may present biological and chemical signals with spatio-temporal accuracy (). These signals are of high significance to the regulation of cell effectiveness and function and tissue regeneration. To help in the restoration of a tissue’s entire function and structure, tissue-engineered structures must resemble some of the natural complexity of a tissue [Citation67]. A scaffold should act as a temporary construction that over time, will be gradually decayed or recycled back in line with the rate at which new tissue is developing. The potential of a biomaterial to regulate human host reactions to externally transplanted cells may alter cellular mechanisms and function and in turn, have a significant impact on the formation of the desired tissue [Citation68]. The developments in the field of biomaterials, along with recent advances in the understanding of the biology of the (ECM) and the significance of environmental factors in tissue development, have resulted in the new design of material templates that are altered to provide suitable structural support and in some cases, to promote the safe and successful regeneration of tissue [Citation69]. Using scaffolding materials to build artificial organs and tissue or as material devices or implants to cure or replace original organs or tissues are the main application of biomaterials [Citation70]. Tissue-engineered scaffolds aim to at least partially resemble the biological ECM and produce a supportive environment to promote tissue development. The development of many medical implants for clinical rejuvenation treatments and the bioengineering design paradigm both owe much to the biomaterials that support and encourage restorative cell growth. Natural and synthetic biomaterials made of two or more materials are only a few of the many alternatives available for constructing a specific biomaterial to be utilised as a matrix pattern. It is necessary to assess the benefits and drawbacks of using these biomaterials as well as their compatibility for use. These biomaterials are classified into natural and synthetic-based biomaterials.
Naturally derived biomaterial
Due to their inherent structural similarity to native tissue ECM, biocompatibility, mechanical dynamics and degradation, natural biomaterials make up an essential subgroup of biomaterials for use as bioengineering templates. These natural biomaterials are synthesised using eco-friendly methods [Citation72]. Environmentally friendly aqueous-based processing techniques are frequently used to handle natural biopolymers. They are not toxic when applied to biological systems and the beginning formulation and processing conditions may be changed to alter the rate of degradation [Citation73]. Natural biomaterials have the property of naturally stimulating cellular recognition, which may help to increase cell attachment and functioning. A broad range of hierarchically organised materials in nature depend on polymers including chitin, collagen and cellulose for their growth and activity [Citation72]. Protein-based biomaterials include gelatin, collagen, fibrin, keratin, eggshell membrane and silk fibroin [Citation74]. Polysaccharide-based biomaterials are cellulose, hyaluronan, alginate, glucose, chondroitin and chitin. While polysaccharide-based biomaterials are mostly derived from microbial or algal sources, such as in the case of dextran and its derivative, protein-based biomaterials are often derived from human and animal sources and contain bioactive compounds that resemble the extracellular environment [Citation72]. Decellularized tissue-derived biomaterials are a category of natural biomaterials that are produced by removing all nuclear and cellular components from native organs and tissues. Examples of these materials include decellularized skin, blood vessels, heart valves and the liver [Citation75]. Natural polymers have been investigated in the development of tissue engineering templates due to their key advantage in supporting cell attachment, propagation, and differentiation [Citation76]. Frequently combined with molecular and mechanical signals, these templates are used for applications ranging from tissue regeneration to functional organ replacement. These polymers are often prepared for transplantation as porous scaffolds, thin membranes or hydrogel particles and are mainly enzymatically biodegradable into non-toxic end products for medical or therapeutic uses [Citation77]. These biomaterials are still effective if local, immediate action is adequate, despite the kinetics of the destruction being difficult to manage. Injectable hydrogel, for example, is a unique type of natural polymer that may be given non-invasively to a target region of tissue injury.
Synthetically derived biomaterial
In comparison to naturally derived biomaterials, the use of synthetically derived biomaterials such as matrix and templates in tissue engineering offers several significant advantages, including appealing options for the architecture and control of shape to produce acceptable substitutes for limitations of ECM systems-based biomaterial on a human origin that mimic biomaterial functions [Citation78]. Polyglycolic acid (PGA), (Poly-hydroxy acids) which include polylactic acid (PLA) and their copolymer, poly(lactic-co-glycolic acid) (PLGA), are the most often employed synthetic polymers for tissue regeneration [Citation78]. Glycolic acid and lactic acid, the harmless breakdown products of these polymers, are produced via chemical hydrolysis of the polymeric materials and eliminated through regular metabolic processes. Chemical hydrolysis may be simpler to predict and regulate than enzymatic decay in vivo due to its independence from local enzyme concentrations [Citation79]. By adjusting the lactide ratio and polymerisation conditions, synthetic polymers’ characteristics, such as mechanical modulus, degradation rate and tensile strength may be easily adjusted for specified applications [Citation80]. These materials have been effectively used in the clinic to replace or create urethral tissue in patients. The wide range of biomedical applications of several synthetic polymers, such as PEG, PCL, PLGA, PLA and PVA are due to the tissue regeneration of ECM-like biomaterial because of its biocompatibility [Citation70]. While scaffolds made of synthetically derived biomaterials can have fully interlinked holes, some types, such as polyhydroxy esters have the potential to release acidic breakdown products that can change the pH of the tissues around them. Consequently, this pH fluctuation may have a severe effect on cell activity and viability and may result in tissue damage and inflammation. However, due to a lack of physiologically functioning features, synthetic biomaterials normally do not pose a threat of eliciting an immune reaction [Citation81]. Integrating biological domains into synthetic biomaterial templates using a variety of synthesis processes that have been developed and perfected makes it possible to create scaffold biomaterials with a known and adjustable structure. The most effective method for inducing desirable cell-material interactions is to place bioactive substances on synthetic biomaterial templates.
ECM (Extracellular Matrix) based biomaterial
The ECM or extracellular macromolecule matrix is a multi-component structural element that is created and put together by the effector cells. It consists of a variety of multidomain biopolymers as well as common structural biomolecules [Citation82]. Its protein network controls the fibre width, structure, and arrangement, maintaining stability with the cells around it. In most organs and tissues in the human body, the ECM complex, biomaterial 3D network of proteins and carbohydrates, cell composition and set of physical characteristics. The extracellular matrix (ECM) of living things can be distributed as an undifferentiated ‘organic matrix’ or organised into interconnecting fibre structures that are ordered in a cell or tissue-specific way [Citation83]. It is well recognised that the living-tissue ECM has several roles in tissue formation and restoration, including determining the stability and structure of tissues, acting as a reservoir for morphogens, and supporting resident cells physically [Citation83]. ECMs are the subject of extensive research work carried out in various parts of the world. These projects aim to demonstrate the nature and special characteristics of ECMs in physiology and composites science as well as for tissue engineering and applications in regenerative medicine [Citation84–86]. Understanding the biological data and elements of ECM for biomaterials design has received a lot of interest over the past few years. These tissues have ECM biomaterial arrangement and composition which reflect adaptation to pressurised conditions and each ECM substance interacts with tissue-forming cells in a distinctive way that has been researched for application in various regeneration approaches [Citation87]. The development of medical devices for tissue regeneration depends on the design and structure of the biomaterials used. Recently, there has been a rise in interest in the ECM-based biomaterials of a certain tissue composition as well as in each matrix component’s physiological and developmental responsibilities. The chemical, physical and structural characteristics of numerous ECM components and naturally occurring proteins and substrates that could facilitate the application of studies on scaffold biomaterials have been and the overall results are successful in tissue regeneration [Citation88]. The highly organised pattern of any ECM-embedded and cell surface-associated constituents suggests essential structural and regulatory functions in tissue function as well as translational effects for the diagnosis and treatment of disease [Citation89].
Challenges in developing biomaterial
The injury due to illness or infection of each tissue frequently exhibits a distinct sequence of tissue regeneration mechanisms. However, comparable cellular and molecular activities during tissue regeneration do occur. The tissue regeneration stages comprise several signalling elements while maintaining strict temporal and spatial control, resulting in the optimum potential tissue regeneration [Citation90]. The cellular scaffold is a biomaterial with mechanical and physical properties that match with the target tissue and that contain a variety of growth factors and adhesion molecules from cells that can stimulate a regenerative extracellular matrix for the right cell populations and alters their behaviour in addition to biocompatibility [Citation91]. A template can not fulfill the criteria for a regenerative extracellular matrix because it lacks the required bioavailability and mechanical characteristics, and more critically, it lacks the target cell interactions to regulate the expression of genes, structure, and kinetics [Citation70].
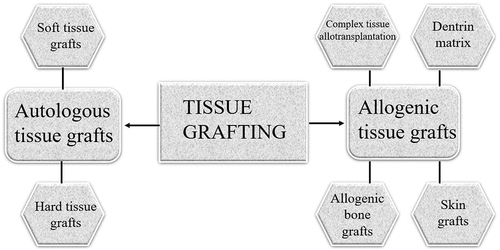
Tissue grafting
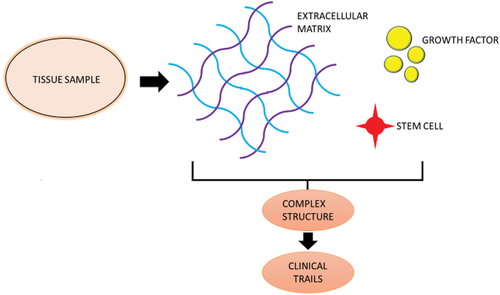
Currently, a wide range of substances can be considered biomaterials, including man-made structures, therapeutic cells, and even a variety of living tissues or organs utilised in transplantation [Citation71]. Consequently, tissue grafts can be viewed as the standard ‘biomaterials’ for reconstructive therapies [Citation92]. Tissue grafting is a transplant of tissues or cells from one part of a person’s body to another. Soft and hard tissue grafts for tissue reconstruction in clinics have increased in popularity during the past two decades [Citation93]. It is evolved in a desire to develop and improve bionic organs and to recover damaged or impaired organs. The use of cells and biomaterials in tissue grafting to create lab-grown tissue/organ replacements that mimic the functionality of native tissue has been driven by efforts in the field of organ transplants, as well as in 3D bioprinting [Citation94]. The previous research was carried out over several years, and the scientists working in the modern bioengineering fields, whether it be tissue (or organ) auto/allo grafting or regenerative medicine or tissue engineering, still adhere to some of the ideas set by those pioneering researchers, the primary objectives are still the same. Here, we have a general understanding of the benefits and drawbacks of various tissue resources and graft types that can give surgeons verified guidelines for graft selection in medical research [Citation95]. Additionally, knowing the composition and structure of native tissue and reviewing the clinical advantages of tissue grafts can provide practical tissue engineers with crucial knowledge on the development of cutting-edge biomaterials and new design innovations ().
Autologous tissue grafts
Tissue grafts are the first application of human-origin biomaterials for the improvement of bionic organs and therapeutic purposes. Even now, a lot of biomedical professionals still claim that autologous tissue is the ideal tissue for repairing the majority of tissue damage [Citation96]. Autologous tissue grafts (autografts) retain significant quantities of living cells and have all the characteristics necessary for new tissue development and structural restoration, they are the standard to which all other implanted biomaterials are compared. The main objective of tissue engineering techniques is to create a tissue composite that, when implanted, performs biologically in a manner identical to or comparable to that of an autologous tissue graft [Citation97]. The application of autologous tissue grafts remains the clinical gold standard for tissue regeneration and reconstruction; a primary vasculature in the implants or bionic organ is frequently grafted to the graft as a resource of blood perfusion [Citation98]. There’s been a lot of research on soft-tissue grafts, especially the use of autologous grafts for the treatment of cutaneous injuries (skin injury), the treatment of soft-tissue volume deficits, and the rebuilding of artificial human body parts [Citation99]. However, the method’s application has been severely constrained due to worries regarding graft survival after in vivo transplantation. To overcome these challenges, recent research has concentrated on the fabrication of cellularized artificial grafts. A healthy endothelium membrane is the best anti-thrombogenic component, as is widely recognised. To assess the theory, autologous endothelial cells (EC) obtained from the saphenous vein were inoculated into the non-heparinised blood and employed to pre-clot the graft before implantation [Citation100]. This technique raised the patency rate of dacron prostheses. Dacron is a polyester graft that is usually employed in the fabrication of artificial organs.
In past years, the target of dentistry research has been to enhance the quality of tooth and dental implant grafts [Citation101]. Gingival recession treatment is a common demand from patients because of its major impact on dentin hypersensitivity and aesthetics. In this regard, gingival recession can be effectively addressed with a free gingival graft (FGG), which is most frequently combined with a coronally positioned graft [Citation102]. The free gingival graft (FGG) is a soft tissue transplant obtained from the palate that has the epithelium still attached [Citation103]. As [Citation104] previously described, an FGG can be used directly to fill the root and restore the gingival margin to its appropriate configuration. In the attempt to increase root coverage capacity and mucogingival junction alignments, customised procedures based on FGGs be effective in the treatment of isolated and numerous gingival recessions along both the upper and lower incisors and premolars. This effective implementation is especially applicable in the fields of implant surgery and periodontics [Citation105].
Allogenic tissue grafts
Even though an autograft is the greatest option for managing artificial organs, there are drawbacks to autologous grafts, including the lack of graft supply and the toxicity of the grafts. Numerous alternative grafting components that fall under the following classifications can be employed to overcome the restrictions associated with the acquisition of autologous tissue grafts:
(i) Xenografts are components obtained from another species and are commonly referred to as xenogenic grafts or heterografts (Namekawa et al., 2019).
(ii) Synthetic grafts, also known as alloplastic grafts, are artificial materials that are classified according to their origin of production and chemical configuration (Popov et al., 2020).
(iii) Allografts are made of components obtained from another member of the same species and are also referred to as homografts, homologous or allogenic grafts (Prat-Vidal et al., 2020).
Effective allogenic material transplantation into individuals relies on minimising risks while maximising benefits. An important key factor influencing the rejection of grafts in individuals is the immunological response. However, with optimal enhanced immunosuppressive medication and improved human leukocyte antigen (HLA), the allograft prognosis can be improved [Citation106].
3D bioprinting
Three-dimensional tissue engineering
A significant number of tissue engineering research has been carried out on the three main components which are growth factors, cells and scaffolds. However, there are several drawbacks to employing scaffolds. The idea of the scaffold has fallen out of favour in recent years, and attempts to synthesise 3D tissue using cells and growth factors are now regularly documented [Citation107]. This technology is known as biofabrication, and it includes the techniques of organ printing and bioprinting [Citation108,Citation109]. Furthermore, while 3D structures employing inkjet printer technology have already emerged as the quick prototype, a 3D printer with an inkjet nozzle out of which droplets with a volume identical to that of cells are embossed and can be handled in a sterile atmosphere has been designed [Citation110]. This could pave the way for the development of 3D tissues [Citation111].
3D printing of artificial organs
3D bioprinting is a revolutionary approach capable of designing and manufacturing tissue-specific constructs by constructing complex, heterocellular formations with micro-scale precision. With the use of 3D bioprinting, a variety of biologics, such as genes, cells, hydrogels, growth factors and neo-tissues with ECM protein modifications, can be deposited [Citation96]. Even though these discoveries have resulted in significant advancement over time, bioprinting has drawn criticism for its limited survival durations, capacity to only produce thin tissues, and difficulty to replicate complex composite tissues [Citation112]. However, bioprinting is gaining popularity in the fields of regenerative medicine and pharmaceuticals. This technique especially serves as the foundation for designing and enhancing bionic organs, tissues, organoids, and organs-on-chip models [Citation114]. Bionic organs currently play a significant part in several areas, offering a viable method to create biological substitutes for natural human tissues or organs for a variety of applications, including tissue synthesis, drug screening and organ transplantation in the field of biomedical engineering [Citation113]. Artificial organs and tissues help to address the issue of limited organ donation for organ transplantation. Numerous ways have been employed to create artificial organs and tissues using palliative techniques [Citation115]. Nevertheless, the morphologies and material systems of organs and tissues in humans are complex. Most existing manufacturing techniques can only accurately reproduce the early stages of the disease, and there are numerous barriers to achieving the living organism’s normal tissue functions [Citation116]. Even though some techniques can simulate several types of tissues, they weren’t ideal for high-throughput validation because of their significant expense, several intricate fabricating steps, and time-consuming trials [Citation117].
Advanced bioprinting has been extensively researched and employed to attempt to build artificial tissues and organs, including the heart, skin, liver and muscle. These organs and tissues can be utilised for disease modelling, drug testing, organ transplantation and toxicity testing [Citation118,Citation119]. For instance, airflow-assisted 3D bioprinting is a cutting-edge technique to fabricate tissues on spiral-based microspheres with great resolution and complex microarchitectures. By embedding multiple cells into the spiral-based spheroids, which were geometrically multiple scalars and cell-orientational, it was possible to create viable organoids in vitro and create new, asymmetrical biomimetic models for regenerative medicine and basic biomedical research [Citation106]. Additionally, heterogeneous spheroids provide benefits like multi-components, adjustable morphology, and usability [Citation120].
In recent years, 3D bioprinting has been utilised to deposit layers of bio-inks made of biocompatible polymer matrix and living cells to create sophisticated artificial tissues and organs [Citation121,Citation122]. However, it’s not always possible to replicate the organs. Overall, most of the stated problems of bioprinting technologies must be addressed to mass-produce artificial tissues and organs, and printable biomaterials that are expected to imitate the material constituent of target tissues and organs.
Methods of 3D bioprinting
Bioprinting allows for the controllable and automatic fabrication of live biological models [Citation123]. However, it depends on the selected carrier. The critical factors of this entail:
Production of a bio-ink for bioprinting that accurately resembles the environment of native cells.
Enhancing the atmosphere for the bioprinting procedure.
Appraisal of the produced bioconstruct.
Assessing the status of bioprinted cells.
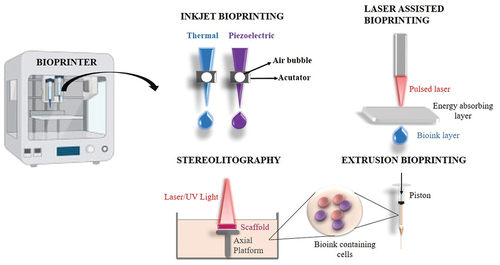
As shown in , Inkjet bioprinting, Laser assisted bioprinting, Stereolithography bioprinting and Microextrusion bioprinting were all widely employed in the construction of artificial organs and tissue. provides a brief comparison of various approaches in 3D bioprinting techniques.
Table 3. Comparison of various 3D bioprinting techniques.
Functionalization of bionic organs by regenerative medicine
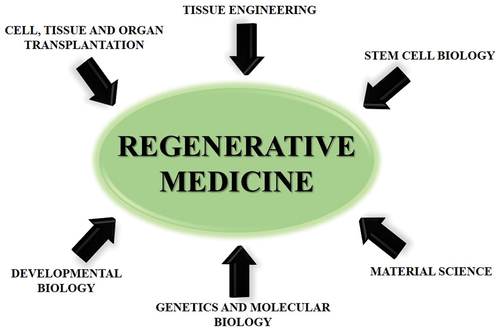
Regenerative medicine is the next phase in the progression of bionic organs. It is generally prompted by the same medical demands as for the development of artificial organs, transplants and replacement therapy, but has much more ambitious goals than traditional methods [Citation135]. It seeks not only to replace what is defective, but also to offer the materials essential for in-vivo restoration, to design replacements that integrate perfectly with the live body, and to stimulate and assist the body’s inherent abilities to regenerate and heal oneself [Citation136]. The essence of regenerative medicine is recognising and utilising the latent potential of cells, and it leads to a huge amount of research in the area [Citation137–139]. It goes beyond conventional transplantation and replacement therapies by combining a number of convergent techniques, in both traditional and developing approaches [Citation140]. One of the main challenges in the development of the field of tissue engineering is the requirement for cell sources. The phrase ‘regenerative medicine’ was coined as a result of the increased need for renewable cells, such as stem cells () [Citation8].
Stem cells in regenerative medicines
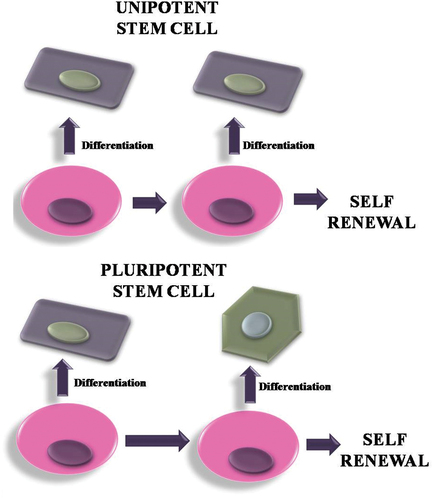
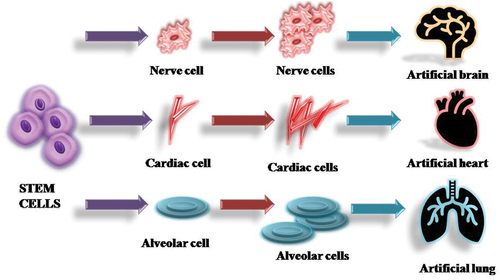
In the discipline of regenerative medicine, stem cells are employed to regenerate the body’s damaged parts, their capacity to self-renew and specialise into every type of cell in the body demonstrates their vital significance [Citation141]. Stem cells offer the ability to create human disease models, which would improve understanding of the pathogenetic mechanism of human diseases and allow for advancements in cell-based treatment for degenerative disorders [Citation142]. According to a study published in 2010 [Citation143], human-derived stem cells grown on polymer scaffolds weren’t any longer noticeable after a few days of implantation in an immune-deficient mice recipient. Instead, murine monocytes rapidly overpopulated the scaffolds, followed by smooth muscle and endothelial cells. The pre-seeded and transplanted stem cells are believed to have produced a large number of monocyte chemoattractant protein-1, which has increased early monocyte recruitment in the recipient, according to the authors’ original hypothesis. These observations imply that tissue regeneration is not solely a cell-restoration mechanism, but also involves an inflammatory mechanism. However, it remains completely unclear what biological function and regulatory mechanisms are engaged in this secretion-induced inflammatory process. Various sub-types of stem cells are employed in the tissue regeneration mechanism, embryonic stem cells are one of them. The blastocyst is where embryonic stem cells are produced following fertilisation. They are naturally pluripotent and also can produce practically every organ. Induced pluripotent stem cells (iPSC) and Somatic stem cells are widely used cell sources in regenerative medicines ( and ).
Stem cells involved in regenerative medicine for the development of bionic organ
Induced pluripotent stem cells (iPSC)
Since the term ‘Regenerative medicine’ was introduced, clinical applications have grown significantly, beginning with the studies on human embryonic stem cells (ESC). Amid disappointment that human ESC could neither be employed in biological research or medicine. To overcome it, the phenomenon of nuclear transplantation was accomplished in an extensively organised experiment involving four gene transfers [Citation1], These cells are generally referred to as induced pluripotent stem cells (iPSC). iPSC seems to be the main focus of study in regenerative medicine. Studies into the clinical use of iPSC showed that mice models for haemophilia [Citation144], sickle cell anaemia [Citation145], and parkinson’s disease [Citation146] were successfully treated using iPSC. These studies suggest that human diseases might be treated using the same approach.
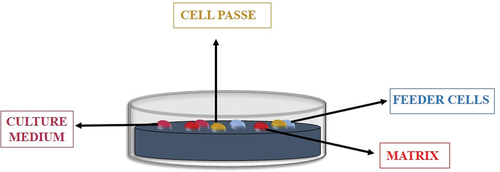
Xenogeneic materials are currently utilised in several procedures in standard ESC/iPSC culture. Both ESC and iPSC require feeder cells to preserve their undifferentiated phase; typically, these cells are mouse embryonic fibroblasts (MEF) that have had their development arrested by mitomycin C (). It was suspected that xenogeneic cell contamination could result in infection if these xenogeneic cells were employed in therapeutic conditions [Citation147]. Multiple researchers have reported coating cell culture dishes with completely synthetic materials and chemically defined-culture media to prevent xenogeneic contamination. And also chitosan and alginate combined in a 3D porous biopolymer – based scaffold assist human ESC self-renewal. On the other hand, human iPSCs could be cultured employing autologous fibroblasts as feeder cells.
However, drawbacks to the usage of iPSCs involve the creation of teratomas from undifferentiated cells, carcinomas by gene transfer and diseases with xenogeneic materials employed in cell cultures. Nearly the whole reprogramming procedure in iPSC is yet unclear.
Somatic stem cells
For a very prolonged time, it was considered that adult cardiomyocytes were in a condition of terminal differentiation and that the heart was incapable of self-healing or regaining its homoeostatic functions. These characteristics sparked a rise in interest in somatic stem cells, such as mesenchymal stem cells, bone marrow-derived stem/progenitor cells and adipose-derived stem cells, among researchers studying heart regeneration. Because foetal tissue-related cells (e.g. amniocytes) have a multipotent capacity. Amniocyte transplantation resulted in heart regeneration in rats with myocardial infarction [Citation148]. Meanwhile, other scientists have consistently claimed that, in the adult heart, cardiomyocytes can be born again. This assertion was based on estimating the age of cardiomyocytes by measuring carbon-14. Following that, several researchers reported that the heart contains stem cells and progenitor cells. The discovery of a method that appeared to be based on embryonic bodies provided the fuel for the extension of this field of study: by constructing a sphere with cardiac-tissue-derived cells, a cluster of almost undifferentiated cells could be enriched. The procedure is injecting cardiac stem cells intramuscularly while performing coronary artery bypass grafting. The cardiac tissue removed during a prior biopsy from the right ventricular septum is where the injected stem cells are extracted from. Recombinant basic fibroblast growth factor (bFGF) is employed during cell culture in place of xenogeneic components, and blood serum is obtained from autologous blood. A simple prolonged gelatin sheet of bFGF is applied to the injection sites before the cells are injected through the epicardium. After this experiment, it is anticipated that more cases will be collected for multi-facility clinical trials and that this technique will grow into a highly advanced biomedical technique.
Conclusion
A rising body of research indicates that biomaterials derived from humans are producing an increasing number of goods and useful clinical techniques to preserve, improve, or regenerate tissues and organs. The future era of biomaterials for healthcare are being created by the integration of sophisticated manufacturing and biomimetic design of structure and material systems. To create biomedical functional equipment with biomimetic architecture and bionic material systems, researchers and scientists have widely developed a variety of advanced additive manufacturing technologies. An enhanced library of biomaterials will open up more opportunities for future biomimetic medical application. Novel biomaterials are a crucial component enabling the formation of natural extracellular matrix. By treating serious, incurable illnesses like diabetes, stroke, and paralysis, regenerative medicine has the potential to save millions of lives. Additionally, it can be used to replace amputated limbs or treat congenital malformations. In a few years, it’s anticipated that some traditional therapy lines will be replaced by techniques including gene editing, 3D bioprinting, living robots, soft nanorobotics, and blends of these techniques based on artificial intelligence. With the use of biofabrication technology, stem cells may be differentiated and given microenvironmental cues that will allow them to develop into adult tissue. The requirement for individual organ donation would therefore be reduced or eliminated as it would be possible to create organs on needed in vitro. Given the potential uses of stem – cell organs, this manufacturing technique has the potential to completely alter how current medicine and healthcare are provided.
Author contributions
Conceptualisation: D.K.S., Y.M., S.M. and D.C.V.; literature search: Y.M., D.K.S., S.C.A. and V.S.; data extraction: D.K.S. and S.C.A.; formal analysis: Y.M. and V.S.; original draft preparation: Y.M., D.K.S., S.S. and D.C.V.; manuscript review and editing: S.M. and D.C.V.; supervision: D.C.V. and S.M. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
Data sharing does not apply to this article.
Additional information
Funding
References
- Gojo S, Toyoda M, Umezawa A. Tissue engineering and cell-based therapy toward integrated strategy with artificial organs. J Artif Organs. 2011;14(3):171–18.
- Zhu G, Hou Y, Xu J, et al. Reprintable polymers for digital light processing 3D printing. Adv Funct Mater. 2021;31:2007173. doi: 10.1002/adfm.202007173
- Chung JJ, Im H, Kim SH, et al. Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Front Bioeng Biotechnol. 2020;8. doi: 10.3389/fbioe.2020.586406
- Chen F-M, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064
- Bajaj P, Schweller RM, Khademhosseini A, et al. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155
- Barthes J, Özçelik H, Hindié M, et al. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances. BioMed Res Int. 2014;2014:921905. doi: 10.1155/2014/921905
- Dzobo K, Thomford NE, Senthebane DA, et al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. 2018;2018:1–24.
- Klak M, Bryniarski T, Kowalska P, et al. Novel strategies in artificial organ development: what is the future of medicine? Micromachines. 2020;11(7):646.
- Altomare L, Bonetti L, Campiglio CE, et al. Biopolymer-based strategies in the design of smart medical devices and artificial organs. Int J Artif Organs. 2018;41(6):337–359.
- Yambe T, Yoshizawa M, Tanaka A, et al. Recent progress in artificial organ research at Tohoku University. Artif Organs. 2003;27(1):2–7.
- Sharma P, Kumar P, Sharma R, et al. Tissue engineering; current status & futuristic scope. J Med Life. 2019;12(3):225–229.
- Lanza RP, Cibelli JB, Blackwell C, et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288(5466):665–669.
- Design of artificial extracellular matrices for tissue engineering. Prog Polym Sci. n.d.;36(2):238–268.
- Dobelle WH. Artificial vision for the blind by connecting a television camera to the visual cortex. ASAIO J. 2000;46(1):3–9.
- Wilbey A. Membrane processing: dairy and beverage applications (2013), edited by A.Y. Tamime, John Wiley & Sons Ltd, The atrium, southern gate, Chichester, West Sussex PO19 8SQ, UK. Price £130.00. Int J Dairy Technol. 2013;66(3):452–453.
- Nosé Y, Malchesky PS. Future of artificial organs; therapeutic artificial organs. Mrs Proc. 1987;110. doi: 10.1557/proc-110-723
- Boesel L, Reis R. Injectable biodegradable systems. In: Biodegradable systems in tissue engineering and regenerative medicine. CRC Press; 2004. doi: 10.1201/9780203491232.ch2
- Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. n.d.;41(2):349–354.
- Shin’oka: Transplantation of a tissue-engineered … n.d. Google Scholar. http://refhub.elsevier.com/B978-0-12-812258-7.00013-7/rf0010
- Szycher M. Prosthetic and biomedical devices. In: Kirk-othmer encyclopedia of chemical technology. John Wiley & Sons, Inc; 2000. doi: 10.1002/0471238961.1618151919262503.a01
- Sonstegard DA, Matthews LS, Kaufer H. The surgical replacement of the human knee joint. Sci Am. 1978;238(1):44–51.
- Lukas JS. Prospects for the state of an art: future goals of engineering in biology and medicine. Proceedings of an international conference, Washington, D.C., Sept. 1967. James, F. Dickson III, and J. H. U. Brown, Eds. Academic Press, New York, 1969. xvi + 360 pp., illus. $16. Science. 1969;166(3913):1614.
- Hill DW. Advances in biomedical engineering and medical physics. Med Biol Eng. 1968;6(6):687.
- Kim HJ, Huh D, Hamilton G, et al. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165.
- Wagner I, Materne E-M, Brincker S, et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip. 2013;13(18):3538.
- Maoz BM, Herland A, Henry OYF, et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip. 2017;17(13):2294–2302.
- Verhulsel M, Vignes M, Descroix S, et al. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials. 2014;35(6):1816–1832.
- Luni C, Serena E, Elvassore N. Human-on-chip for therapy development and fundamental science. Curr Opin Biotechnol. 2014;25:45–50.
- Meli L, Barbosa HSC, Hickey AM, et al. Three dimensional cellular microarray platform for human neural stem cell differentiation and toxicology. Stem Cell Res. 2014;13(1):36–47.
- Pandey PK, Sharma AK, Gupta U. Blood brain barrier: an overview on strategies in drug delivery, realisticin vitromodeling andin vivolive tracking. Tissue Barriers. 2015;4(1):e1129476.
- Doryab A, Amoabediny G, Salehi-Najafabadi A. Advances in pulmonary therapy and drug development: lung tissue engineering to lung-on-a-chip. Biotechnol Adv. 2016;34(5):588–596.
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662–1668.
- Aubin H, Nichol JW, Hutson CB, et al. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31(27):6941–6951.
- Zhang YS, Yue K, Aleman J, et al. 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng. 2016;45(1):148–163.
- Bhise NS, Manoharan V, Massa S, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8(1):014101.
- Biomaterials in regenerative medicine: Engineering to recapitulate the natural. Curr Opin Biotechnol. n.d.;23(4):579–582.
- Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. n.d.;30(12):2252–2258.
- Zakhariants AA, Burmistrova OA, Shkurnikov MY, et al. Development of a specific substrate—inhibitor panel (liver-on-a-chip) for evaluation of cytochrome P450 activity. Bull Exp Biol Med. 2016;162(1):170–174.
- Marsano A, Conficconi C, Lemme M, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16(3):599–610.
- LI W-X, Liang G-T, Yan W, et al. Artificial uterus on a microfluidic chip. Chin J Anal Chem. 2013;41(4):467–472.
- Kauffman A, Hoffmann H. Faculty opinions recommendation of A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Faculty Opin – Post-Pub Peer Rev Biomed Literat. 2017. doi: 10.3410/f.727450954.793533987
- Rice JJ, Martino MM, Laporte LD, et al. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. n.d.;2(1):57–71.
- Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785.
- Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105–121.
- Wenger R, Giraud M-N. 3D printing applied to tissue engineered vascular grafts. Appl Sci. 2018;8(12):2631.
- Wang X, Yan Y, Zhang R. Recent trends and challenges in complex organ manufacturing. Tissue Eng Part B Rev. 2010;16(2):189–197.
- Saroia J, Yanen W, Wei Q, et al. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des Manuf. 2018;1(4):265–279.
- Deliormanlı AM. Direct write assembly of graphene/poly(ε-caprolactone) composite scaffolds and evaluation of their biological performance using mouse bone marrow mesenchymal stem cells. Appl Biochem Biotechnol. 2019;188(4):1117–1133.
- Vienken J. Polymers in nephrology characteristics and needs. Int J Artif Organs. 2002;25(5):470–479.
- Zhang YS, Arneri A, Bersini S, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59.
- Ehlers LJ, Bouwer EJ. RP4 plasmid transfer among species of pseudomonas in a biofilm reactor. Water Sci Technol. 1999;39(7):163–171.
- Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. n.d.;5(1):1–13.
- Moore WE, Holdeman LV, Cato EP, et al. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983;42(2):510–515.
- Yarwood JM, Paquette KM, Tikh IB, et al. Generation of virulence factor variants in staphylococcus aureus biofilms. J Bacteriol. 2007;189(22):7961–7967.
- Rachid S, Cho S, Ohlsen K, et al. Induction of staphylococcus epidermidis biofilm formation by environmental factors: the possible involvement of the alternative transcription factor sigb. In: Advances in experimental medicine and biology. Kluwer Academic Publishers; n.d. p. 159–166. doi: 10.1007/0-306-46840-9_22
- von Eiff C, Jansen B, Kohnen W, et al. Infections associated with medical devices. Drugs. 2005;65(2):179–214.
- Hausner M, Wuertz S. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl Environ Microbiol. 1999;65(8):3710–3713.
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193.
- O’Donnell BT, Ives CJ, Mohiuddin OA, et al. Beyond the present constraints that prevent a wide spread of tissue engineering and regenerative medicine approaches. Front Bioeng Biotechnol. 2019;7. doi: 10.3389/fbioe.2019.00095
- Kim B-S, Mooney DJ, Atala A. Genitourinary system. In: Principles of tissue engineering. Elsevier; 2000. p. 655–667. doi: 10.1016/b978-012436630-5/50050-7
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926.
- Olson JL, Atala A, Yoo JJ. Tissue engineering: current strategies and future directions. Chonnam Med J. 2011;47(1):1.
- Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8(10):839–845.
- Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. n.d.;8(6):457–470.
- Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng R Rep. n.d.;34(4–5):147–230. doi: 10.1016/S0927-796X(01)00035-3
- Williams DF. The biomaterials conundrum in tissue engineering. Tissue Eng Part A. 2014;20(7–8):1129–1131.
- Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J Royal Soc Interface. 2010;8(55):153–170.
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. n.d.;4(7):518–524.
- Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. n.d.;8(1):15–23.
- Chen F-M, Wu L-A, Zhang M, et al. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32(12):3189–3209.
- Stoppel G, McNamara III, McNamara, et al. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng. 2014a;43(3):657–680.
- Renth AN, Detamore MS. Leveraging “raw materials” as building blocks and bioactive signals in regenerative medicine. Tissue Eng Part B Rev. 2012 May 14;18(5):341–362. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref1190
- Peptide-based biopolymers in biomedicine and biotechnology. Mater Sci Eng R Rep. n.d.;62(4):125–155.
- Self-assembly of mineralized collagen composites. Mater Sci Eng R Rep. n.d.;57(1–6):1–27. doi: 10.1016/j.mser.2007.04.001
- Barnes CP. Cross-Linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007 July 15. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref1325
- Atala: engineering complex tissues. n.d. Google Scholar. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref1170
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55.
- Coburn JM, Gibson M, Monagle S, et al. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Nat Acad Sci. 2012;109(25):10012–10017.
- Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog Polym Sci. n.d.;38(10–11):1487–1503.
- Vacanti NM. Localized delivery of dexamethasone from electrospun fibers reduces the foreign body response. ACS Publications; 2012 Sep 11. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref1400
- Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Springer Netherlands; 2014 Jan 1. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref2350
- Scopus preview - Scopus. n.d. Welcome to Scopus.
- Nakayama KH, Hou L, Huang NF. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv Healthc Mater. n.d.;3(5):628–641.
- Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. n.d.;35(24):6143–6156
- New insights into and novel applications of release technology for periodontal reconstructive therapies. J Control Release. n.d.;149(2):92–110.
- Bonnans C, Chou, Werb J, et al. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801.
- Li X. Coating electrospun poly(ε-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. ACS Publications; 2008 Nov 16. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref1410
- Pradhan S, Farach-Carson MC. Mining the extracellular matrix for tissue engineering applications. Regen Med. 2010 Nov 17;5(6):961–970. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref2420
- Gurtner GC, Callaghan MJ, Longaker MT. Progress and potential for regenerative medicine. Ann Rev. 2007 Jan 11;58(1):299–312. http://refhub.elsevier.com/S0079-6700(15)00038-6/sbref1000
- Rossi F, van Griensven M. Polymer functionalization as a powerful tool to improve scaffold performances. Tissue Eng Part A. 2014;20(15–16):2043–2051.
- Kaur A, Midha S, Giri S, et al. Functional skin grafts: where biomaterials meet stem cells. Stem Cells Int. 2019;2019:1–20.
- Patel S, Caldwell J, Doty SB, et al. Integrating soft and hard tissues via interface tissue engineering. J Orthop Res. 2018;36(4):1069–1077.
- Daar AS. The future of replacement and restorative therapies: from organ transplantation to regenerative medicine. Transplant Proc. 2013;45(10):3450–3452.
- Lee LN, Quatela O, Bhattacharyya N. The epidemiology of autologous tissue grafting in primary and revision rhinoplasty. Laryngoscope. 2018;129(7):1549–1553.
- Naegeli KM, Kural MH, Li Y, et al. Bioengineering human tissues and the future of vascular replacement. Circ Res. 2022;131(1):109–126.
- Papadimitropoulos A, Scotti C, Bourgine P, et al. Engineered decellularized matrices to instruct bone regeneration processes. Bone. 2015;70:66–72.
- Kitsuka T, Hama R, Ulziibayar A, et al. Clinical application for tissue engineering focused on materials. Biomedicines. 2022;10(6):1439.
- Giannobile WV, Jung RE, Schwarz F. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: osteology foundation consensus report part 1-effects of soft tissue augmentation procedures on the maintenance of peri-implant soft tissue health. Clin Oral Implants Res. 2018;29:7–10.
- Colazo JM, Evans BC, Farinas AF, et al. Applied bioengineering in tissue reconstruction, replacement, and regeneration. Tissue Eng Part B Rev. 2019;25(4):259–290.
- Zuhr O, Bäumer D, Hürzeler M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: critical elements in design and execution. J Clin Periodontol. 2014;41(Suppl 15):S123–S142. doi: 10.1111/jcpe.12185
- Lin JC-Y, Nevins M, Kim DM. Laser de-epithelialization of autogenous gingival graft for root coverage and soft tissue augmentation procedures. Int J Periodontics Restorative Dent. 2018;38:405–411. doi: 10.11607/prd.3587
- Meza-Mauricio J, Cortez-Gianezzi J, Duarte PM, et al. Comparison between a xenogeneic dermal matrix and connective tissue graft for the treatment of multiple adjacent gingival recessions: a randomized controlled clinical trial. Clin Oral Investig. 2021;25:6919–6929. doi: 10.1007/s00784-021-03982-w
- Nabers JM. Free gingival grafts. Periodontics. 1966;4:243–245.
- Parvini P, Obreja K, Becker K, et al. The prevalence of peri-implant disease following immediate implant placement and loading: a cross-sectional analysis after 2 to 10 years. Int J Implant Dent. 2020;6:63. doi: 10.1186/s40729-020-00259-x
- Zhao H, Chen Y, Shao L, et al. Airflow-assisted 3D bioprinting of human heterogeneous microspheroidal organoids with microfluidic nozzle. Small. 2018;14(39):1802630.
- Batalov I, Stevens KR, DeForest CA. Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels. Proc Nat Acad Sci. 2021;118(4). doi: 10.1073/pnas.2014194118
- Jakab K, Norotte C, Marga F, et al. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2(2):022001.
- Visconti RP, Kasyanov V, Gentile C, et al. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin Biol Ther. 2010;10(3):409–420.
- Nishiyama Y, Nakamura M, Henmi C, et al. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2008;131(3). doi: 10.1115/1.3002759
- Banerjee K, Radhakrishnan J, Ayyadurai N, et al. Advances in neoteric modular tissue engineering strategies for regenerative dentistry. J Sci. 2022;7(4):100491.
- Jessop ZM, Al-Sabah A, Gardiner MD, et al. 3D bioprinting for reconstructive surgery: principles, applications and challenges. J Plast Reconstruct Aesthetic Surg. 2017;70(9):1155–1170.
- Zhu W, Ma X, Gou M, et al. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 2016;40:103–112.
- Vijayavenkataraman S, Yan W-C, Lu WF, et al. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev. 2018;132:296–332.
- Colla G, Porto LM. Development of artificial blood vessels through tissue engineering. BMC Proc. 2014;8(S4). doi: 10.1186/1753-6561-8-s4-p45
- Jang J, Yi H-G, Cho D-W. 3D printed tissue models: present and future. ACS Biomater Sci Eng. 2016;2(10):1722–1731.
- Park JY, Jang J, Kang H-W. 3D bioprinting and its application to organ-on-a-chip. Microelectron Eng. 2018;200:1–11.
- McCormack A, Highley CB, Leslie NR, et al. 3D printing in suspension baths: keeping the promises of bioprinting afloat. Trends Biotechnol. 2020;38(6):584–593.
- Saleh Alghamdi S, John S, Roy Choudhury N, et al. Additive manufacturing of polymer materials: progress, promise and challenges. Polymers. 2021;13(5):753.
- Huang L, Wu K, Zhang R, et al. Fabrication of multicore milli- and microcapsules for controlling hydrophobic drugs release using a facile approach. Ind Eng Chem Res. 2019;58(36):17017–17026.
- Yu J, Park SA, Kim WD, et al. Current advances in 3D bioprinting technology and its applications for tissue engineering. Polymers. 2020;12(12):2958.
- Zadpoor AA, Malda J. Additive manufacturing of biomaterials, tissues, and organs. Ann Biomed Eng. 2016;45(1):1–11.
- Aljohani W, Ullah MW, Zhang X, et al. Bioprinting and its applications in tissue engineering and regenerative medicine. Int J Biol Macromol. 2018;107:261–275.
- Garreta E, Oria R, Tarantino C, et al. Tissue engineering by decellularization and 3D bioprinting. Mater Today. 2017;20(4):166–178.
- Daly AC, Critchley SE, Rencsok EM, et al. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication. 2016;8(4):045002.
- Dai G, Lee V. Three-dimensional bioprinting and tissue fabrication: prospects for drug discovery and regenerative medicine. Adv Health Care Techno. 2015;23. doi: 10.2147/ahct.s69191
- Malda J, Visser J, Melchels FP, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25(36):5011–5028.
- Pinos R, Sbrana FV, Scielzo C. Bioprinting functional tissues: cell types and a focus on cancer modeling. In: Bioprinting. Elsevier; 2022. p. 247–269. doi: 10.1016/b978-0-323-85430-6.00005-4
- Knowlton S, Onal S, Yu CH, et al. Bioprinting for cancer research. Trends Biotechnol. 2015;33(9):504–513.
- Derakhshanfar S, Mbeleck R, Xu K, et al. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3(2):144–156.
- Koch L, Deiwick A, Schlie S, et al. Skin tissue generation by laser cell printing. Biotechnol Bioeng. 2012;109(7):1855–1863.
- Irvine S, Venkatraman S. Bioprinting and differentiation of stem cells. Molecules. 2016;21(9):1188.
- Hopp B. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt Eng. 2012;51(1):014302.
- Ozbolat IT, Yin Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60(3):691–699.
- Daar AS, Greenwood HL. A proposed definition of regenerative medicine. J Tissue Eng Regen Med. 2007;1(3):179–184.
- King NMP. Ethics in regenerative medicine and transplantation. In: Regenerative medicine applications in organ transplantation. Elsevier; 2014. p. 977–986. doi: 10.1016/b978-0-12-398523-1.00071-9
- Tam PKH, Wong KKY, Atala A, et al. Regenerative medicine: postnatal approaches. Lancet Child Adolesc Health. 2022;6(9):654–666.
- The extracellular matrix as a biologic scaffold material. Biomaterials. n.d.;28(25):3587–3593.
- Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater Sci Eng C. n.d.;45:620–634. doi: 10.1016/j.msec.2014.06.003
- Edgar L, Pu T, Porter B, et al. Regenerative medicine, organ bioengineering and transplantation. Br J Surg. 2020;107(7):793–800.
- Ilic D, Polak JM. Stem cells in regenerative medicine: introduction. Br Med Bull. 2011;98:117–126. doi: 10.1093/bmb/ldr012
- Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respir Int Rev Thorac Dis. 2013;85:3–10. doi: 10.1159/000345615
- Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci USA. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107
- Rose M, Gao K, Cortez-Toledo E, et al. Endothelial cells derived from patients’ induced pluripotent stem cells for sustained factor VIII delivery and the treatment of hemophilia A. Stem Cells Transl Med. 2020;9(6):686–696.
- Demirci S, Uchida N, Tisdale JF. Gene therapy for sickle cell disease: an update. Cytotherapy. 2018;20(7):899–910.
- Stoddard-Bennett T, Pera R. Stem cell therapy for Parkinson’s disease: safety and modeling. Neural Regen Res. 2020;15(1):36.
- Martin MJ, Muotri A, Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11(2):228–232.
- Tsuji H, Miyoshi S, Ikegami Y, et al. Xenografted human amniotic membrane–derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 2010;106(10):1613–1623.