ABSTRACT
The lack of a viable vaccine and the emergence of novel Mycobacterium tuberculosis (MTB) strains that are particularly resistant to treatments, presage a complicated future situation. Biosynthesized nanomaterials are currently proving to be a viable antibacterial therapeutic option, including for MTB infection treatment. The goal of this work is to synthesize silver nanoparticles (AgNPs) from Syzygium aromaticum seeds and investigate their antimicrobial, anti-tubercular, and cytotoxic properties using zebrafish embryos and Artemia salina. The UV spectrophotometer, SEM-EDAX, TEM and FTIR measurements were used to characterize the AgNPs. Antibacterial activity was performed against S. aureus, P. aeruginosa, E. coli and K. pneumoniae and exhibited potential inhibitory activity towards the bacterial cultures. The MABA assay was used to investigate the anti-mycobacterial activity, and the AgNPs showed the highest percentage of inhibition in both test concentrations (250 and 500 µg/ml).
Introduction
Tuberculosis is a severe public health problem, with over 2 billion people worldwide afflicted and over 1.3 million deaths expected in 2019 [Citation1]. Mycobacterium tuberculosis is the pathogen that causes tuberculosis and is transferred from person to person by aerosols [Citation2]. They are residues and multiplies in alveolar macrophages as it has developed several pathways to evade digestion in acidic phagosomes and may persist intracellularly in a dormant phase. In developing countries, including India, tuberculosis remains a leading cause of mortality [Citation3–5]. In 2019, rifampicin-resistant MTB produced an estimated 464,000 new cases of human TB, with 78% of MDR-TB strains resistant to the two first-line medicines, isoniazid and rifampicin. However, when compared to the annual total of 1.4 million deaths attributable to tuberculosis in general, the number of MDR-TB-related deaths is minimal [Citation1,Citation6]. The increase in the incidence of drug resistance TB around the world urges the need to develop a new drug derived from natural product such as higher plants to fight against tuberculosis. Medicinal plants usually have the property of curing disease for more than centuries. Usually, plants have been used as traditional medicine for the treatment of various diseases. Only few species of medicinal plants have been investigated for revealing its medicinal properties and others are yet to be discussed. Syzygium aromaticum L., also known as Eugenia caryophyllata L., is a tropical evergreen tree with crimson blooms that belongs to the Myrtaceae family [Citation7], endemic to the Maluku Islands in Indonesia, and is mostly used as a spice. They are mostly harvested in India, Pakistan, Indonesia, Madagascar, Zanzibar, Sri Lanka, and Tanzania for commercial use. However, the biggest clove buds oil producers are Indonesia and Madagascar; Buds, leaf, and stem oil are all used to extract oil [Citation8]. A review of previous reports suggests that the Syzygium aromaticum (Clove) possess biologically active component which are effective against many types of diseases. Clove buds were chosen as a ligneous, representative substrate in this study because of its importance in cuisine, traditional medicine, pharmaceutics, and cosmetics [Citation7,Citation8]. Clove and its derivatives of have numerous properties such as antimicrobial activity, anti-viral activity, anti-inflammatory hepatoprotective activity, chemoprotective activity, anti-diabetic and antioxidant activity, anti-inflammatory, anti-carcinogenic effects [Citation7,Citation9,Citation10], neuroprotective ability, hypolipidemic efficiency and anti-diabetic activity etc [Citation8,Citation9,Citation11]. Polyphenols present in clove buds have antibacterial and antiviral properties. In recent studies researchers found that Quercetin has been discovered to have a possible role against coronavirus disease 2019. Along with the plant extract, nanomaterials are currently showing to be a potential alternative as antimicrobial treatments, including the treatment of MTB infection [Citation7]. Nanoparticles have been extensively used for pharmaceutical and industrial purposes. As the nature consists of several metals, only few of them are used for the synthesis of nanoparticles [Citation9,Citation10,Citation12,Citation13]. Among these silver nanoparticles holds a special place because of their expensive properties which is utilized in many industries such as agriculture, textile industry, air filtration etc [Citation14]. These silver nanoparticles have the potential to penetrate bacterial cell walls and affect the shape of cell membranes, resulting in cell growth inhibition or even cell death [Citation15,Citation16]. This is due to their nanoscale size and high specific surface area. Furthermore, biosynthesis of AgNPs using various sources such as microorganisms and plant extracts has been recognized as the green technique and has become an unavoidable trend when compared to other synthetic methods. Without requiring hazardous solvents or generating detrimental byproducts, the reducing agents in these green sources would transfer their electrons to reduce silver ions into silver nanoparticles [Citation17,Citation18]. These biological molecules would also cover the produced silver nanoparticles and act as capping agents, preventing agglomeration, reducing toxicity, and improving silver nanoparticle antibacterial activity. Probing deep into literature, it also proves that these components flow a unique mechanism to provide a potential action against Tuberculosis (TB). Therefore, the nanoparticles of clove will have impact on TB with unique mechanism. Hence, the present study was carried out to synthesize silver nanoparticles from Syzygium aromaticum (Clove) and exploring its Anti-mycobacterial property.
Materials and methods
Sample collection and extract preparation
The seeds of Syzygium aromaticum (cloves) were purchased from nearby shop in Solinganallur, Chennai, Tamilnadu. Seeds were washed with tween 20 detergent followed by distilled water 2 to 3 times to remove the impurities. Then, they were dried for 24 h under shade. The dried materials were ground into fine powder using a mixer grinder. Take 1 g of ground clove powder; add 50 ml of double distilled water. Boil the mixture using a water bath at 60◦C for 10–15 min method proposed by Krithiga et al. 2015. After cooling down filter the extract using Whatmann No.1 filter paper. Then, the filtrate was stored in 4°C for further use [Citation19,Citation20].
Biological synthesis of silver nanoparticles
Two ml of clove extract was added in to 98 ml of 0.1 mM aqueous AgNO3 solutions and kept in a dark condition at room temperature for about 24 h. The bio reduction of Ag + ions in aqueous solution was monitored by colour change from pale yellow to brown [Citation19]. The solution is centrifuged at 4000 rpm for 15 min and the pellet was transferred into petri plates and allowed to dry. The powder was scrapped and stored in sterile Eppendorf tubes and used for further characterization and application studies.
Characterization of silver nanoparticles
UV-visible absorption spectroscopy
The nanoparticles synthesized were characterized using different techniques. X-ray diffraction (XRD) with KαCu (= 1.5406 Å) was utilized to study the crystalline nature of silver nanoparticles. The Scherer equation Kλ/βCosθ was used to calculate the size of the silver nanoparticles. UV – Vis spectrophotometer (Agilent 8453) recorded absorption spectra. FT-IR spectra were collected between 4000 and 400 cm−1. High-resolution scanning electron microscopy (HR-SEM)/transmission electron microscopy (TEM) was utilized to analyse morphology, size, and particle size distribution. Image J was used for image processing and analysis. EDAX was used to determine elemental composition of silver nanoparticles.
Antimicrobial activity
The agar well diffusion method was used to determine the antibacterial activity of the extracts prepared from plant Syzygium aromaticum using different solvents such as Hexane, Ethyl acetate and Methanol. The test microorganism used was Escherichia coli, Pseudomonas aeruginosa Klebsiella pneumonia, Staphylococcus aureus. Nano synthesized silver samples of Syzygium aromaticum were tested for antibacterial activity. The plates were incubated at 37◦C for 16–18 h. The zone of inhibition (mm) was measured, and the results were tabulated [Citation21,Citation22].
Anti-mycobacterial activity microplate alamar blue assay (MABA)
Mycobacterium smegmatis was obtained from the Microbial Type Culture Collection and Gene Bank (MTCC) India. They were maintained on Lowenstein-Jensen (LJ) slopes and cultured on Middlebrook 7H9 broth. Cultures were grown aerobically on 7H9 broth at 37°C adjusted spectrophotometrically to a no.1 McFarland tube standard and further diluted 1:10 in 7H9 broth for the test (Palomino et al. 2002). Briefly, 100 μL of 7H9 broth was dispensed in each well of a sterile flat-bottom 96-well plate and serial twofold dilutions of the crude extracts and each positive control drug were prepared directly in the plate. In growth control wells, 100 µl of Middlebrook 7H9 broth was added. In DMSO control wells, 100 µl of 10% DMSO containing M7H9 broth was added. In drug control wells, 100 µl Rifampicin containing M7H9 broth (2 µg/ml) was added. In test wells, 100 µl of desired concentration of test samples was added to the dissolved M7H9 broth. In the negative control wells, 200 µl of M7H9 broth was added. Furthermore, 100 µl of M. smegmatis suspension was added to all the above wells except the negative control well. The plates were incubated at 37°◦C for 24 hr. After incubation, 32.5 µl of prepared dye was added to all the other remaining wells. No colour change was observed, and the plates were further incubated for 24 hr. All the experiments were carried out in triplicates and were conducted in a Biosafety Level Three (BSL-3) laboratory [Citation23].
Cytotoxicity assay using zebra fish embryos
Zebra fish embryos were purchased from the zebra fish aquarium in Kanchipuram district. For toxicity studies, 20 healthy post hatched zebra fish were transferred to the wells of a 24-well plate along with 1 ml of embryo water (60 mg of sea salt/litre of ultrapure water). Different concentrations of nano synthesized plant extracts at (5, 10, 25, 50 and 100 μg ml−1 concentration) were added to the wells and incubated for 72 hr at 28.5°C. Tests were performed in duplicate and repeated thrice. Mortality of the zebra fish was noted after 24, 48 and 72 hr. The embryos that appeared opaque and white in colour were observed. The dead embryos were degraded quickly, whereas the structures of intact embryos were more visible by 48 hr that allowed for a clear distinction between the dead and alive. At the end of the incubation period, the embryos were photographed using a light contrast microscope, and the mortality rate was calculated [Citation24].
Cytotoxicity assay using Artemia salina
Artemia salina eggs were purchased from the nearby aquarium in Kanchipuram district. Dried cysts were placed in a bottle containing artificial seawater which was prepared by dissolving 35 g of sodium chloride in 1 litre of distilled water. After 36–48 hr incubation at room temperature (28–30°C) under conditions of strong aeration and continuous illuminations, the larvae hatched within 48 hr. The evaluation of cytotoxicity of nanoparticles in A. salina was performed according to the previous methods. The assay was carried out on larvae of brine shrimp. Around 200µl of plant extract from each concentration was taken and added in test wells at 5,25,50,75 and 100 concentrations where artificial seawater serves as control. Twenty nauplii per well was added in all the wells, including control wells. The plate was incubated at room temperature under strong aeration. After 24 hr of incubation, the survival rate of nauplii and the percentage of lethality were determined using the following. The numbers of surviving nauplii in each well were counted under a stereoscopic microscope after 24 hr. The experiments were conducted in triplicate for each concentration [Citation25].
Percentage of lethality = [(Test – control)/control] x 100
Results and discussion
Synthesis of silver nanoparticles
Syzygium aromaticum constitutes the taxonomically most extensive group of plants. The silver nanoparticles were synthesized using a green synthesis approach. The precursor, silver nitrate, was mixed with the plant extract and incubated at room temperature. The colour change was tracked and recorded. Triplicate reactions were used in the tests. Formation of nanoparticles is described in , and it was validated by the yellowish-brown colour change. No colour change was observed in the Control silver nitrate solution [Citation20].
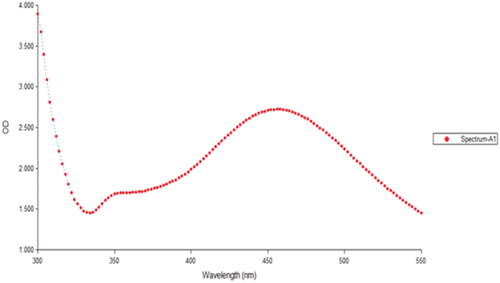
Analysis of the silver nanoparticles using UV-spectrophotometer
A UV-spectrophotometer was used to confirm the produced silver nanoparticles. With the use of a UV–vis spectrophotometer, the formation of the silver nanoparticles was further confirmed. depicts the UV–Vis spectrum. At 450 nm, Syzygium aromaticum displayed a substantial absorption. Surface plasmon resonance is the name given to this absorption band (SPR). Bio-reduction of silver particle was measured using UV–Visible spectroscopy based on the surface plasmon resonance leading to an increase in colour intensity. The UV-visible spectrum of nanoparticles synthesized from Syzygium aromaticum shows at 450 nm. A similar study was done by Vijayaraghavan et al., (2012) evaluated that the nanoparticles synthesized from Syzygium aromaticum showed plasmon excitation at 430 nm [Citation26]. The SPR is highly influenced by the shape and size of the nanoparticles [Citation27]. Majeed et al., (201) explained that the reduction of silver nitrate by Syzygium aromaticum extract results in the formation of silver nanoparticles. After 24 hr of reaction, particles ranging in size from 20 to 149 nm were produced [Citation28].
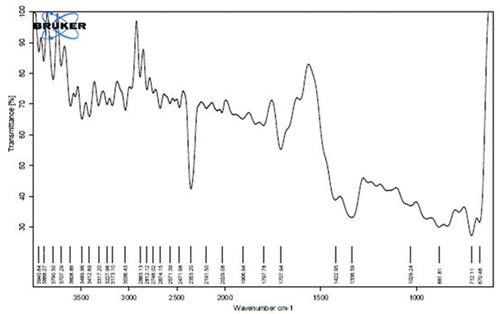
Fourier transform infrared spectroscopy analysis (FTIR) of silver nanoparticles
FT-IR spectroscopic analysis of Syzygium aromaticum mediated silver nanoparticles is shown in . The results of FTIR analysis of this study show different stretches of bonds shown at different peaks; 3608.86 cm−1 showed O-H stretch which has medium intensity, 2883.13 (-C-H alkane stretch), 2812.12 (-C-H aldehyde weak intensity), 2746.02, 2674.15, 2571.39 cm−1 (-O-H carboxylic acids with strong intensity), 1906.94 cm−1 (=C-H aromatics), 1797.78 cm−1 (=C-H), 1707.94 (-C=O ketone), 1422.95, 1029.24 (C-H alkene strong bend). The analysis was evaluated and confirmed attachment of the functional group of the silver nanoparticles. FTIR spectroscopic analysis confirmed the reducing and the capping capacity of S. aromaticum on the synthesis of silver nanoparticles [Citation22,Citation29]. Our results support the existence and interaction of aromatic compounds such as phenols, flavonoids, terpenoids, and others, with silver nanoparticles derived from Syzygium aromaticum, that contribute to their potential stabilization. FTIR analysis indicates that the secondary structure of proteins remains unaltered following their interaction with silver ions, which is consistent with the findings reported in prior studies [Citation30,Citation31].
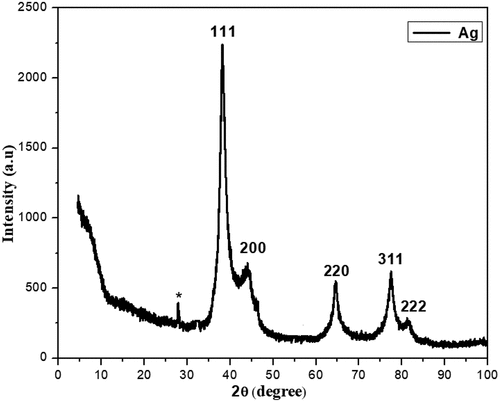
XRD of silver nanoparticles
The graph of the XRD spectrum for the silver samples made from biological extract of clove is shown in . The graph shows the 2θ values for crystalline planes (111), (200), (220), and (311) facets, respectively, at 38.34, 44.27, 64.64, and 77.94° that was identified from the JCPDS card no 89–3722 and the peaks were consistent with the face centre cubic (FCC) crystal structure of Ag NPs. With respect to the Ag structure, the Scherrer’s equation (D = Kλ/βcosθ) was used to determine the average size of the particles of silver nanoparticles, and the nanoparticles size was determined to be 28 nm. The crystalline substance that was produced in the nm range, according to the observable lines broadening of the XRD analysis peaks [Citation31]. The Ag phase, which indicates that it contains a lot of nano-Ag particles, is what causes the most powerful peak to be detected at 38. In most cases, particle size effects are to blame for the widening of the peaks in solids’ XRD patterns. Broader peaks reflect the influence of the experimental circumstances on the formation and development of the crystal nuclei and indicate smaller particle size [Citation32,Citation33].
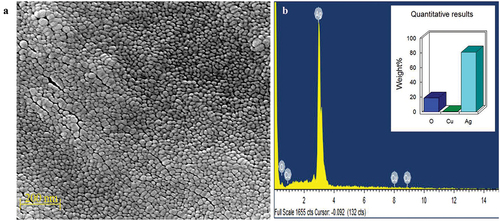
SEM-EDAX of silver nanoparticles
The dimensions and form of the silver nanoparticles were verified by SEM examination. The diameter of the spherical and uniformly scattered Ag NPs ranged from 20 to 45 nm (). The sizes and forms of the Ag NPs produced from Syzygium aromaticum varied; large and small-sized silver nanospheres are depicted in . This is explained by the concurrent reduction of Ag NPs caused by the phytochemicals found in S. aromaticum. The regulated NP agglomeration caused by optimized reaction conditions may be advantageous for long-term storage and other biological applications. EDAX was used to look at the elemental makeup of silver NPs, as seen in . Ag is the only element found, and thus distinguishes the phase purity of the NPs [Citation34,Citation35]. The oxygen signal suggested that the extracellular organic material was likely adsorbed on the surface of the Ag NPs, and EDX analysis verified the presence of silver. Nanoparticles vary in size and shape depending on the type of plant utilized, the portion employed, and the phytoconstituents such as alkaloids, flavonoids, tannins, and cardiac glycoside involved at the time of formation [Citation32,Citation36].
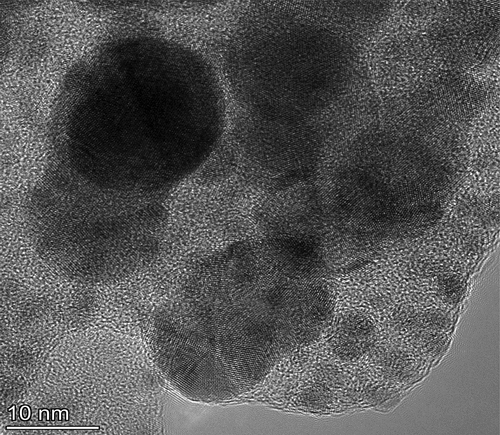
TEM of silver nanoparticles
To more clearly identify the crystalline characteristics as well as the size of the produced nanoparticles, a TEM investigation was conducted. The SEM results are consistent with the TEM photographs of Ag, which demonstrated that the particles are largely spherical as shown in . The majority of the Ag NPs are depicted in this picture to be spherical, with typical particle dimensions of 100 nm. The results of TEM coincide with the SEM and XRD analysis that represents the morphological structure of nanoparticles [Citation22,Citation26].
Antibacterial activity of nano silver particle and plant extract
The antibacterial effects of nano synthesized compounds and plant extracts on four microorganisms viz., Staphylococcus aureus, Klebsiella pneumonia, Escherichia coli and Pseudomonas aeruginosa were determined using agar well diffusion method are represented in .
Figure 7. Antibacterial activity of plant extract on different microorganisms.
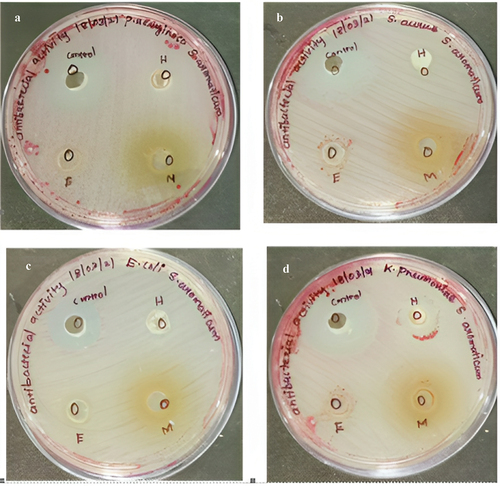
Figure 8. Antibacterial activity of silver nanoparticles.
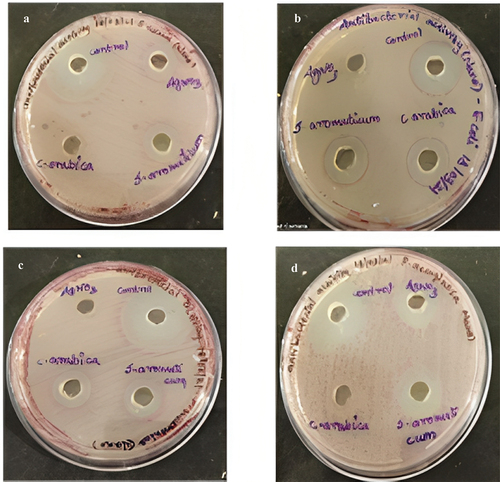
The zone of inhibition representing the antibacterial activity for nano synthesized compounds on green synthesized silver nanoparticles of Syzygium aromaticum is reported in . In that, all the organisms were inhibited at a maximal level. Whereas in crude extracts of Syzygium aromaticum showed minimal activity against all the pathogens are described in and . Penicillin streptomycin would be the control for all pathogens. The antimicrobial activity of nanosynthesized and crude extracts of Syzygium aromaticum showed inhibition ranges from 4 mm to 11 mm with different solvent extracts. The maximum inhibitory activity was observed in methanolic extracts of Syzygium aromaticum against K. Pneumonia (11 mm) was reported by Saikumari et al. (2016) [Citation19,Citation37,Citation38]. Prasad and Vyshnava (2013) demonstrated broad-spectrum antimicrobial activity of AgNPs from Syzygium cumini against Gram-positive and Gram-negative microorganisms. The investigation of the antibacterial activity of silver nanoparticles synthesized with cumini extract against Staphylococcus aureus and Bacillus licheniformis depicted a high potential as an antimicrobial agent [Citation39].
Table 1. Antibacterial activity of synthesized silver nanoparticles.
Table 2. Antibacterial activity of plant extracts.
It has been hypothesized that silver nanoparticles obtained from Syzygium aromaticum bind to the outer membrane of bacteria and increase its permeability, resulting in cell death [Citation40,Citation41]. It is commonly accepted that the interaction of the silver ions with negatively charged species present in the cytoplasm layer is the mechanism by which silver exerts its antibacterial effect [Citation28]. Silver ions are released in the aqueous solution as the Ag nanostructure is in contact with the bacteria and preferentially attaches to the cell wall. Oxygen and hydroxyl groups in aqueous media stabilize silver nanoparticles by having a powerful interaction with the surface metal atoms, in addition to securely anchoring silver ions onto the cell wall through ion–dipole interactions [Citation42,Citation43]. According to this interaction, the cell membrane may contract or separate from the wall of the cell. As a result of the cytoplasm membrane contracting or separating from the cell wall, the DNA molecules become condensed and lose their capacity to multiply when silver ions are introduced. Additionally, it has been proposed that the silver ions may interact with proteins’ thiol groups, leading to the inactivation of bacterial proteins. Last but not least, the membrane’s contraction makes it less likely that nutrients will enter the cell for continued growth and proliferation. Further studies on the mechanism of silver nanoparticles need to be analysed using microscopic studies to confirm the adhering property of silver nanoparticles [Citation31,Citation44].
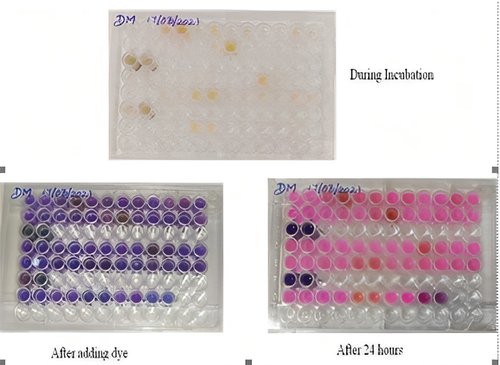
Antimycobacterial activity – microplate alamar blue assay
The plant extracts did not show enhanced reduction of Alamar Blue as compared to the medium control. The nano synthesized plant extract of Syzygium aromaticum at 250 µg/ml showed activity against M. smegmatis. The study showed the inhibitory activity at 250 µg/ml concentration by MABA (). It was denoted by the colour of the dye that remained blue, indicating their capacity to inhibit the growth of M. smegmatis. The change of dye colour from blue to pink colour indicates the growth of M. smegmatis which means no inhibition. Kaur et al., (2015) determined the anti-mycobacterial activity of Syzygium aromaticum along with many other medicinal with an indicator organism of M. tuberculosis H37RV [Citation15]. The anti-mycobacterial activity of Syzygium aromaticum with an indicator organism such as M. smegmatis, the MIC of Syzygium aromaticum and silver nanoparticles was observed to be at 250 µg/ml by MABA as shown in which is similar to the study done by Gupta et al., 2018 [Citation23]
Table 3. Quantitative analysis on antimycobacterial activity and plant extract.
These findings suggest that both the aqueous extracted AgNPs and the clove extract have promising antimycobacterial properties. Further research and exploration of these agents could pave the way for innovative approaches in combating mycobacterial infections, including tuberculosis, a global health challenge.
Cytotoxicity assay on zebrafish embryo and Artemia salina
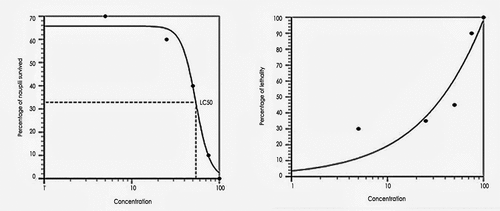
In this study, five different concentrations of green synthesized silver nanoparticles were used from 5, 25, 50, 75to 100 mg/ml concentrations. There is no significant mortality at 5 and 25 mg/ml than the other concentrations. Whereas the 50 µg/ml shows moderate and 75 and 100 µg/ml shows high toxicity level. Where the silver nanoparticles of Syzygium aromaticum showed less toxicity in zebra fish larvae at minimal concentration represented in and [Citation6,Citation45].
Figure 10. Zebra fish embryo – morphological change was noticed with high concentration of nanodrug.
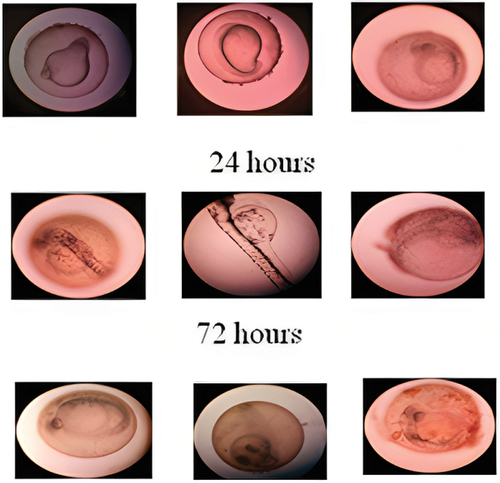
Table 4. The cytotoxicity assay of plant extract Syzygium aromaticum was assessed by zebra fish embryo.
The brine shrimp lethality assay was also used to determine the toxicity of nano particles. Five different concentrations of nano synthesized plant extract of Syzygium aromaticum are screened as shown in . A substance with a lower LC50 value is more toxic than one with a higher LC50 value. According to the results, the 5 µg and 25 µg concentration have low toxicity, whereas 50 µg concentration has moderate toxicity and 75 µg and 100 µg concentration have higher toxicity level. The graph was plotted for calculating LC50 value of nano synthesized plant extract of Syzygium aromaticum represented in ). In our study, we have analysed the cytotoxicity of the plant Syzygium aromaticum. Till now, a few studies have been reported the toxicity effect of nanomaterials on Artemia salina. Rajabi et al. (2015). Five different concentrations were used for both test 5 and 25 µg/ml concentration shows low cytotoxicity. Whereas the 50 µg/ml shows moderate concentration and 75 and 100 µg/ml show high cytotoxicity level. The study done by Rajabi et al., (2015) used 16 nanoparticles to screen the lethality. Only two nanoparticles showed strong toxicity which is LC50 < 100 ug/ml [Citation24,Citation46].
Conclusion
The flower buds of Syzygium aromaticum have a potent antimycobacterial and pharmacological activity. It is recommended to be taken because it has many beneficial effects in human health such as it used to be a carminative, to cure dental problems and fungal infections and to strengthen the immune system and used to cure respiratory illness. Further studies can be carried out to separate potential and pharmacological compounds using column chromatography, HPLC and NMR spectroscopy. The present study could also pave the way towards the possibility of obtaining anti-mycobacterial drugs against other mycobacterial species.
Acknowledgments
The authors are thankful to the Researchers Supporting Project number (RSPD2023R728), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- Mwaba P, Chakaya JM, Petersen E, et al. Advancing new diagnostic tests for latent tuberculosis infection due to multidrug-resistant strains of Mycobacterium tuberculosis — end of the road? Inter J Infect Dis. 2020;92:S69–13. doi: 10.1016/j.ijid.2020.02.011
- Jafari AR, Mosavi T, Mosavari N, et al. Mixed metal oxide nanoparticles inhibit growth of Mycobacterium tuberculosis into THP-1 cells. Int J Mycobacteriol. 2016;5:S181–S183. doi: 10.1016/j.ijmyco.2016.09.011
- Bihon A, Zinabu S, Muktar Y, et al. Human and bovine tuberculosis knowledge, attitude and practice (KAP) among cattle owners in Ethiopia. Heliyon. 2021;7(3):e06325. doi: 10.1016/j.heliyon.2021.e06325
- Lee KK, Bing R, Kiang J, et al. Adverse health effects associated with household air pollution: a systematic review, meta-analysis, and burden estimation study. Lancet Glob Health. 2020;8(11):e1427–e1434. doi: 10.1016/S2214-109X(20)30343-0
- Zhao L, Cao B, Borghi E, et al. Data gaps towards health development goals, 47 low- and middle-income countries. Bull World Health Org. 2022;100(1):40–49. doi: 10.2471/BLT.21.286254
- Chen C-C, Chen Y-Y, Yeh C-C, et al. Alginate-capped silver nanoparticles as a potent anti-mycobacterial agent against Mycobacterium tuberculosis. Front Pharmacol. 2021;12:746496. doi: 10.3389/fphar.2021.746496
- Asghar MA, Yousuf RI, Shoaib MH, et al. Green synthesis and characterization of carboxymethyl cellulose fabricated silver-based nanocomposite for various therapeutic applications [retraction]. IJN. 2022;17:987–988. doi: 10.2147/IJN.S364552
- Bajpai SK, Kumari M. A green approach to prepare silver nanoparticles loaded gum acacia/poly(acrylate) hydrogels. Int j biol macromol. 2015;80:177–188. doi: 10.1016/j.ijbiomac.2015.06.048
- Hussain M, Nafady A, Sirajuddin, et al. Biogenic silver nanoparticles for trace colorimetric sensing of enzyme disrupter fungicide vinclozolin. Nanomaterials. 2019;9(11):1604. doi: 10.3390/nano9111604
- Mehmood Y, Farooq U, Yousaf H et al. Antiviral activity of green silver nanoparticles produced using aqueous buds extract of Syzygium aromaticum. Pak J Pharm Sci. 2020; 33: 839–845.
- Venugopal K, Rather HA, Rajagopal K, et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J Photochem Photobiol, B. 2017;167:282–289. doi: 10.1016/j.jphotobiol.2016.12.013
- Nartop P. Effects of surface sterilisation with green synthesised silver nanoparticles on Lamiaceae seeds. IET Nanobiotechnol. 2018;12(5):663–668. doi: 10.1049/iet-nbt.2017.0195
- Acharya D, Satapathy S, Somu P, et al. Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from Marine algae chaetomorpha Linum extract against human colon cancer cell HCT-116. Biol Trace Elem Res. 2021;199(5):1812–1822. doi: 10.1007/s12011-020-02304-7
- Robinson AM, Zhao L, Shah Alam MY, et al. The development of “fab-chips” as low-cost, sensitive surface-enhanced raman spectroscopy (SERS) substrates for analytical applications. Analyst. 2015;140(3):779–785. doi: 10.1039/C4AN01633E
- Jaryal N, Kaur H. Plumbago auriculata leaf extract-mediated AgNPs and its activities as antioxidant, anti-TB and dye degrading agents. J Biomater Sci Polym Ed. 2017;28(16):1847–1858. doi: 10.1080/09205063.2017.1354673
- Yin IX, Zhang J, Zhao IS, et al. The antibacterial mechanism of silver nanoparticles and its application in dentistry. IJN. 2020;15:2555–2562. doi: 10.2147/IJN.S246764
- Jafari A, Nagheli A, Foumani AA, et al. The role of metallic nanoparticles in inhibition of Mycobacterium tuberculosis and enhances phagosome maturation into the infected macrophage. Oman Med J. 2020;35(6):e194–e194. doi: 10.5001/omj.2020.78
- Sampath S, Bhushan M, Saxena V, et al. Green synthesis of Ag doped ZnO nanoparticles: study of their structural, optical, thermal and antibacterial properties. Mater Technol. 2022;1–10.
- Saikumari D, Rani SKS, Saxena N. Antibacterial activity of Syzygium aromaticum L (clove). Int J Curr Microbiol App Sci. 2016;5(11):484–489. doi: 10.20546/ijcmas.2016.511.056
- Lee K-G, Shibamoto T. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) merr. et Perry]. Food Chem. 2001;74(4):443–448. doi: 10.1016/S0308-8146(01)00161-3
- Daoud A, Malika D, Bakari S, et al. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of date palm pollen (DPP) from two Tunisian cultivars. Arabian J Chem. 2019;12(8):3075–3086. doi: 10.1016/j.arabjc.2015.07.014
- Roy A, Bulut O, Some S, et al. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673–2702. doi: 10.1039/C8RA08982E
- Gupta VK, Kaushik A, Chauhan DS, et al. Anti-mycobacterial activity of some medicinal plants used traditionally by tribes from Madhya Pradesh, India for treating tuberculosis related symptoms. J Ethnopharmacol. 2018;227:113–120. doi: 10.1016/j.jep.2018.08.031
- Asharani PV, Lian Wu Y, Gong Z, et al. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19(25):255102. doi: 10.1088/0957-4484/19/25/255102
- Sangian H, Faramarzi H, Yazdinezhad A, et al. Antiplasmodial activity of ethanolic extracts of some selected medicinal plants from the northwest of Iran. Parasitol Res. 2013;112(11):3697–3701. doi: 10.1007/s00436-013-3555-4
- Vijayaraghavan K, Nalini SPK, Prakash NU, et al. Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum. Mater Lett. 2012;75:33–35. doi: 10.1016/j.matlet.2012.01.083
- Link S, El-Sayed MA. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu Rev Phys Chem. 2003;54(1):331–366. doi: 10.1146/annurev.physchem.54.011002.103759
- Majeed A, Ullah W, Anwar AW, et al. Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: a review. Mater Technol. 2018;33(5):313–320. doi: 10.1080/10667857.2015.1108065
- Prakash MVD, Sampath S, Amudha K, et al. Eco-friendly green synthesis of copper nanoparticles from Tinospora cordifolia leaves: optical properties with biological evaluation of anti-microbial, anti-inflammatory and anti-oxidant applications. Mater Technol. 2023;38(1):2247908. doi: 10.1080/10667857.2023.2247908
- Jardón-Romero EA, Lara-Carrillo E, González-Pedroza MG, et al. Antimicrobial activity of biogenic silver nanoparticles from Syzygium aromaticum against the Five most common microorganisms in the oral cavity. Antibiotics. 2022;11(7):834. doi: 10.3390/antibiotics11070834
- Mohammed SSS, Lawrance AV, Sampath S, et al. Facile green synthesis of silver nanoparticles from sprouted Zingiberaceae species: spectral characterisation and its potential biological applications. Mater Technol. 2022;37(8):533–546. doi: 10.1080/10667857.2020.1863571
- Dashora A, Rathore K, Raj S, et al. Synthesis of silver nanoparticles employing polyalthia longifolia leaf extract and their in vitro antifungal activity against phytopathogen. Biochem Biophys Rep. 2022;31:101320. doi: 10.1016/j.bbrep.2022.101320
- Chaitanyakumar A, Yadav KK, Gomez LA, et al. Biogenically engineered silver nanoparticles using bael leaf extract and evaluation of its therapeutic potential. Mater Technol. 2022;37(11):1617–1628. doi: 10.1080/10667857.2021.1965701
- Singhal G, Bhavesh R, Kasariya K, et al. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13(7):2981–2988. doi: 10.1007/s11051-010-0193-y
- Arumai Selvan DS, Keerthi M, Murugesan S, et al. In vitro cytotoxicity efficacy of phytosynthesized Ag/ZnO nanocomposites using Murraya koenigii and Zingiber officinale extracts. Mater Chem Phys. 2021;272:124903. doi: 10.1016/j.matchemphys.2021.124903
- Gul R, Saddique M, Khan MA, et al. Eco-friendly synthesis of silver nanoparticles and its biological evaluation using Tamarix aphylla leaves extract. Mater Technol. 2022;37(9):962–969. doi: 10.1080/10667857.2021.1908770
- Nirmala MJ, Durai L, Gopakumar V, et al. Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. IJN. 2019;14:6439–6450. doi: 10.2147/IJN.S211047
- Hadidi M, Pouramin S, Adinepour F, et al. Chitosan nanoparticles loaded with clove essential oil: characterization, antioxidant and antibacterial activities. Carbohydr Polym. 2020;236:116075. doi: 10.1016/j.carbpol.2020.116075
- Prasad R, Vyshnava SS. Antibacterial activity of silver nanoparticles synthesized by bark extract of Syzygium cumini. J Nanoparticles. 2013;2013:1–6. doi: 10.1155/2013/431218
- Gholami M, Azarbani F, Hadi F, et al. Eco-friendly synthesis of copper nanoparticles using Mentha pulegium leaf extract: characterisation, antibacterial and cytotoxic activities. Mater Technol. 2022;37(10):1523–1531. doi: 10.1080/10667857.2021.1959214
- Bernardo WDC, Boriollo MFG, Tonon CC, et al. Biosynthesis of silver nanoparticles from Syzygium cumini leaves and their potential effects on odontogenic pathogens and biofilms. Front Microbiol. 2022;13:995521. doi: 10.3389/fmicb.2022.995521
- Muthu K, Rajeswari S, Akilandaeaswari B, et al. Synthesis, characterisation and photocatalytic activity of silver nanoparticles stabilised by Punica granatum seeds extract. Mater Technol. 2021;36(11):684–693. doi: 10.1080/10667857.2020.1786786
- Dejen KD, Kibret DY, Mengesha TH, et al. Green synthesis and characterisation of silver nanoparticles from leaf and bark extract of Croton macrostachyus for antibacterial activity. Mater Technol. 2023;38(1):2164647. doi: 10.1080/10667857.2022.2164647
- Girase B, Depan D, Shah JS, et al. Silver–clay nanohybrid structure for effective and diffusion-controlled antimicrobial activity. Mater Sci Eng C. 2011;31(8):1759–1766. doi: 10.1016/j.msec.2011.08.007
- Palomino J-C, Martin A, Camacho M, et al. Resazurin microtiter assay plate: simple and Inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46(8):2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002
- Rajabi S, Ramazani A, Hamidi M, et al. Artemia salina as a model organism in toxicity assessment of nanoparticles. DARU, J Pharm Sci. 2015;23(1):20. doi: 10.1186/s40199-015-0105-x