ABSTRACT
The objective of this study is to obtain precise medication delivery by combining the drug xovoltib with chitosan nanoparticles that are synthesized from the cuttlebone of sepia. The effectiveness of this drug delivery system will be evaluated by examining its ability to inhibit the growth of cancer cells using the MCF-7 cell line. This study synthesized chitosan from the cuttlebone of sepia and complexed it with Tri Polyphosphate to form Chitosan-Tri Poly phosphate-nanoparticles (CS-TPP-NPS) using the ionotropic gelation method. The UV-visible absorption study showed a strong characteristic band ranging between 200–800 nm. The 2θ value at 20.0 degrees and 20.63 degrees indicates the crystalline nature of CS-TPP-NPS loaded with xovoltib. HR-SEM image but the morphology of chitosan nanoparticles was magnified with a high voltage of 20,000 KV and observed as spherical, porous and smooth. The in vitro drug delivery of xovoltib-loaded CS-TPP was tested against MCF-7 which had lowered the cell viability rate at 300 µl/mL.
Introduction
One of the biggest causes of death worldwide continues to be cancer. The potentially fatal word ‘cancer’ focuses everyone’s attention on finding a cure or a more effective and reasonable course of therapy. In a lifetime, cancer lines represent division and growth. Significant progress has been achieved in the fundamental understanding of cancer biology over the past few decades, improving approaches for diagnosis and treatment. One of the main causes is our inability to accurately deliver therapeutic drugs to specific areas while avoiding side effects on healthy tissue. Most malignancies are treated with chemotherapy, radiation therapy, and surgical excision [Citation1]. The two most common cancers in India are liver and breast cancer. Cancer cells are tested in cancer treatments and used for research. 1950 BT-20 was developed as the first breast cancer cell line [Citation2]. However, it took another 20 years before creating breast cancer cell lines became more popular [Citation3]. MCF-7, created in 1973 at the Michigan Cancer Foundation [Citation4], is still the world’s most widely used breast cancer cell line. The popularity of the oestrogen receptor (ER) expression makes it a perfect model for studying hormone response [Citation5].
Chitosan nanoparticles are drug carriers with wide development potential and have the advantage of controlled drug release, which improves drug solubility and stability, enhances efficiency, and reduces toxicity. Because of their small size, they can pass through biological barriers and deliver the drug to the targeted area [Citation6,Citation7]. Chitosan nanoparticles were tested in vitro for their ability to suppress tumour growth, and the results showed 27% inhibition of cervical cancer Hela cells and 23% inhibition of liver SMMC-7721 cells when used at a concentration of 500 mg/L. Using BGC-823 cells for gastric cancer, 29%, and MCF-7 cells for breast cancer, 55% [Citation8,Citation9]. They claimed that chitosan possessed anticancer properties, providing a strong case for its use as an additional antitumor medication and drug carrier. Studies have also shown considerable variations in the anticancer activity of chitosan nanoparticles produced by various manufacturers, as well as chitosan nanoparticles’ selectivity for tumour cells [Citation10,Citation11].
Nanocarriers can be employed in both passive and active targeting approaches. The cytotoxicity is enhanced by the increased permeability and retention effect (EPR), nanoparticle size, and unique characteristics. Chitosan nanoparticles (ChNPs) containing anticancer medications infiltrate tumour cells by exploiting the gaps in the Enhanced Permeability and Retention (EPR) effect on tumour angiogenic blood vessels, which have a size range of 600–800 nm [Citation12]. ChNP can trigger cellular death by disrupting cellular metabolism and inhibiting tumour-induced cell proliferation. Prior studies on ChNPs have discovered that chitosan and chitooligosaccharide with low molecular weight can stimulate the growth of tumour cells. The process of adsorbing blood plasma and other tissues will accelerate the detection of the reticuloendothelial system, as it is recognized as an alien entity by human antibodies. Macrophages will phagocytose ChNP and eliminate it via the body’s circulatory system. ChNP’s surface charge facilitates the adsorption of plasma proteins, enabling rapid identification by macrophages. This procedure facilitates the fast infiltration or ingress of the medication into the tumour tissue [Citation13].
Chitin, a common natural polysaccharide found in the exoskeleton of crustaceans like crabs, shrimp, and cuttlebone, is deacetylated to produce chitosan, a linear amino polysaccharide made up of randomly distributed [Citation1–4] linked D-glucosamine and N-acetyl-D-glucosamine units [Citation14]. Chitin is a white, rigid, and inelastic nitrogenous polymer that is present in both the interior and exoskeleton components of invertebrates. The naturally occurring, renewable polymers chitin and chitosan offer great qualities, including biodegradability, biocompatibility, non-toxicity, and adsorption [Citation15]. Xovoltib drugs are used to treat cancer; this tablet contains xovoltib, a tyrosine kinase inhibitor (4-anilinoquinazoline).
Chitosan, a naturally occurring cationic polysaccharide, is commonly employed as a biomaterial because of its chemical stability, safety, lack of toxicity, compatibility with living organisms, and capacity to break down naturally. Chitosan is commonly employed as a carrier substance for the formulation of drug-loaded nanoparticles [Citation16]. Prior research has demonstrated that chitosan can encapsulate protein and peptide medications, safeguarding them from degradation by proteolytic enzymes. This results in an extended duration of drug presence in the body. Furthermore, the process of manufacturing chitosan nanoparticles loaded with drugs is relatively straightforward, requiring only mild conditions. The bioactivity of proteins or peptides is minimally affected by the method, as it does not entail the use of organic solvents [Citation17]. Furthermore, there have been reports indicating that chitosan and its derivatives had inhibitory properties against cervical cancer, stomach cancer, and other types of tumour cells [Citation18]. Considering the exceptional carrier characteristics of chitosan, we hypothesized that utilizing xovoltib-loaded chitosan nanoparticles could potentially augment the anticancer efficacy of xovoltib.
This study aims to achieve targeted drug delivery by incorporating the drug xovoltib with chitosan nanoparticles synthesized from the cuttlebone of sepia and testing its anticancer activity using MCF-7 cell lines [Citation19].
Materials and methods
Sample collection and preparation
Sepia was gathered from the seashores of ‘Pazhaverkadu and Arambakkam’, where the cuttlebone was taken out. The cuttlebone was cleaned in running tap water, then with double-distilled water, and lastly, it was air dried in the shade. The cuttlebone was homogenized by a mortar and pestle after drying. The chitosan nanoparticles are to be synthesized using the protocol proposed by Lam et al. [Citation20].
Extraction of chitin from cuttlebone
Deprotenization
Eight grams of sodium hydroxide was weighed and dissolved in 200 mL of distilled water. The cuttlebone powder of 20 g was added with 1.0 M NaOH and incubated at 60°C. After 24 h, the samples were centrifuged at 4000 rpm for 10 min and the supernatant was discarded. The pellets were washed using distilled water until they reached the neutral pH and stored in a separate container.
Demineralization
Samples from the deproteinization process were added with 1.0 M HCL in the ratio 1:6 (W/V) and allowed to stand for 24 h [Citation21] with pH values ranging from 1.0 to 2.5 at room temperature. The solution was filtered, and the samples were washed with distilled water until a neutral pH (6.5–8.0) was achieved.
Procedure
Sixteen grams of NaOH was weighed and dissolved in 40 mL of distilled water. The samples were mixed with the NaOH solution and kept in a hot air oven for 16 h at 100 ̊C. It was then washed about three times using distilled water and centrifuged at 4000 rpm for 10 min, and the pellets obtained were known as chitin.
Extraction of chitosan from chitin
Deacetylation
Preparation of acetic acid and NaOH
Four mL of acetic acid was dissolved in 40 mL of distilled water
0.80 g of NaOH was mixed in 5 mL of distilled water.
Procedure
The deacetylation process was conducted by mixing the acetic acid solution with the chitin extracted, and the pH of the solution was increased by 10 by the addition of 1.4 mL of 4 M NaOH. This solution was centrifuged and transferred to the conical flask.
Dialyzing
The dialysis membrane was soaked in water, then one end of the membrane was tied with the thread, and the chitosan solution was poured into the membrane, and another end was also tied. The solution was dialysed against distilled water for 24 h and the dialysed solution was centrifuged at 4000 rpm for 10 min, and the pellets were taken in a container and freeze-dried. The obtained chitosan has met all the pharmacopeial requirements [Citation22].
Synthesis of CS-TPP nanoparticles
The synthesis of nanoparticles was performed as described by Anand et al., 2018 [Citation23].
Chitosan solution preparation
The freeze-dried chitosan of 40 mg was taken into a beaker and dissolved in 20 distilled water along with 4% acetic acid solution and was thoroughly mixed using the magnetic stirrer under 4000 rpm for 15 minutes15 min.
Preparation of sodium tri poly phosphate solution (TTP)
Twenty milligrams of TPP was mixed with 20 mL of distilled water.
Preparation of CS-TPP nanoparticles
Chitosan -Tri Poly Phosphate (CS-TPP) nanoparticles were synthesized by adding 40 g of chitosan in 20 mL of distilled water, and 40 µL of acetic acid was dissolved and sonicated before the solution became transparent. The addition of the TPP solution at a concentration of 5 mL to CS solution with stirring at room temperature produced the formation of CS-TPP nanoparticles by ionic gelation mechanism. For the preparation of CS-TPP nanoparticles loaded with the extract, 20%(W/V) will be added to the chitosan solution before adding the TPP solution.
Drug preparation
The xovoltib tablet (10 No.) was made into a powdered form. Twenty milligrams of the xovoltib powder was taken and dissolved in 10 ml of distilled water.
CS-TPP nanoparticles loaded with drug
Chitosan solution, TPP with drug, is added in a dropwise manner while it is in the magnetic stirrer itself at 4000 rpm. The solution is centrifuged at 4000 rpm for 10 minutes. The pellet is collected and kept for freeze-drying. After freeze drying, it is kept under a hot air oven at 60°C for 10 minutes.
Freeze drying
Both the CS-TPP solution with drug and without drug were taken in a separate tube and centrifuged for 10 min at 4000 rpm. The pellet was taken, and it was freeze-dried. After freeze-dried, the sample was kept in a hot air oven at 60°C for 10 min, and the pellet became powdered in form. Then, the CS-TPP NPS are given for SEM, UV, XRD and FTIR for analysis
Characterization of nanoparticles
The synthesized nanoparticles were characterized based on their size, morphology and surface charge by HR-SEM, UV-visible spectrophotometer, X-ray diffraction, and FTIR [Citation24–27].
High resolution scanning electron microscope (HR-SEM)
A scanning electron microscope scans a focused electron beam over a surface to create an image. The electrons in the beam interact with the samples, producing various signals that can be used to obtain information about the surface topology and composition. Sample preparation can be minimal or elaborate for SEM analysis, depending on the nature of the samples and the data required. Minimal preparation includes the acquisition of a sample that will fit into the SEM chamber and some accommodation to prevent charge build-up on electrically insulating samples. Most electrically insulating samples are coated with a thin layer of conducting material, commonly carbon, gold, or some other metal alloy. The choice of material for conductive coating depends on the data to be acquired: carbon is most desirable if element analysis is a priority, while metal coatings are most effective for high-resolution electron imaging applications. Alternatively, electrically insulating samples can be examined without a conductive coating in an instrument capable of low vacuum operation.
UV-Vis spectra
UV-VIS spectra were recorded using a UV-VIS spectrophotometer (Shimadzu, Japan) between 200 and 800 nm. Methanol was used as a blank. UV spectroscopy obeys the Beer-lambert law, which states that: when a beam of monochromatic light passes through a solution of an absorbing substance, the rate of decrease of intensity of radiation with the thickness of the absorbing solution is proportional to the incident radiation as well as the concentration of the solution.
X-Ray diffraction
X-ray diffraction is the method to determine the crystalline structure or phase of a crystal. Dried chitosan powder was used for analysis, and their diffraction patterns were recorded by PAN analytical XPRT PRO, D-8 advanced Brucker instrument.
Fourier transformed infrared spectroscopy (FTIT)
FTIR is extensively used for quantitative analysis and chemical structure determination of compounds to investigate the structure of polymers and to analyse the possible interactions between their functional groups. FTIR spectrum for the chitosan and drug-loaded chitosan nanoparticles spectrum was recorded using a Perkin-Elmer 1600 series FTIR spectrometer using potassium bromide (kBr) pellets. The pellets were placed in a light path, and the spectra were analysed.
Culturing the MCF-7 cells
The anticancer activity was performed on both chitosan nanoparticles with and without the drug xovoltib using MCF-7, the human breast cancer cell lines. A subculture of MCF-7 in Dulbecco’s modified Eagle’s Medium (DMEM) was trypsinized separately after discarding the culture medium. To the disaggregated cell in the flask, 25 mL of DMEM with 10% of foetal calf serum (FCS) was added. The cells were suspended in the medium by a gentle passage with the pipette, and the cells homogenized [Citation28].
Cytotoxicity assay
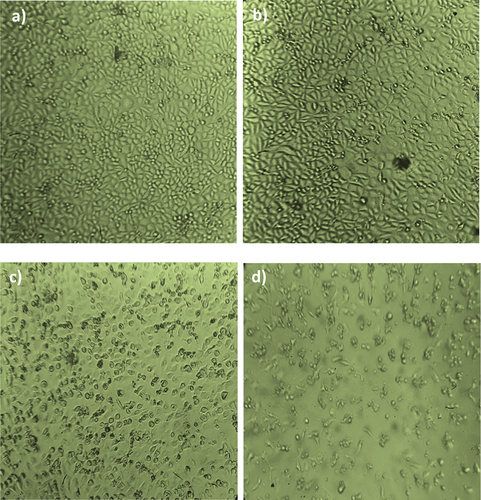
The cytotoxicity assay was carried out using (3-(4, 5-dimethyl thiazol- 2yl)-2, 5-diphenyl tetrazolium bromide (MTT). MTT is cleaved by mitochondrial succinate dehydrogenase and reductase of viable cells, yielding a measurable purple product, formazan. This formazan production is directly proportional to the viable cell number and inversely proportional to the degree of cytotoxicity. The homogenized cell suspension of 1 mL was added to each well of a 24-well culture plate along with different concentrations of CS-TPP nanoparticles and Xovoltib-loaded CS-TPP nanoparticles (0 to 300 µg/mL) and incubated at 37°C in a humidified CO2 incubator with 5% CO2. After 48 h of incubation, the cells were observed under an inverted tissue culture microscope with 80% confluence of cells cytotoxicity assay was carried out as previously described by Abondanza in the year 2008 [Citation29]. After 48 h of incubation, the wells were added with MTT and left for 3 h at room temperature. All wells have removed the content using the pipette, and 100 µl SDS in DMSO was added to dissolve the formazan crystals; absorbance s was read in lark LIPR- 9608 microplate reader at 540 nm [Citation30]. The absorbance at 570 nm was measured with a UV-Spectrophotometer using wells without Nanoparticles containing cells as blanks. The effect of the samples on the proliferation of MCF-7 was expressed as the %cell viability using the following formula:
% of cell viability = A570 of treated cells/A570 of control cells X 100
Statistical analysis
All measurements were conducted in triplicates, and the resulting experimental data are presented as mean ± standard deviation (SD). Statistical analyses were performed using SPSS
package v 23 SAS software version 9.4 (SAS Institute Inc., Cary, NC, U.S.A.). The statistical
significance of the data was assessed through two-sample independent t-tests and one-way ANOVA with a significance limit set at a 5% probability level [Citation31].
Results and discussion
Characterization of nanoparticle CS-TPP- NPS
High resolution scanning electron microscope
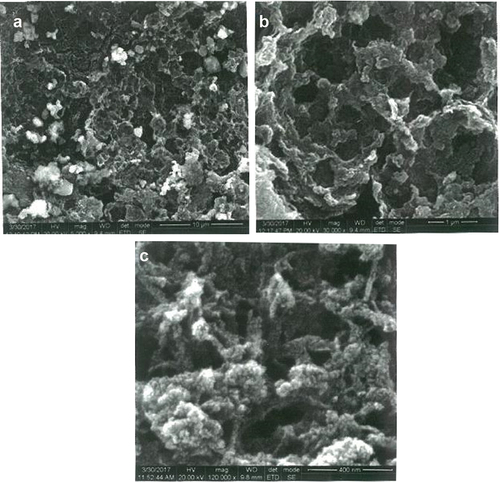
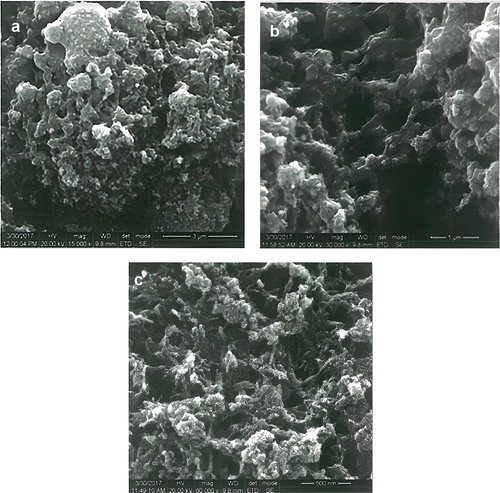
The structural morphology of synthesized Chitosan Triphosphate nanoparticles (CS-TPP) from the cuttlebone of sepia was characterized by HR-SEM. The normal chitosan nanoparticles size was 65 nm, and the average distribution size ranged starting from 43 to 82 nm. The chitosan nanoparticles were magnified and studied with a high voltage of 20,000 KV by varying magnification. The xovoltib drug loaded with synthesized nanoparticles were also analysed with different magnification, which confirmed the formation of particles by the appearance of special, porous, smooth and the particle size ranges from 10 to 30 nm. The current SEM analysis study revealed particles’ interaction after loading with chitosan nanoparticles (CS-TPP). The magnification of about 3000 X for chitosan nanoparticles with xovoltib and the nanoparticles which were not loaded without the drug also showed greater difference by the long chain of interacting rod-shaped particles. This is clearly shown in .
UV-Vis spectroscopic analysis
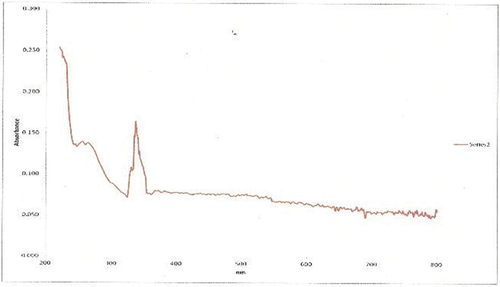
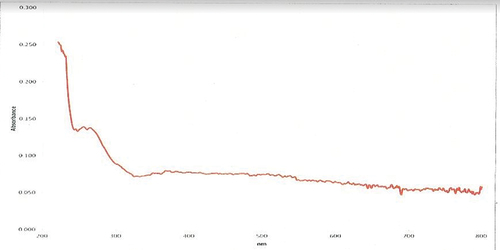
The UV-Visible absorption study showed a strong characteristic band ranging between 200-800 nm for chitosan nanoparticles synthesized and xovoltib loaded with CS-TPP. This clearly indicates the strong surface plasmon resonance (SPR) and shows a significant difference by comparing nanoparticles loaded with xovoltib drug and without the drug. This study revealed good absorbance observed by a sharp peak at 335 nm. The absorbance range for the nanoparticles with and without drugs is shown in .
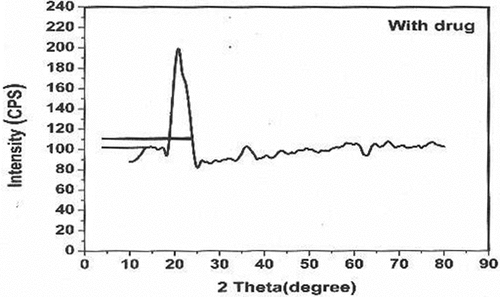
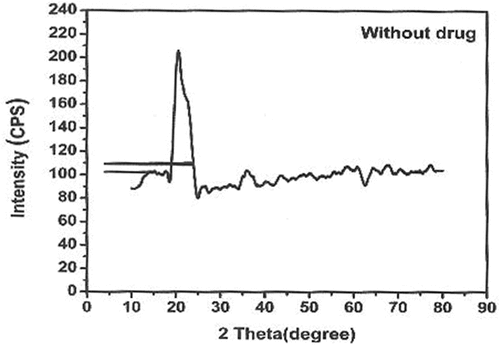
X-ray diffraction method
XRD patterns revealed the nature of crystallinity of the CS-TPP nanoparticles and CS-TPP xovoltib-loaded nanoparticles. The XRD patterns of CS-TPP NPS showed 20.0 degree and 35.0 degree prominent crystalline peaks (2θ) with high intensity. The 2θ value of CS-TPP loaded with xovoltib at 20.0 degrees and 20.63 degree shows strongly indicates crystalline nature. The distinct differences in the diffraction patterns of CS-TPP nanoparticles and CS-TPP loaded with Xovoltib could be attributed to the modification in the arrangement of molecules in the crystal lattice. It was well known that the CS-TPP nanoparticles exhibit a semi-crystalline structure with a peak at the 2θ angles of 20.0̊. The XRD patterns for the nanoparticles with and without a drug is shown in .
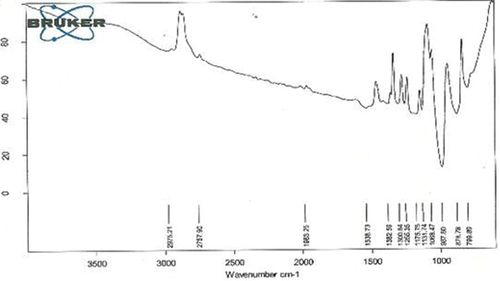
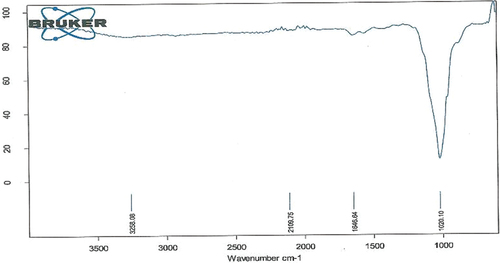
Fourier transformed infrared spectroscopy (FTIR)
To investigate CS-TPP nanoparticles formation, FTIR spectra of CS-TPP nanoparticles and Xovoltib loaded CS-TPP nanoaprticles were recorded. The IR spectrum of CT-PP nanoparticles with and without drug showed several obvious characteristic peaks is shown in and their corresponding functional groups is been discussed in .
Table 1. Characteristic FTIR absorption frequency of CS-TPP nanoparticles loaded with xovoltib.
Table 2. Characteristic FTIR absorption frequency of CS-TPP nanoparticles without xovoltib.
Cytotoxicity assay
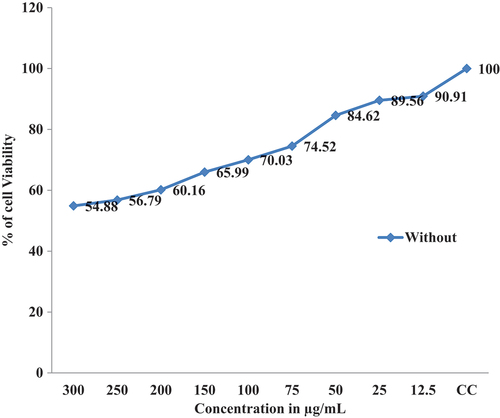
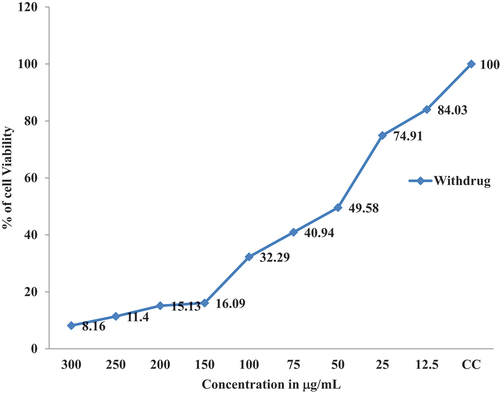
The Invitro cytotoxicity activity proved that cancer cell lines were inhibited significantly with the increasing of CS-TPP Nanoparticles and Xovoltib-loaded CS-TPP nanoparticles concentration. In MCF-7 cell lines, more cytotoxicity effect was observed in both CS-TPP Nanoparticles and xovoltib-loaded CS-TPP nanoparticles also cells without the addition of any of these nanoparticles taken as control after 48 hours of treatment. In comparison with the results of the MTT assay, it revealed that increased concentration of Xovoltib-loaded CS-TPP Nanoparticles showed superior toxicity over the cancer cells at higher concentration of 300 µg/mL while CS-TPP nanoparticles showed less toxicity against the cell lines at 300 µg/mL. This effect is due to the samples having more specific activity in cancer cell lines. The cell viability (%) was calculated for control, xovoltib loaded CS-TPP nanoparticles. It was recorded that half maximal inhibitory concentration (IC50) for xovoltib-loaded CS-TPP nanoparticles showed 50 µg/mL concentration, then unloaded CS-TPP nanoparticles did not attain IC50 at test concentrations. The cytotoxicity activity of chitosan nanoparticles with and without a drug ()
Discussion
Natural and non-toxic biopolymers chitin and chitosan are now widely produced commercially from cuttle bone of sepia. During the past few decades, chitin and chitosan have attracted significant interest in view of a wide range of proposed novel applications. Their unique properties, biodegradability, biocompatibility and non-toxicity make them useful for a wide range of applications [Citation32]. The chitin obtained after deproteinization and demineralization was about 29.57% and 13.45% and the chitosan obtained after de-acetylation was about 7.29%. Similarly, the chitosan yield of crab shell was determined as 4.65% from grinded crab shell after demineralization (yield was 34.32%), deproteinization (yield is 7.25%) and de-acetylation process.
β-Chitin can be obtained from cuttlefish and then deacetylated using alkaline hydrolysis to yield chitosan, which is better suitable for many applications [Citation33]. Shushizadeh et al. (2015) documented the production of chitosan derived from cuttlefish [Citation34]. In recent years, there has been an increase in studies focusing on the extraction and characterisation of β-chitin from squid and cuttlefish bones due to its bioactive qualities [Citation35]. Chitin can be obtained using two regularly employed methods: chemical extraction and biological extraction using microbes. When compared to several ecologically benign chemical extraction technologies [Citation17], old strong acid/alkali methods for extracting chitin have drawbacks and cause different environmental issues. The manufacture of chitin from crustacean waste employs biological processes due to their convenient, uncomplicated, and rapid benefits. Additionally, they may typically be controlled by optimising process parameters, ambient temperature, and minimising solvent usage, resulting in a reduced environmental effect and cost. However, up until now, it has only been conducted on a small scale in laboratory settings [Citation36].
Nanoparticle chitosan has the ability to form a bond with CPP. CPP, or cell-penetrating peptide, is a peptide that is classified as short, water-soluble, and somewhat hydrophobic. It consists of at most 30–35 amino acid residues and has a positive charge [Citation37]. Furthermore, CPP is defined at physiological pH. Cationic cell-penetrating peptides (CPPs) has the unique ability to enter cell membranes at low micromolar concentrations in both living organisms and laboratory settings, without the need for chiral receptors and without inflicting significant membrane harm. CPP enhances cellular uptake, as demonstrated in a study. A rise will ensue from the increase in cellular absorption in cytotoxicity [Citation38].
CS-TPP nanoparticles are sensitive towards its shape, size, pH, chitosan and TPP concentration, agglomeration state and refractive index near the nanoparticles surface which makes UV-Vis is valuable tool for identifying and characterizing the nanomaterial. The surface of CS-TPP nanoparticles loaded with Xovoltib drug became rougher and studded with dense granules depicted in HR-SEM image but the morphology of chitosan nanoparticles were magnifies with high voltage 20,000 KV observed as spherical, porous and smooth. The present study concluded that the drug was incorporated in its amorphous or disordered crystalline phase inside the nanoparticle matrix. The presence of granular particles of varying sizes was also noticed and attributed to a chemical activity sufficient to the formation of agglomerates whose morphology is different from the structure of the polymer alone [Citation39]. This study was found that the synthesized chitosan nanoparticles were shown greater difference after loaded with xovoltib drug by their shape and size. Chitosan is a polycation which expands at lower pH values due to higher protonation and electrostatic repulsive interactions between polymer chains and produces nanoparticles with large particle size [Citation3]. The UV-Vis absorption spectra of CS-TPP loaded with xovoltib showed absorbance peak at 335 nm which indicates the particles are polydispersed in low wavelength. The X-ray diffraction patterns of CS-TPP nanoparticles and CS-TPP nanoparticles loaded with Xovoltib depicted the crystalline nature of the particles with intense reflections at 2θ degree. XRD analysis provides the crystal lattice arrangements and gives the information regarding the degree of crystallinity in the formulation. XRD results confirmed the cross-linking reaction between CS and TPP in the formation of nanoparticles. It was reported that after ionic cross-linking CS with TPP, no peak is detected in the diffract grams of CS nanoparticles, reflecting the destruction of the native CS packing structure. From the XRD patterns of CS-TPP nanoparticles and CS-TPP nanoparticle loaded with xovoltib, molecular level dispersion of xovoltib in CS – TPP nanoparticles is clearly indicated from the XRD bands. This implies that the addition of xovoltib into chitosan polymer matrix cross-linked with TPP greatly augmented the domain of amorphous regions (i.e. the intensity of XRD crystal peak decreased) Yang and Wu in the year 2009 [Citation40] also reported similar XRD patterns for the characterization of microprous poly(vinyl alcohol)/poly (vinyl chloride) (PVA/PVC)) composite polymer membrane for use on the capacitors. CS-TPP nanoparticles and Xovoltib (drug) loaded CS-TPP Nanoparticles were analysed using FTIR spectrophotometer for the characteristic absorption bands [Citation39]. IR spectroscopy is perhaps to identify the functional groups. This is possible because different functional groups vibrate at different frequencies allowing their identification. The frequency of vibration however depends on the additional factors such as delocalization of electrons, H-Bonding and substitutions at the nearby groups. The absorption bands in 4000–1500 cm−1 region allow identification of functional groups of the IR spectrum. The lower energy portion of the mid -IR region (1500-400 cm−1) usually contains a very complicated set of peaks arising due to complex vibrations involving several atoms. This region is unique to a particular compound and therefore is known as the fingerprint regions of the IR spectrum. Though it is difficult to assign the vibrational modes to these peaks, these are useful to identify a compound if the spectrum of the compound is already known. According to the FTIR absorption peaks for CS-TPP nanoparticles and CS-TPP nanoparticles loaded with xovoltib provides the identification of functional group. In comparison with the FTIR spectrum of CS-TPP nanoparticles and xovoltib loaded CS-TPP nanoparticles, showed shift Amine group from 3258.08 cm−1 and 2109.75 cm−1 to 1382.59 cm−1 and 1255.35 cm−1. Because of strong dipole molecules the IR absorbance 1020.10 cm-1 of the ether functional group with C-O-C stretching vibration. MTT was the most sensitive viability assay for detecting the cytotoxic effects of the nanoparticles, regardless of the cell line. This implies that although nanoparticles primarily exerted toxicity on the mitochondrial compartment after cellular internalization, the plasma membranes remained intact. These findings could be related to the mechanism of action of xovoltib. The drug xovoltib is a type of biological therapy called a Tyrosine Kinase Inhibitor (TKI). It is a treatment for non-small cell lung cancer that has spread outside the lung or to the other parts of the body. It works by blocking particular proteins on cancer cells that encourage the cancer growth. Xovoltib blocks protein called epidermal growth factor receptors (EGFR). Cancers that have this receptor are called EGFR positive (Cancer research UK).Garcia in the year 2006 [Citation41] examined the cell death of MCF-7 human breast cancer cell induced by EGFR activation in the absence of other growth factors. The epidermal growth factor (EGF) receptor (EGFR) plays an important role in the growth and progression of breast cancer. Overexpression of EGFR or high activity of EGFR signal pathway has been related with increases in cell proliferation and a poor prognosis in patients with breast cancer. In this study MCF-7 human breast cancer cells were stimulated with EGF and the effects on cell proliferation, signal pathways and cell cycle progression were determined. The result demonstrated that EGF stimulation in the absence of others growth factors induced a modest effect on cell proliferation and the induction of a cellular arrest in the G1 phase of the cell cycle. Hence xovoltib loaded CS-TPP nanoparticles is an enhanced way for drug delivery to the targeted regions which avoids the other effects of administration of xovoltib through orally [Citation42].
Conclusion
Nanotechnology provides a wide range of options in the treatment of cancer. In this study, the chitosan was extracted from cuttle bone, a thrown-away material while processing the sepia was utilized for the preparation of polymeric nanoparticles with TPP by using the ionic gelation method. Xovoltib is a tyrosine kinase inhibitor drug which was loaded with CS-TPP nanoparticles. The characterizations of formulated nanoparticles were studied using SEM, UV-VIS, XRD and FTIR. The results suggested the morphological, size, shape and functional group of the xovoltib loaded CS-TPP. The in vitro drug delivery CS-TPP was tested against MCF-7 breast cancer cell lines. The result suggested that xovoltib-loaded CS-TPP should lower the cell viability rate at 300 µl/mL. This is a result of higher anticancer activity shown by the xovoltib loaded CS-TPP nanoparticles. Therefore, it can be concluded that CS-TPP nanoparticles and xovoltib-loaded CS-TPP nanoparticles are potential carriers and drug delivery systems, respectively.
Acknowledgments
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2024R398) King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- Ruoslahti E, Sangeeta N, Sailor, et al. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188(6):759–13. doi: 10.1083/jcb.200910104
- Lasfargues EY, Ozello L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958;21:1131–1147.
- Caillaeu R, Olive M, Cruciger QV. LLong term human breast carciboma cell lines of metastatic origin: preliminary characterization. In vitro. 1978;14(11):911–915. doi: 10.1007/BF02616120
- Soule HD, Vasquez J, Long A, et al. A human cell line from a pleural effusion derived from a breast carcinoma 2. J Natl Cancer Inst. 1973;51(5):-1409–1413. doi: 10.1093/jnci/51.5.1409
- Levvenson SA, Jordan VC. T-cell recognition of tumor-associated carbohydrates: the nature of the glycan moiety plays a decisive role in determining glycopeptide immunogenicity. Perspectives Cancer Res. 1997;57(15):-3071–3078.
- Agarwal M, Nagar DP, Srivastava N, et al. Chitosan nanoparticles based drug delivery. Int J Adv Multidiscip Res. 2015;2(4):-1–13.
- Praseetha PK, Godwin MA, AlSalhi MS, et al. Porous chitosan-infused graphitic carbon nitride nanosheets for potential microbicidal and photo-catalytic efficacies. Int J Biol Macromol. 2023 May 31;238:124120. doi: 10.1016/j.ijbiomac.2023.124120
- Aruna U, Rajalakshmi R, Muzib IY, et al. Role of chitosan nanoparticles in cancer therapy. Int J Innovative Pharm Res. 2013;4(3):318–324.
- Zhou SH, Hong Y, Fang GJ. Preparation, characterization and anticancer effect of chitosan naaoparticles. J Clin Rehabilitative Tissue Eng Resource. 2007;111(48):9688–9691.
- Fang GJ, Hong Y, Jiang YY. Comparision of antitumor effects of chitosan nanoparticles from different sourse invitro. J Clin Rehabilitative Tissue Eng Resource. 2007;11(48):9696–9699.
- Karthika V, AlSalhi MS, Devanesan S, et al. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci Rep. 2020 Nov 3;10(1):18912. doi: 10.1038/s41598-020-76015-3
- Gholami N, Cohan RA, Razavi A, et al. Cytotoxic and apoptotic properties of a novel nano-toxin formulation based on biologically synthesized silver nanoparticle loaded with recombinant truncated pseudomonas exotoxin A. J Cell Physiol. 2020;235(4):3711–3720. doi: 10.1002/jcp.29265
- Mushtaq A, Li L, Grøndahl L. Chitosan nanomedicine in cancer therapy: targeted delivery and cellular uptake. Macromol Biosci. 2021;21(5):2100005. doi: 10.1002/mabi.202100005
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2(5):-347–360. doi: 10.1038/nrd1088
- Hudson SM, Smith C. Polysaccharides: Chitin and Chitosan: Chemistry and Technology of Their Use As Structural Materials. In: Biopolymers from Renewable Resources. Springer; 1998. p. 96–118.
- Carter KC, Puig-Sellart M. Nanocarriers made from non-ionic surfactants or natural polymers for pulmonary drug delivery. Curr Pharm Des. 2016;22(22):3324–3331. doi: 10.2174/1381612822666160418121700
- Amidi M, Mastrobattista E, Jiskoot W, et al. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82. doi: 10.1016/j.addr.2009.11.009
- Zhang L, Hu Y. Alphastatin-loaded chitosan nanoparticle preparation and its antiangiogenic effect on lung carcinoma. Int J Polym Sci. 2019;2019:e2751384. doi: 10.1155/2019/2751384
- Bamrungsap S, Zhao Z, Chen T, et al. Nanotechnology in therapeutics, a focus on nanoparticles as a drug delivery system. Nanomedicine. 2012;7(8):1253–127. doi: 10.2217/nnm.12.87
- Lam TD, Hoang VD, Lien LN, et al. Synthesis and characterization of chitosan nanoparticles used as drug. J Chem. 2006;44:105–109.
- Puvvada YS, Vankayalapati S, Sukhavasi S. Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Int Curr Pharm J. 2012;1(9):258–263. doi: 10.3329/icpj.v1i9.11616
- Căprărescu S, Zgârian RG, Tihan GT, et al. Biopolymeric membrane enriched with chitosan and silver for metallic ions removal. Polymers. 2020;12(8):1792. doi: 10.3390/polym12081792
- Anand M, Sathyapriya P, Maruthupandy M, et al. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front Lab Med. 2018;2(2):72–78. doi: 10.1016/j.flm.2018.07.003
- Veena S, Devasena T, Sathak SSM, et al. Green synthesis of gold nanoparticles from vitex negundo leaf extract: characterization and in vitro evaluation of antioxidant–antibacterial activity. J Clust Sci. 2019;30(6):1591–1597. doi: 10.1007/s10876-019-01601-z
- Mohammed SSS, Lawrance AV, Sampath S, et al. Facile green synthesis of silver nanoparticles from sprouted zingiberaceae species: spectral characterisation and its potential biological applications. Mater Technol. 2020;37(8):533–546.
- Dhanislas M, Sampath S, Shamya M, et al. Green synthesis of biofabricated silver nanoparticles from syzygium aromaticum seeds: spectral characterization and evaluation of its anti-mycobacterial activity, cytotoxicity assessment on zebrafish embryo and artemia salina. Mater Technol. 2023;38(1):2269358. doi: 10.1080/10667857.2023.2269358
- Sampath S, Sunderam V, Madhavan Y, et al. Facile green synthesis of zinc oxide nanoparticles using artocarpus hirsutus seed extract: spectral characterization and in vitro evaluation of their potential antibacterial-anticancer activity. Biomass Conv Bioref [Internet]. 2023 cited 2023 Jul 19. doi: 10.1007/s13399-023-04127-7
- Yasasve M, Kumaran PM, Manoj D, et al. Evaluation of arjunolic acid against brucella melitenis and in vitro cytotoxic study of lung adenocarcinomic cell line (A549). Indian J Exp Biol (IJEB). 2022;60:510–513.
- Abondanza TS, Oliveria CR, Barbosa CMV. Bcl-2 expression and apoptosis induction in human HL-60 leukaemic cells treated with a novel organotellurium compound RT-04. Food Chem Toxicol. 2008;46(7):2540–2545. doi: 10.1016/j.fct.2008.04.010
- Mosamann T. Rapid colorimetric assay for cellular growth and survival: application to proliferate and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):-55–63. doi: 10.1016/0022-1759(83)90303-4
- How to cite IBM SPSS statistics or earlier versions of SPSS [Internet]. [cited 2022 Jun 10]. Available from: https://www.ibm.com/support/pages/how-cite-ibm-spss-statistics-or-earlier-versions-spss
- Wang SL, Chang TJ, Liang TW. Conversion and degradation of shellfish wastes by serratia species. TKU016 fermentation for the production of enzymes and bioactive materials. Biodegradation. 2010;10532:009–9303.
- Hazeena SH, Hou C-Y, Zeng J-H, et al. Extraction optimization and structural characteristics of chitosan from cuttlefish (S. pharaonis sp.) bone. Materials. 2022;15(22):7969. doi: 10.3390/ma15227969
- Shushizadeh MR, Pour EM, Zare A, et al. Persian gulf β-chitin extraction from Sepia pharaonis sp. cuttlebone and preparation of its derivatives. Bioact Carbohydrates Dietary Fibre. 2015;6(2):133–142. doi: 10.1016/j.bcdf.2015.09.003
- Subhapradha N, Ramasamy P, Shanmugam V, et al. Physicochemical characterisation of β-chitosan from sepioteuthis lessoniana gladius. Food Chem. 2013;141(2):907–913. doi: 10.1016/j.foodchem.2013.03.098
- Kaur S, Dhillon GS. Recent trends in biological extraction of chitin from marine shell wastes: a review. Crit Rev Biotechnol. 2015;35(1):44–61. doi: 10.3109/07388551.2013.798256
- Herdiana Y, Wathoni N, Shamsuddin S, et al. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polym (Basel). 2021;13(11):1717. doi: 10.3390/polym13111717
- Wicaksono PA, Name S, Martien R, et al. Formulation and cytotoxicity of ribosome-inactivating protein mirabilis Jalapa L. Nanoparticles using alginate-low viscosity chitosan conjugated with anti-epcam antibodies in the T47D breast cancer cell line. Asian Pac J Cancer Prev. 2016;17(4):2277–2284. doi: 10.7314/APJCP.2016.17.4.2277
- Marreco PR, Moreira PL, Genari SC, et al. Effect of different sterilization methods on the morphology, mechanical properties and cytotoxicity of chitosan membranes used as wound dressing. J Biomed Mater Res. 2004;71(2):-268–277. doi: 10.1002/jbm.b.30081
- Yang CC, Wu GM. Study of microporous PVA/PVC composite polymer membrane and its application to MnO2 capacitors. Mater Chem Phys. 2009;114(2–3):-948–955. doi: 10.1016/j.matchemphys.2008.11.009
- Murugan K, Nataraj D, Madhiyazhagan P, et al. Carbon and silver nanoparticles in the fight against the filariasis vector culex quinquefasciatus: genotoxicity and impact on behavioral traits of non-target aquatic organisms. Parasitol Res. 2016 Mar;115(3):1071–1083. doi: 10.1007/s00436-015-4837-9
- Garcia R, Franklin RA, McCubrey JA. Cell death of MCF-7 human breast cancer cell induced by EGFR activation in the absence of other growth factors. Cell Cycle. 2006;5(16):-1840–1846.