ABSTRACT
Reactive aggression refers to aggressive behaviour evoked by threat, provocation or frustration. While not all adolescents display reactive aggressive behaviour, these behaviours peak during adolescence. This review discusses whether typical patterns of adolescent brain development, particularly in circuitry of relevance to reactive aggression and which underpin emotional reactivity, regulation and social behaviour, may render some adolescents vulnerable to exhibiting reactive aggression. As highlighted by theories of aggression developed in adults, individual differences play a key role in determining the likelihood of aggressive behaviour. We therefore also consider factors such as hyper-responsivity to threat, poor emotion regulation and high levels of irritability, which characterise adolescents exhibiting clinical levels of reactive aggression. It is likely that normative development of the relevant neural circuitry interacts with individual and social risk factors to increase vulnerability to externalising conditions in a minority of adolescents.
1. Introduction
Reactive aggression refers to aggressive behaviour in response to real or perceived threat, provocation or frustration, and is typically impulsive, immediate, and directed toward the perceived perpetrator (Berkowitz, Citation1993). Reactive aggressive behaviours often escalate or emerge for the first time during adolescence (Moffitt, Citation1993; Raine et al., Citation2006), with significant potential for long-term socio-legal consequences for both perpetrator and victims (Erskine et al., Citation2014). This period of life is characterised by significant brain development in regions underpinning processes relevant to reactive aggression, such as threat evaluation and self-control (e.g. Mills, Goddings, Clasen, Giedd, & Blakemore, Citation2014). It is therefore perhaps surprising that relatively little research has engaged in understanding the neural basis of reactive aggression and its typical and atypical development during adolescence. This review aims to synthesise existing literature on the neurocognitive bases of reactive aggression and their development and presentation during adolescence. It will start by identifying the neural networks involved in aggression, also known as the ‘aggression network’. It will then explore the development of reactive aggression and its neural bases throughout ‘typical’ adolescence. Finally, it will review the current neuroimaging literature in adolescent clinical samples characterised by high levels of reactive aggression, e.g. conduct disorder. It is for these individuals that the phenomenon of increased susceptibility to reactive aggression during adolescence appears to be most prominent. According to some models (e.g. Casey et al., Citation2010; Moffitt, Citation2003), transient aggressive behaviour and mood volatility are typical features of normative adolescent neurocognitive and social development. However, the vast majority of adolescents do not demonstrate an increase in clinically significant reactive aggression over this period. It is therefore necessary to explore both typical and atypical trajectories of reactive aggression and its neural bases, in order to understand why only a subset of individuals develop harmful reactive aggressive behaviours, and how this may be prevented.
2. What is reactive aggression?
2.1. Theoretical frameworks
Reactive aggression occurs, by definition, in the absence of pre-planned intention, in contrast to proactive aggression which is goal-directed or instrumental in nature (Dodge & Coie, Citation1987). There are also key differences in the cognitive processes underpinning reactive and proactive aggression; reactive aggression tends to be associated with poor emotion regulation and executive control, whereas proactive aggression is more strongly associated with callous-unemotional traits characterised by lack of empathy, guilt, and shallow affect (Frick & Viding, Citation2009). Behavioural models of aggression, e.g. the General Aggression Model (Anderson & Bushman, Citation2002; see also Allen, Anderson, & Bushman, Citation2018) and the I3 (‘I-cubed’) model (Finkel & Hall, Citation2018; Finkel & Slotter, Citation2009; Slotter & Finkel, Citation2011) provide theoretical frameworks for studying aggression, from which specific models may be tested, and which can be used as a guide to interpret the neuroimaging literature on reactive aggression. These models aim to account for individual differences in susceptibility to reactive aggression as they take into account different factors which may increase or decrease the likelihood that a situation will provoke a reactive aggressive response.
The General Aggression Model (Allen et al., Citation2018; Anderson & Bushman, Citation2002) considers the roles of a broad range of person-related and situational factors, including social, cognitive, biological, developmental and environmental factors on aggression. Person-related factors refer to individual differences in traits, e.g. trait anger, cognitive biases, and impaired executive functions. Situational factors include components such as frustration, provocation, social stress and social rejection. Each of these component factors are considered modifiers of the likelihood of an aggressive response by mediating cognitive and affective processes such as affect appraisal and decision-making. Through repeat exposure, these factors create knowledge structures which build aggressive ‘personalities’, in turn influencing the likelihood of an individual to aggress.
Similarly, the I3 theory (Finkel & Hall, Citation2018; Slotter & Finkel, Citation2011; see also Perfect Storm Theory; Finkel, Citation2014) does not focus on one ‘root’ cause of aggression but on a multitude of influencing factors. The I3 theory provides an organisational model of aggression whereby the likelihood of an aggressive response is determined by the culmination of the interacting effects of three overarching factors (see ): Instigation (stage 1), Impellence (stage 2) and Inhibition (stage 3). Instigation is the necessary first step in the process of aggression and refers to situations or circumstances which may trigger an aggressive impulse in some individuals, e.g. goal-obstruction or peer-rejection. Stage 2 of the model is Impellence. These are factors which determine the strength of the aggressive impulse, and include both person-related (e.g. personality, attitudes and beliefs) and situational (e.g. temperature, pain) factors. The net value of both Instigating and Impellence factors determine an individual’s proclivity to aggress. The final stage of the model, Inhibition, refers to factors such as inhibitory control and frontal lobe function, which may serve to override the proclivity to aggress.
Figure 1. I3 Model of aggression: Proclivity to aggress (y-axis) is determined by the net strength of the interaction between Instigating (x-axis) and Impellence factors. Aggressive behaviour only manifests when the strength of proclivity to aggress exceeds the strength of Inhibition factors, shown here as the behaviour thresholds. Taken from Finkel and Hall (Citation2018). Reprinted from Current Opinion in Psychology, 19, Finkel & Hall, The I3 Model: a metatheoretical framework for understanding aggression, 125-130, Copyright (2018), with permission from Elsevier.
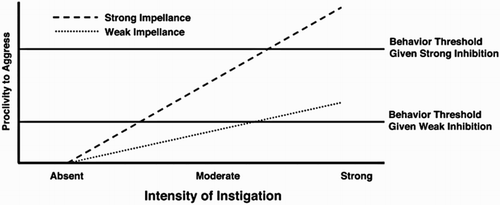
Both models provide a detailed framework of factors at multiple levels of analysis (e.g. social, cognitive, biological) which may interact to culminate in an aggressive response. In addition, the I3 model makes an important distinction between factors related to risk for an aggressive response (e.g. trait narcissism or hostile rumination) and those related to resilience (e.g. inhibitory control ability). However, as noted by the authors (e.g. Finkel & Hall, Citation2018), these models of aggression currently constitute ‘meta-theories’, i.e. general-purpose frameworks laying down a foundation of ‘true assumptions’ as opposed to falsifiable propositions. Moreover, while these theories acknowledge the importance of both development and the neurobiological underpinnings of aggressive behaviour, specific models of the development of the neural underpinnings of susceptibility to reactive aggression are currently lacking. According to the prominent neuroconstructivist approach to developmental disorder (Dekker & Karmiloff-Smith, Citation2011; Karmiloff-Smith, Citation1998) and the related ‘causal model’ of developmental disorder (Morton & Frith, Citation1995), atypical behaviour should be understood as arising from an interplay between genes, environment, brain, cognition and behaviour over developmental time. Applied to reactive aggression, we would argue it is important to understand the context in which aggression-relevant cognitive processes (e.g. emotional reactivity and regulation) are developing. As discussed below, adolescence is a key time for the development of the neural bases of such processes, and as such could constitute a ‘sensitive period’ for the emergence of both adaptive and maladaptive patterns of response to aggression triggers. In the next section we will review the neural circuitry involved in the elicitation and regulation of reactive aggressive responding, before discussing this circuitry in relation to typical and atypical development.
2.2. Neural bases
Animal studies have provided a useful basis for studying the neural underpinnings of reactive aggression. Much of this research has been done using lesion studies in rodents and non-human primates (see Bartholow, Citation2018; Nelson & Trainor, Citation2007, for comprehensive reviews). In adult male rodents, lesions to the anterior hypothalamus (Kruk, Citation1991) and medial amygdala (Vochteloo & Koolhaas, Citation1987) resulted in reduced aggression, with similar effects also found in non-human primates (e.g. hypothalamus lesions, Lloyd & Dixson, Citation1988). This suggests that these regions are crucial for the aggression response. In contrast, electrical stimulation of the anterior hypothalamus in male rodents (Kruk, Citation1991; Kruk et al., Citation1984), to the ventromedial hypothalamus in non-human primates (Lipp & Hunsperger, Citation1978) and to the amygdala in male rodents (Potegal, Hebert, DeCoster, & Meyerhoff, Citation1996) all increased the number of species-specific aggressive behaviours, e.g. vocal displays of dominance in primates. Furthermore, electrical stimulation of the anterior hypothalamus or periaqueductal gray (PAG) in cats induced defensive rage behaviours, mirroring naturally elicited behaviours exhibited in response to threat (Siegal, Roeling, Gregg, & Kruk, Citation1999). A number of prefrontal cortex areas have also been implicated in the aggression network, notably the orbitofrontal cortex (OFC). Lesions to the OFC in male rats and dominant rhesus monkeys resulted in increased aggression (De Bruin, Van Oyen, & Van De Poll, Citation1983; Machado & Bachevalier, Citation2006), suggesting these regions may regulate aggression via inhibitory control function.
Human lesion and brain injury studies have found largely similar results to those in the animal literature. Lesions to the orbitofrontal cortex (OFC) in humans have been associated with high levels of reactive aggression in individuals identified as having ‘acquired sociopathy’ (e.g. Blair, Citation2001). Case studies of patients with OFC lesions have also reported explosive and impulsive aggressive outbursts (Anderson, Bechara, Damasio, Tranel, & Damasio, Citation1999; Blair & Cipolotti, Citation2000). Likewise, in a review of the frontal brain injury literature, brain injury to focal OFC was specifically associated with increased levels of aggression compared to other areas of frontal brain injury (Brower & Price, Citation2001). Additionally, lesions to the adjacent ventromedial prefrontal cortex (vmPFC) was associated with increased aggressive behaviours in veterans compared to veterans with lesions to other regions and healthy controls (Grafman et al., Citation1996). Increased aggression occurring post-removal or damage to the OFC and vmPFC therefore suggests these areas regulate aggressive responding, such that greater activation in areas such as the OFC and vmPFC would more strongly suppress an aggressive response (Davidson, Putnam, & Larson, Citation2000).
Within human neuroimaging there has been some converging evidence to that found across animal models of aggression and human lesion studies. Adults with increased levels of reactive aggression have been found to have hyper-responsivity, particularly within the amygdala, when viewing negatively valenced or threatening images, e.g. angry faces (Nomura et al., Citation2004) compared to neutral faces. In contrast, a meta-analysis of fMRI studies using emotional face viewing paradigms in typical individuals found that angry faces had no significant effect on the amygdala (Fusar-Poli et al., Citation2009). Rather, angry faces selectively activated the insular cortex. The amygdala, insular cortex and hypothalamus (implicated in animal studies) form part of the limbic system, one of the oldest systems evolutionarily, and is responsible for threat and salience processing (Adolphs, Citation2008). In addition, neuroimaging studies have found increases in vmPFC/OFC activity during aggression for typically developing adults (Lotze, Veit, Anders, & Birbaumer, Citation2007), but a decrease in activity in individuals with high levels of reactive aggression (Blair, Citation2004; see this paper also for a comprehensive review of roles of the amygdala and OFC in reactive aggression).
Broadly, aggressive responding therefore appears to depend on limbic areas, namely amygdala, hypothalamus and PAG (e.g. Panksepp, Citation2005), with prefrontal regions (primarily OFC and vmPFC) playing a largely regulatory role. However, human reactive aggression is a complex phenomenon that can be elicited by several antecedent triggers, broadly conceptualised as threat, provocation and frustration (Gilam & Hendler, Citation2017). While the behaviour elicited (e.g. hitting) may appear similar across contexts, the underlying neurocognitive processes and subjective experiences likely differ. Indeed, constructionist conceptions of emotion (e.g. Barrett & Satpute, Citation2017; Lindquist & Barrett, Citation2012) posit that specific emotional experiences are constructed from brain networks that encode a set of more basic operations, e.g. internal and external sensations, knowledge based on past experience, and understanding of the current context. It therefore makes sense to consider how superficially similar reactive aggressive responses can arise as a consequence of differing triggers and underlying networks.
Of the three key antecedent processes, threat has received the most attention in the context of the ‘freeze, flight or fight’ response. This ‘defensive’ aggression is thought to rely on a separable neural network from that of ‘predatory’ aggression, which has been more strongly associated with proactive aggression (Haller, Citation2017). The ‘fight’ response is thought to be in part mediated by the brainstem threat system (Bartholow, Citation2018; Blair, Citation2001) interacting with top-down control mediated by prefrontal cortex (Davidson et al., Citation2000). In line with animal literature on threat and reactive aggression (e.g. Potegal et al., Citation1996), threat paradigms used in human neuroimaging, e.g. viewing of stimuli signalling threat such as fearful faces, or threat-induction via fear-conditioning, have found increased activity in the amygdala (Buchel, Morris, Dolan, & Friston, Citation1998; Morris et al., Citation1996; Whalen et al., Citation1998), insula and dorsolateral PFC (Schienle et al., Citation2002).
The neural circuitry mediating the path from a frustrating event to an aggressive response differs from that of threat, with recent studies implicating anterior cingulate cortex (ACC), insular cortex, and the ventral PFC (Abler, Walter, & Erk, Citation2005; Yu, Mobbs, Seymour, Rowe, & Calder, Citation2014). While threat typically signals immediate danger, frustration aggression typically occurs when one’s goal is blocked (Berkowitz, Citation1989; Dollard, Doob, Miller, Mowrer, & Sears, Citation1939). For example, Abler et al. (Citation2005) asked participants to make simple left/right decisions in response to presented stimuli to win monetary rewards. Correct responses gave participants a 60% chance of obtaining the reward; however on 40% of correct trials, participants did not obtain a reward despite a correct response, inducing frustration. Results indicated increased activity in the ventral PFC and anterior insula during omitted reward (i.e. frustrating trials) compared to reward (i.e. non-frustrating) trials. However, it is worth noting that this study could not disambiguate neural responses associated with reward omission from those associated with the subjective experience of frustration.
A slightly different approach was taken by Yu et al. (Citation2014), using a paradigm that modulated the level of subjective frustration induced. Typical adult participants made simple left/right responses to presented stimuli (arrows pointing left or right) to continue through a pre-determined number of stages to complete a trial and earn a reward. To induce frustration, on some trials participants’ progression through the trial was blocked at one of the different stages, with the participant subsequently losing the reward for that trial. The design enabled the researchers to manipulate the proximity of the reward as well as the effort expended in an effort to obtain the reward at the moment the participant was blocked. Both closer proximity and greater expended effort were independently associated with greater self-reported levels of frustration, as well as activation in regions implicated in reactive aggression, namely amygdala, PAG and anterior insula.
Finally, provocation refers to the incitement of an individual to aggress, usually through unfair treatment such as opponents ‘stealing’ earned points from the participant (e.g. point-subtraction aggression paradigm, Cherek, Moeller, Schnapp, & Dougherty, Citation1997) or unfair monetary ‘punishments’ from opponents (e.g. Taylor Aggression Paradigm, Taylor, Citation1967). The Taylor Aggression Paradigm also manipulates the level of provocation induced by altering the degree of unfairness in the opponent’s punishments; high provocation opponents will consistently punish with very unfair offers while low provocation opponents will consistently punish with less unfair offers. The advantage of using these paradigms in studying reactive aggression is that they allow participants to make an aggressive response, therefore the neural bases of both the antecedent process (provocation) and the aggressive response can be measured independently of each other. This is not as easily achieved with threat or frustration paradigms. Neuroimaging studies of adult samples have found both overlapping and distinct neural activations during the provocation and aggression segments of the paradigms (Krämer, Jansma, Tempelmann, & Mϋnte, Citation2008; Pincham, Wu, Killikelly, Vuillier, & Fearon, Citation2015; Repple et al., Citation2017), suggesting the neural activation of the antecedent process (provocation) may be preparing the individual for an aggressive response (Repple et al., Citation2017).
For example, Repple et al. (Citation2017) used the Taylor Aggression Paradigm in healthy adults, with participants able to take anything from 10 to 100 cents from their opponent as a punishment. Behaviourally, participants chose a more severe punishment for the high-provocation opponent compared to the low-provocation opponent. During the provocation stage, high compared to low provocations revealed increased activation in the rostral ACC (rACC), medial PFC (mPFC) and thalamus. During the aggression stage however, high versus low provocation comparisons revealed increased activity in rACC, mPFC and OFC, insular cortex, dorsolateral PFC and ventrolateral PFC. Both dlPFC and clPFC are associated with control and management of cognitive processes, (Levy & Wagner, Citation2011; Elliot, Citation2003), suggesting an increased recruitment of regulatory regions during the aggression stage.
Together, these studies provide the basis for a neural model of reactive aggression with regard to both the antecedent processes and the aggressive response. Research identifies an ‘aggression network’ comprising limbic (amygdala, hypothalamus, insular, ACC and periaqueductal gray; e.g. Panksepp, Citation2005) and PFC regions (e.g. OFC and vmPFC; Davidson et al., Citation2000), with the PAG acting as a possible interface between the emotional reactivity (limbic) and emotion regulation (PFC) regions via functional and structural connections (Benarroch, Citation2012). However, this ‘aggression network’ is based on adult studies so may not be representative of the functioning of the developing adolescent brain. The following section will discuss structural and functional maturation of the neural circuitry underpinning these processes during adolescence.
3. Typical development of reactive aggression and its neural bases
Adolescence is stereotypically referred to as a time of ‘storm and stress’ characterised by increased mood volatility (e.g. Larson, Moneta, Richards, & Wilson, Citation2002) and sensation seeking and risk-taking (Romer & Hennessy, Citation2007; Steinberg, Citation2008). Epidemiological and developmental data also show a peak in antisocial behaviours during this time, driven by a minority of individuals (e.g. Barker, Tremblay, Nagin, Vitaro, & Lacourse, Citation2006; Moffitt, Citation1993; Moffitt, Caspi, Dickson, Silva, & Stanton, Citation1996), with the majority of aggressive acts being impulsive or reactive in nature (Raine et al., Citation2006). At the same time, evidence from structural and functional neuroimaging suggests that adolescence might be a key time for neurocognitive maturation of circuitry relevant for reactive aggression, for example regions underlying emotional reactivity, emotion regulation, decision-making and social cognition (e.g. Blakemore & Mills, Citation2014; Crone & Dahl, Citation2012).
The identification of distinct developmental trajectories of brain regions underlying ‘reactivity’ and ‘regulation’ (broadly defined) has led some researchers to conclude that adolescence represents a period of ‘developmental mismatch’ or ‘imbalance’ (e.g. Casey, Getz, & Galvan, Citation2008; Steinberg, Citation2008). While evolutionarily older regions of the brain such as those in the limbic system (e.g. amygdala, striatum) undergo rapid, broadly linear development and are thought to reach maturity during adolescence (Romer, Reyna, & Satterthwaite, Citation2017), some regions within prefrontal and temporal cortices do not fully mature until late adolescence or early twenties (Gogtay et al., Citation2004; Somerville, Citation2016). As such, increases in emotional reactivity, sensation-seeking and emotional lability driven by the maturation of limbic regions and the concomitant remodelling of dopaminergic circuitry (Nelson, Jarcho, & Guyer, Citation2016; Nelson, Leibenluft, McClure, & Pine, Citation2005; Telzer, Citation2016) may not yet be paralleled by efficient regulatory circuitry. Complimenting this theory, Scherf, Smyth, and Delgado (Citation2013) suggest that maturation of the amygdala and its connections drives a reorganisation of neural networks involved in social processing. Tracing studies in rats, for example, have found bottom-up amygdala-PFC projections to emerge earlier than the inverse top-down PFC-amygdala projections (Bouwmeester, Smits, & van Ree, Citation2002; Bouwmeester, Wolterink, & van Ree, Citation2002). This overall state of flux in the developing brain may contribute to adolescence as a vulnerable period in which reactive aggression is more likely to occur, due both to poor regulation of negative emotion in response to perceived threat or frustration; and due to a lower threshold for impulsive aggression in the context of peer group influence.
While the majority of typically developing adolescents do not exhibit clinically meaningful aggression (see Vitaro, Brendgen, & Barker, Citation2006 for a review of normative trajectories of aggression; see also Barker et al., Citation2006), one interpretation in line with mismatch/dual-systems models of adolescent brain development is that the overall increase in reactive aggressive behaviours seen during adolescence reflects a phase of normative neurocognitive development and social maturation. As such, developmental shifts in typical adolescent behaviours reflecting these processes might be expected across the full spectrum of individual differences, i.e. some behavioural or affective change would be measurable even in temperamentally calm individuals. Clinically significant reactive aggression would therefore reflect an exaggeration of normative neurocognitive development and behaviours (Moffitt, Citation2003). Alternatively (though these models are not entirely mutually exclusive), normative maturational processes may confer a window of increased vulnerability (Steinberg, Citation2005), but this would only have overt behavioural consequences for a minority, interacting with the presence of additional risk and resilience factors both intrinsic to the individual (e.g. individual differences in the sensitivity and function of neural circuitry involved in reactive aggression) and extrinsic (e.g. social and environmental factors such as parenting style and violence exposure). Either way, it is important to consider both normative neurocognitive developmental trajectories and the contribution of individual differences at neural, cognitive and behavioural levels of explanation that might confer clinical risk in order to formulate a model of adolescent reactive aggression. The following sections will review the typical development of key neurocognitive processes subserving reactive aggression, specifically emotional reactivity and regulation, while later sections will consider individual differences that may underpin atypical reactive aggressive behaviour.
3.1. Emotional reactivity
Emotional reactivity has been shown to increase during adolescence across multiple paradigms and brain regions (see Guyer, Silk, & Nelson, Citation2016; Scherf et al., Citation2013 for comprehensive reviews). Of particular relevance to threat-related reactive aggression, Stroud et al. (Citation2009) found increased reactivity in systems implicated in the fight, flight or freeze response (sympathetic system and hypothalamic-pituitary-amygdala axis) during a stressful task in 13–17 years olds compared with 9–12 year olds. This suggests a peak in reactivity of these systems during mid-adolescence (Dahl & Gunnar, Citation2009), although it would have been ideal to include an additional adult comparison group. Self-report and experience-sampling studies have also shown a peak in frequency, volatility and intensity of emotional experiences during adolescence relative to childhood or adulthood (Casey et al., Citation2010; Guyer et al., Citation2016; Larson et al., Citation2002). At the neural level, fMRI studies have demonstrated that adolescents show greater amygdala activity in response to emotional stimuli, e.g. fearful, happy and calm faces, relative to both children and adults (Hare et al., Citation2008). Similarly, increased reactivity to emotional faces has been demonstrated longitudinally at age 13 compared to age 10 in amygdala and ventral striatum (Pfeifer et al., Citation2011), with the magnitude of increased reactivity between age 10 and 13 found to positively correlate with pubertal status (Moore et al., Citation2012). Given the amygdala’s role in processing socially and emotionally salient information (Adolphs, Citation2008), increased activity implies greater sensitivity or reactivity to emotional stimuli.
The ventral striatum is also implicated in heightened emotional reactivity in adolescence, with this brain region considered a key node in reward-related circuitry. Hyper-reactivity in this area during adolescence may therefore contribute to increased risk-taking and sensation-seeking behaviours during this time (Luciana, Wahlstrom, Porter, & Collins, Citation2012). For example, several fMRI studies have shown increased ventral striatum response in adolescents in risky but rewarding contexts such as during risky gambling, for example selecting high-risk rewards of a large value but small attainment probability (Van Leijenhorst et al., Citation2010), and risk-taking in the presence of peers (Chein, Albert, O’Brien, Uckert, & Steinberg, Citation2011). One interpretation of these data are in terms of mismatch theories, i.e. the increase in ventral striatum activity during adolescence coupled with poor regulatory control, drives the increase in risk-taking behaviours. However, alternative models have recently been proposed that allow for the impact of social factors (e.g. peer influence) and individual differences (e.g. in proclivity towards sensation-seeking). During adolescence, there is a greater emphasis on peer group interaction than at other points in the lifespan (Steinberg & Silverberg, Citation1986) and social rewards such as peer approval are particularly potent (Davey, Yücel, & Allen, Citation2008). Increased sensitivity of ventral striatum and amygdala to social reward in adolescence (e.g. peer approval) may mean that the potential for social reward plays a disproportionate role when weighing up the costs and benefits of risk behaviours that typically play out in a social context (e.g. whether to drive recklessly, experiment with drugs, or take part in a fight; Blakemore & Mills, Citation2014). Another model, the Life-Span Wisdom Model (Romer et al., Citation2017) suggests that peaks in adolescent risk-taking occur predominantly in the context of sensation-seeking, i.e. exploration of novel stimuli where risk is ambiguous, as opposed to contexts where risk are fully known. Under this model, the peak in sensation-seeking found across cultures in late adolescence (Steinberg et al., Citation2017) could be adaptive, driving adolescents to gain necessary life experience.
The inclusion of individual differences such as sensation-seeking (and their neural bases) and contextual factors such as peer pressure are in line with both the GAM and I3 theories of aggression, suggesting these are important factors to consider when theorising about the development of reactive aggression. Irrespective of the prevailing model, the findings reviewed thus far with regard to emotional reactivity (increased amygdala and ventral striatum activity in response to socioaffective stimuli with age; increased reactivity on behavioural and self-report measures) have clear implications for the reactive aggression literature. Firstly, reactive aggression has been associated with greater emotional reactivity (Hubbard et al., Citation2002), e.g. greater skin conductance and heart rate responses to stressful stimuli in participants with higher levels of teacher-reported reactive aggression (Hubbard et al., Citation2004). Secondly, striatal activity and the role of peer pressure seems particularly relevant; most antisocial behaviours and law-violating behaviours occur in groups for adolescent offenders, but not for adults (Sickmund & Puzzanchera, Citation2014; Zimring, Citation1998).
3.2. Emotion regulation
Research also suggests an improvement in emotion regulation abilities during adolescence (see Ahmed, Bittencourt-Hewitt, & Sebastian, Citation2015 for a review). Behavioural studies have found increased ability to efficiently deal with stressful situations, to manage emotional experiences by selecting and implementing effective regulation strategies (Silvers et al., Citation2012), and to express emotions in socially appropriate ways (Cole, Michel, & Teti, Citation1994) during this time. The neural underpinnings of emotion regulation continue to develop during adolescence, in particular prefrontal engagement during regulation and connectivity with limbic regions (Gee et al., Citation2013; Sebastian et al., Citation2011). For example, using fMRI, Gee et al. (Citation2013) found that mPFC-amygdala connectivity during an emotional face processing task became more strongly negative across ages 4–22 years, suggesting age-related improvement in prefrontal ‘top-down’ regulation; this was paralleled by improvements in task performance. Similar results have been found when participants are instructed to use a deliberate strategy such as cognitive reappraisal to downregulate negative affect in response to aversive images. For example, in a sample spanning ages 6–23, Silvers et al. (Citation2017) found increasingly negative connectivity between amygdala and ventromedial PFC with age during reappraisal, as well as decreased negative affect. The relationship between age and amygdala response was additionally found to be mediated by left ventrolateral PFC response. These findings suggest that tighter negative coupling between prefrontal and limbic regions across the course of childhood and adolescence may serve to underpin improving emotion regulation abilities.
3.3. Frustration and provocation
While surprisingly little research has investigated the typical development of reactive aggression circuitry in adolescence directly, existing studies do suggest functional development in the ability to manage frustrating events or provocation. In one study, Lewis, Lamm, Segalowitz, Steiben, and Zelazo (Citation2006) used EEG with 5–16-year-olds on a go/no-go task where points were either won based on performance (blocks 1,3) or were systematically lost (block 2) to induce frustration. Self-report measures confirmed the negative emotion induction during block 2. Focusing on the N2 and P3 components (negative amplitude potential observed at 200 ms and positive amplitude potential observed at 300 ms post stimulus onset respectively, both associated with impulsivity control), Lewis and colleagues found a main effect of decreasing P3 and N2 amplitudes with age across all blocks. However, relative to the performance blocks, the frustration induction block showed increased P3 amplitudes across all ages, and increased N2 amplitudes in an adolescent subgroup only (13–15 years specifically). This suggests an increased recruitment of response inhibition mechanisms at all ages during frustration, but with increased N2 response showing specificity for adolescence. Source modelling of the N2 component additionally found a change in the location of the source from mid or posterior to anterior cingulate with increasing age, further suggesting development in the neural bases of the frustration response with age.
Similar results were found using a provocation paradigm with EEG in younger (10–12 years) and older (14–16 years) adolescent participants, looking particularly at N2 (inhibitory control) and late positive potential (LLP) signals (Pincham et al., Citation2015). LLP has been associated with limbic areas such as amygdala, as well as cingulate cortex and insula, and is thought to reflect emotional evaluations and the processing of arousing stimuli, e.g. larger amplitudes in response to more arousing stimuli (Bradley, Hamby, Löw, & Lang, Citation2007). Behavioural results showed that both younger and older adolescents selected more severe punishments for the high-provocation opponent, i.e. the opponent who consistently ‘punished’ with the more severe aversive noise, than the low-provocation opponent. However, younger adolescents on average selected more severe punishments than the older adolescents, despite the level of unprovoked aggression being similar across ages, i.e. punishment selected prior to facing an opponent. During both the provocation and aggression phase, LLP activation was greater for the younger participants during high provocation only. Increased activity during high provocation for the younger participants therefore could indicate greater emotional reactivity in response to the provocation, consistent with more severe punishments selected by this group. Furthermore, LLP difference scores (difference in activation between low- and high-provocation opponents) were positively correlated with average punishment selection, suggesting a potential association between sensitivity to provocation and proclivity to aggress. N2 activity was also stronger for younger participants than older participants, but there were no effects of level of provocation found. N2 activity is associated with inhibitory control, suggesting potentially more inefficient recruitment of inhibitory mechanisms in the younger adolescents than the older adolescents, as found by Lewis et al. (Citation2006).
Together, these findings suggest that regulatory control of provocation/frustration induced negative affect is still developing during the adolescent period, with young and mid-adolescents requiring greater recruitment of regulatory mechanisms when they were frustrated or provoked than older adolescents. However, individual differences in the LLP difference scores (Pincham et al., Citation2015) further suggest an important role for individual variability in the aggressive response chosen.
Overall, studies in typically developing adolescents suggest that the neurocognitive underpinnings of reactive aggression and its component processes continue to develop during this time. How these general developmental trends interact with individual variation in factors contributing to aggression (such as those identified by the GAM and I3 models) is an important question for future research. In the following section we review studies that seek to understand the neural underpinnings of adolescent reactive aggression by focusing on the extreme tail of the distribution, i.e. individuals exhibiting clinically significant levels of antisocial behaviour, such as Disruptive Behaviour Disorders (DBD), Conduct Disorder (CD) and Conduct Problems (CP). Given that the majority of individuals do not develop clinical levels of reactive aggression during adolescence despite neurocognitive developments potentially creating a window of increased vulnerability during this time, investigating the neural underpinnings of reactive aggression in individuals who do present clinical levels of reactive aggression may provide an insight into additional risk factors associated with reactive aggression.
4. Atypical development
Conduct Disorder refers to a persistent pattern of antisocial and aggressive behaviours that violate social norms and the rights of others (DSM-5), and falls within the broader category of Disruptive Behavioural Disorders. Conduct disorders peak during adolescence (Frick & Viding, Citation2009), predominantly affect males (Bongers, Koot, van der Ende, & Verhulst, Citation2004; Maughan, Rowe, Messer, Goodman, & Meltzer, Citation2004) and entail a significant cost to public health and wider society (Romeo, Knapp, & Scott, Citation2006). Prevalence is estimated at approximately ∼4% of males (2010 sample; Erskine et al., Citation2013) and has been estimated to account for 5.75 million years living with disability (Erskine et al., Citation2014). Years living with disability is a means of quantifying the burden of disability, and is calculated as the cumulated number of years given prevalence rates of the disability in question (see Erskine et al., Citation2013 for a detailed breakdown of this estimation). Additionally, externalising behaviours (dominated by reactive, as opposed to proactive, aggression) feature transdiagnostically across conditions as diverse as ADHD, ODD, Borderline Personality Disorder, Intermittent Explosive Disorder, anxiety and depression (Card & Little, Citation2006; Haller, Citation2017), and as such represent a significant public health concern.
Previous research investigating the neural bases of reactive aggression have found abnormal activations in regions subserving emotional reactivity and emotion regulation in adolescents with CP/CD/DBD relative to typically developing adolescents. However, contradictory findings have often been reported. For example, studies demonstrating abnormal emotional reactivity processing in CP youth compared to typically developing youth have found both hypo-activation (Passamonti et al., Citation2010) and hyper-activation (Herpertz et al., Citation2008; Sterzer, Stadler, Krebs, Kleinschmidt, & Poustka, Citation2005) of the amygdala in response to threat such as fearful or angry faces. Regarding emotion regulation, Herpertz et al. (Citation2008) found no differences in neural response to emotional faces between CD and control participants in regulatory regions of interest including OFC and ACC, suggesting no regulatory deficit. In comparison, relative to typically developing children, children with CP showed reduced P3b amplitude (reflecting inhibition) during a frustrating go/no-go task (Gatzke-Kopp et al., Citation2013). In addition, a recent meta-analysis (Alegria, Radua, & Rubia, Citation2016) found CP individuals showed reduced activation within dmPFC during hot executive functioning tasks, e.g. decision-making in the presence of potential rewards; and within dlPFC during emotion processing tasks, e.g. viewing affective stimuli. These latter results suggest impaired emotion regulation, not consistent with the findings from Herpertz et al. (Citation2008).
This mixed picture seen with regard to atypical affective processing in young people with conduct problems is likely at least in part driven by heterogeneity within the conduct disorder diagnostic category, which encompasses the full spectrum of aggressive behaviours (e.g. from reactive to proactive) and multiple aetiologies (Frick & Viding, Citation2009; Moffitt, Caspi, Harrington, & Milne, Citation2002). To date very few studies have investigated the neural bases of affective processing specifically in young people with CP exhibiting primarily reactive aggressive behaviour. One approach to shedding light on this issue is to subtype adolescents with CP on the basis of callous–unemotional (CU) traits. CU traits are characterised by a lack of guilt and empathy, and a profile of shallow affect (Essau, Sasagawa, & Frick, Citation2006). Children with CP and high levels of CU traits (CP/CU+) typically display high levels of proactive aggression (plus co-occurring reactive aggression; Card & Little, Citation2006), and exhibit hyporeactive behavioural (Sharp, van Goozen, & Goodyer, Citation2006) and neural (Lockwood et al., Citation2013) responses to affective stimuli. In contrast, those with CP and low levels of CU traits (CP/CU−) typically exhibit mainly reactive aggressive behaviour (Frick & Viding, Citation2009) coupled with behavioural and neural hyper-reactivity to affective stimuli (Sebastian et al., Citation2014) and impaired emotion regulation abilities (Frick & Morris, Citation2004). Understanding the neural bases of reactive aggression within this latter group therefore may shed light on the underlying causes of developing harmful reactive aggression.
Studies that have differentiated subgroups of adolescents with CP based on CU traits have found a more consistent pattern of results in CP/CU− (reactive) individuals, with heightened emotional reactivity in limbic regions, and impairments in PFC-mediated emotion regulation performance. For example, Sebastian et al. (Citation2014) found increased responses in amygdala, subgenual ACC and OFC in CP/CU− relative to typically developing youth aged 10–16 when attention was specifically drawn to the most salient eye region of a fearful face by a requirement to locate a target stimulus. Reaction times to locate the target were also slower in this condition for CP/CU− youth, and the size of this RT interference was positively correlated with increased activation in the amygdala. This suggests amygdala hyper-reactivity to affective information may have functional relevance for behavioural performance. Amygdala hyper-reactivity in this group, relative to both control and CP/CU+ groups, has also been found when fearful faces are presented ‘pre-attentively’ for only 17 ms and below the level of conscious awareness (Viding et al., Citation2012). This finding suggests that increased threat reactivity in adolescents with conduct problems and low levels of CU traits extends to the very earliest levels of threat processing. Moreover, these findings cannot be attributed to conduct problems per se, since a very different pattern of results was seen in CP/CU+. Comorbid ADHD and anxiety symptoms also could not explain the findings. Together, these studies suggest that hyper-reactivity of limbic regions in response to threat characterises adolescents exhibiting primarily reactive aggressive conduct problems.
Extending this approach beyond simple threat processing, White et al. (Citation2016) compared groups of youth with DBD and either low or high CU traits and typically developing controls (10–18 years) using a provocation paradigm (Social Fairness Game). Participants were offered either a fair (i.e. equal) or varying levels of unfair (i.e. unequal) split of a $20 reward which they could either accept or reject. The most unfair splits of the reward represented high-provocation trials. Participants could also punish their opponent at a cost to the participant. Behaviourally, both fair (e.g. $10/$10) and extremely unfair offers (e.g. $18 to partner/$2 to participant) were equally as likely to be accepted or rejected respectively by all groups. However, DBD participants responded more severely to slightly unfair offers (e.g. $14/$6). fMRI results showed greater amygdala and PAG activity in DBD/CU− youth relative to controls; as well as reduced attenuation of vmPFC activity (i.e. less reduction in activity); and reduced amygdala-vmPFC functional connectivity specifically during high-provocation trials. Notably, both reduced vmPFC attenuation and reduced amygdala-vmPFC connectivity were negatively correlated with level of punishment selected. Therefore, both hypo-activation and decreased functional connectivity between PFC and limbic areas may result in impaired emotion regulation in clinical groups of adolescents characterised by high levels of reactive aggression.
Insight into reactive aggression can also be gained by exploring the neural bases of related phenotypes, such as irritability (see Brotman, Kircanski, & Leibenluft, Citation2017; Leibenluft, Citation2017 for comprehensive reviews). Clinical irritability has been defined as ‘an increased propensity to exhibit aggression relative to one’s peers’ (Leibenluft, Citation2017, p. 277) and is thought to arise from dysfunctional threat and frustration processing (Brotman et al., Citation2017). Reactive aggressive behaviour is considered the extreme behavioural manifestation of irritability (Leibenluft, Citation2017). In line with the studies so far presented of reactive aggression in adolescents with CP (Sebastian et al., Citation2014; Viding et al., Citation2012), youth with clinical or chronic irritability exhibit increased activation to threat (e.g. angry faces) in the amygdala, insula, cingulate and striatum compared to typically developing controls (Thomas et al., Citation2013), suggesting heightened emotional reactivity as a core component of irritability (although see Deveney et al., Citation2013, which found decreased amygdala response in this group, albeit on a non-affective task). Additionally, amygdala-mPFC functional connectivity was found to inversely correlate with irritability severity in youth viewing angry faces at 150% intensity (Stoddard et al., Citation2017), suggesting a failure of top-down regulation from the mPFC to amygdala. Results therefore suggest similar neural bases could underpin high irritability and threat-reactive conduct problems, though potential overlap across these groups would need to be more closely delineated.
The literature on adolescent atypical reactive aggression to date suggests that such behaviours are at least partially underpinned by hyperreactivity of the limbic system and impaired emotion regulation circuitry. It could be argued that this reflects an exaggeration of patterns seen in normative adolescent development (for example as conceptualised by dual systems or mismatch theories). However, it is currently unclear how normative developmental neurocognitive processes interact with individual and social risk factors (for example parenting, maltreatment and violence exposure, socioeconomic status, and community violence exposure) to increase vulnerability to developing reactive aggressive conduct problems during adolescence. This is an important next step in understanding this phenomenon.
5. Theoretical implications
In forensic and legal settings, immaturity is rightly considered a mitigating factor, and indeed evidence from neuroscience has recently played a key role in ensuring developmental status is taken into account in sentencing decisions (e.g. Steinberg, Citation2013). Neuroscience is now additionally contributing to understanding how and why reactive aggressive behaviour emerges in the first place.
The neuroscience literature converges across animals, adult humans and both typical and atypical adolescents to find a broad neural network involved in reactive aggression. Models of typical adolescent development (e.g. dual systems models or the Lifespan Wisdom model) provide a basis for understanding how normative neurocognitive development during adolescence may confer vulnerability to risk-taking, emotional volatility and reactive aggression. However, only a minority of adolescents actually develop clinically significant levels of reactive aggression. To understand why this is, we need to additionally consider individual differences (at behavioural, cognitive and neural levels of explanation) and social/environmental factors over and above normative neurocognitive development. These may act as moderating risk factors that increase in the likelihood that individuals will a) aggress under specific circumstances on any one given occasion and b) develop a pattern of harmful reactive aggression. This brings us full circle, back to the GAM and I3 models discussed above, in that only with the integration of all of these factors can we understand the conferred vulnerability to reactive aggression during adolescence.
However, these models have yet to formally integrate a developmental angle. Let us consider how development-specific factors (including, but not limited to, neurocognitive development) may be integrated into a framework such as the I3. Can development itself be considered a moderating factor in determining the likelihood and degree of aggressive response? At the stage of Instigation, is there evidence that adolescents respond to situational factors such as goal obstruction or peer rejection with an aggressive response more readily than do adults? To date, evidence suggests that adolescents may have greater negative affective responses and neural reactivity to such stressors (e.g. Lewis et al., Citation2006; Sebastian, Viding, Williams, & Blakemore, Citation2010, Citation2011), although to our knowledge studies have not measured whether such phenomena increase the likelihood of an aggressive response.
Similarly regarding Impellance, i.e. factors determining the strength of an aggressive response, there is limited evidence suggesting that younger adolescents may react to provocation more aggressively than older adolescents (Pincham et al., Citation2015), but further work is needed to examine how individual differences and situational variables interact with developmental status to predict the severity of aggressive response. Finally, there is a wealth of literature documenting ongoing development of Inhibition (e.g. inhibitory control) and its neural bases during adolescence (e.g. Cohen et al., Citation2016), but relatively little linking this with inhibition of aggressive responding specifically. Thus, there is considerable scope for future work looking across the age range from childhood to adolescence to adulthood to understand how neurocognitive and behavioural development interacts with well-characterised theoretical frameworks of aggression.
6. Conclusion
Events that threaten, frustrate or provoke occur on a daily basis, yet only a minority of individuals respond with aggression. During adolescence, ongoing functional development occurs in neural circuitry underpinning processes of key relevance to reactive aggression, including emotional reactivity, emotion regulation, decision-making and social reward; as well as in the response of the ‘aggression network’ itself (including amygdala, hypothalamus, insula, PAG, OFC/PFC). This may at least in part explain the peak in reactive aggressive behaviour seen during these years. However, since the majority of adolescents do not develop clinically significant reactive aggression, it is important to understand how individual differences (in factors such as irritability or emotional reactivity) may interact with canonical trajectories of adolescent neurocognitive trajectories to confer risk or resilience in this area.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Catherine L. Sebastian http://orcid.org/0000-0001-8080-0582
Additional information
Funding
References
- Abler, B., Walter, H., & Erk, S. (2005). Neural correlates of frustration. Neuroreport, 16(7), 669–672. doi: 10.1097/00001756-200505120-00003
- Adolphs, R. (2008). Fear, faces, and the human amygdala. Current Opinion in Neurobiology, 18(2), 166–172. doi: 10.1016/j.conb.2008.06.006
- Ahmed, S. P., Bittencourt-Hewitt, A., & Sebastian, C. L. (2015). Neurocognitive bases of emotion regulation development in adolescence. Developmental Cognitive Neuroscience, 15, 11–25. doi: 10.1016/j.dcn.2015.07.006
- Alegria, A. A., Radua, J., & Rubia, K. (2016). Meta-analysis of fMRI studies of disruptive behavior disorders. American Journal of Psychiatry, 173(11), 1119–1130. doi: 10.1176/appi.ajp.2016.15081089
- Allen, J. J., Anderson, C. A., & Bushman, B. J. (2018). The general aggression model. Current Opinion in Psychology, 19, 75–80. doi: 10.1016/j.copsyc.2017.03.034
- Anderson, S. W., Bechara, A., Damasio, H., Tranel, D., & Damasio, A. R. (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience, 2, 1032–1037. doi: 10.1038/14833
- Anderson, C. A., & Bushman, B. J. (2002). Human aggression. Annual Review of Psychology, 53(1), 27–51. doi: 10.1146/annurev.psych.53.100901.135231
- Barker, E. D., Tremblay, R. E., Nagin, D. S., Vitaro, F., & Lacourse, E. (2006). Development of male proactive and reactive physical aggression during adolescence. Journal of Child Psychology and Psychiatry, 47(8), 783–790. doi: 10.1111/j.1469-7610.2005.01585.x
- Barrett, L. F., & Satpute, A. B. (2017). Historical pitfalls and new directions in the neuroscience of emotion. Neuroscience Letters, 17, 30617–1.
- Bartholow, B. (2018). The aggressive brain: Insights from neuroscience. Current Opinion in Psychology, 19, 60–64. doi: 10.1016/j.copsyc.2017.04.002
- Benarroch, E. E. (2012). Periaqueductal gray: An interface for behavioral control. Neurology, 78(3), 210–217. doi: 10.1212/WNL.0b013e31823fcdee
- Berkowitz, L. (1989). Frustration-aggression hypothesis: Examination and reformulation. Psychological Bulletin, 106, 59–73. doi: 10.1037/0033-2909.106.1.59
- Berkowitz, L. (1993). Aggression: Its causes, consequences, and control. New York: McGraw-Hill.
- Blair, R. J. (2001). Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery and Psychiatry, 71(6), 727–731. doi: 10.1136/jnnp.71.6.727
- Blair, R. J. (2004). The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition, 55, 198–208. doi: 10.1016/S0278-2626(03)00276-8
- Blair, R. J., & Cipolotti, L. (2000). Impaired social response reversal. A case of ‘acquired sociopathy’. Brain, 123(Pt 6), 1122–1141. doi: 10.1093/brain/123.6.1122
- Blakemore, S. J., & Mills, K. L. (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. doi: 10.1146/annurev-psych-010213-115202
- Bongers, I. L., Koot, H. M., van der Ende, J., & Verhulst, F. C. (2004). Developmental trajectories of externalizing behaviors in childhood and adolescence. Child Development, 75, 1523–1537. doi: 10.1111/j.1467-8624.2004.00755.x
- Bouwmeester, H., Smits, K., & van Ree, J. M. (2002). Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. Journal of Comparative Neurology, 450(3), 241–255. doi: 10.1002/cne.10321
- Bouwmeester, H., Wolterink, G., & van Ree, J. M. (2002). Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. Journal of Comparative Neurology, 442(3), 239–249. doi: 10.1002/cne.10084
- Bradley, M. M., Hamby, S., Löw, A., & Lang, P. J. (2007). Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology, 44(3), 364–373. doi: 10.1111/j.1469-8986.2007.00520.x
- Brotman, M. A., Kircanski, K., & Leibenluft, E. (2017). Irritability in children and adolescents. Annual Review of Clinical Psychology, 13, 317–341. doi: 10.1146/annurev-clinpsy-032816-044941
- Brower, M. C., & Price, B. H. (2001). Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: A critical review. Journal of Neurology, Neurosurgery, and Psychiatry, 71(6), 720–726. doi: 10.1136/jnnp.71.6.720
- Buchel, C., Morris, J., Dolan, R. J., & Friston, K. J. (1998). Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron, 20, 947–957. doi: 10.1016/S0896-6273(00)80476-6
- Card, N. A., & Little, T. D. (2006). Proactive and reactive aggression in childhood and adolescence: A meta-analysis of differential relations with psychosocial adjustment. International Journal of Behavioral Development, 30, 466–480. doi: 10.1177/0165025406071904
- Casey, B. J., Getz, S., & Galvan, A. (2008). The adolescent brain. Developmental Review, 28, 62–77. doi: 10.1016/j.dr.2007.08.003
- Casey, B. J., Jones, R. M., Levita, L., Libby, V., Pattwell, S. S., Ruberry, E. J., … Somerville, L. H. (2010). The storm and stress of adolescence: Insights from human imaging and mouse genetics. Development and Psychobiology, 52(3), 225–235.
- Chein, J., Albert, D., O’Brien, L., Uckert, K., & Steinberg, L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14, F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x
- Cherek, D. R., Moeller, F. G., Schnapp, W., & Dougherty, D. M. (1997). Studies of violent and nonviolent male parolees: I. Laboratory and psychometric measurements of aggression. Biological Psychiatry, 41, 514–522. doi: 10.1016/S0006-3223(96)00059-5
- Cohen, A. O., Breiner, K., Steinberg, L., Bonnie, R. J., Scott, E. S., Taylor-Thompson, K. A., … Casey, B. J. (2016). When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychological Science, 27(4), 549–562. doi: 10.1177/0956797615627625
- Cole, P. M., Michel, M. K., & Teti, L. O. (1994). The development of emotion regulation and dysregulation: A clinical perspective. In N. A. Fox (Ed.), The development of emotion regulation: Biological and behavioral considerations (pp. 73–100). Chicago: University of Chicago Press. Monographs of the Society for Research in Child Development, 59 (Serial No. 240).
- Crone, E. A., & Dahl, R. E. (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. doi: 10.1038/nrn3313
- Dahl, R. E., & Gunnar, M. R. (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21, 1–6. doi: 10.1017/S0954579409000017
- Davey, C. G., Yücel, M., & Allen, N. B. (2008). The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews, 32, 1–19. doi: 10.1016/j.neubiorev.2007.04.016
- Davidson, R. J., Putnam, K. M., & Larson, C. L. (2000). Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science, 289(5479), 591–594. doi: 10.1126/science.289.5479.591
- De Bruin, J. P., Van Oyen, H. G., & Van De Poll, N. (1983). Behavioural changes following lesions of the orbital prefrontal cortex in male rats. Behavioural Brain Research, 10(2–3), 209–232. doi: 10.1016/0166-4328(83)90032-3
- Dekker, T., & Karmiloff-Smith, A. (2011). The dynamics of ontogeny: A neuroconstructivist perspective on genes, brains, cognition and behavior. Progress in Brain Research, 189, 23–33. doi: 10.1016/B978-0-444-53884-0.00016-6
- Deveney, C. M., Connolly, M. E., Haring, C. T., Bones, B. L., Reynolds, R. C., Kim, P., … Leibenluft, E. (2013). Neural mechanisms of frustration in chronically irritable children. American Journal of Psychiatry, 170(10), 1186–1194. doi: 10.1176/appi.ajp.2013.12070917
- Dodge, K. A., & Coie, J. D. (1987). Social-information-processing factors in reactive and proactive aggression in children’s peer groups. Journal of Personality and Social Psychology, 53(6), 1146–1158. doi: 10.1037/0022-3514.53.6.1146
- Dollard, J., Doob, L., Miller, N., Mowrer, O., & Sears, R. (1939). Frustration and aggression. New Haven, CT: Yale University Press.
- Elliott, R. (2003). Executive functions and their disorders. British Medical Bulletin, 65(1), 49–59. doi: 10.1093/bmb/65.1.49
- Erskine, H. E., Ferrari, A. J., Nelson, P., Polanczyk, G. V., Flaxman, A. D., Vos, T., … Scott, J. G. (2013). Research review: Epidemiological modelling of attention-deficit/ hyperactivity disorder and conduct disorder for the global burden of disease study 2010. Journal of Child Psychology and Psychiatry, 54, 1263–1274. doi: 10.1111/jcpp.12144
- Erskine, H. E., Ferrari, A. J., Polanczyk, G. V., Moffitt, T. E., Murray, C. J. L., Vos, T., … Scott, J. G. (2014). The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. Journal of Child Psychology and Psychiatry, 55(4), 328–336. doi: 10.1111/jcpp.12186
- Essau, C. A., Sasagawa, S., & Frick, P. J. (2006). Callous-unemotional traits in a community sample of adolescents. Assessment, 13(4), 454–469. doi: 10.1177/1073191106287354
- Finkel, E. J. (2014). The I3 model: Metatheory, theory, and evidence. In Z. Olson & M. Zanna (Eds.), Advances in experimental social psychology (49th ed., pp. 1–94). San Diego: Academic Press.
- Finkel, E. J., & Hall, A. N. (2018). The I3 model: A metatheoretical framework for understanding aggression. Current Opinion in Psychology, 19, 125–130. doi: 10.1016/j.copsyc.2017.03.013
- Finkel, E. J., & Slotter, E. B. (2009). An I3 theory analysis of human sex differences in aggression. Behavioral and Brain Sciences, 32, 279. doi: 10.1017/S0140525X09990410
- Frick, P. J., & Morris, A. S. (2004). Temperament and developmental pathways to conduct problems. Journal of Clinical Child & Adolescent Psychology, 33, 54–68. doi: 10.1207/S15374424JCCP3301_6
- Frick, P. J., & Viding, E. M. (2009). Antisocial behavior from a developmental psychopathology perspective. Development and Psychopathology, 21, 1111–1131. doi: 10.1017/S0954579409990071
- Fusar-Poli, P., Placentino, A., Carletti, F., Landi, P., Allen, P., Surguladze, S., … Politi, P. (2009). Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience, 34(6), 418–432.
- Gatzke-Kopp, L. M., Willner, C. J., Jetha, M. K., Abenavoli, R. M., DuPuis, D., & Segalowitz, S. J. (2013). How does reactivity to frustrative non-reward increase risk for externalizing symptoms? International Journal of Psychophysiology, 98, 300–309. doi: 10.1016/j.ijpsycho.2015.04.018
- Gee, D. G., Humphreys, K. L., Flannery, J., Goff, B., Telzer, E. H., Shapiro, M., … Tottenham, N. (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013
- Gilam, G., & Hendler, T. (2017). Deconstructing anger in the human brain. Current Topics in Behavioral Neurosciences, 30, 257–273. doi: 10.1007/7854_2015_408
- Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., … Thompson, P. A. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. doi: 10.1073/pnas.0402680101
- Grafman, J., Schwab, K., Warden, D., Pridgen, A., Brown, H. R., & Salazar, A. M. (1996). Frontal lobe injuries, violence, and aggression: A report of the Vietnam head injury study. Neurology, 46(5), 1231–1238. doi: 10.1212/WNL.46.5.1231
- Guyer, A. E., Silk, J. S., & Nelson, E. E. (2016). The neurobiology of the emotional adolescent: From the inside out. Neuroscience and Biobehavioral Reviews, 70, 74–85. doi: 10.1016/j.neubiorev.2016.07.037
- Haller, J. (2017). Studies into abnormal aggression in humans and rodents: Methodological and translational aspects. Neuroscience and Biobehavioral Reviews, 76(Pt A), 77–86. doi: 10.1016/j.neubiorev.2017.02.022
- Hare, T. A., Tottenham, N., Galvan, A., Voss, H. U., Glover, G. H., & Casey, B. J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go/no-go task. Biological Psychiatry, 63(10), 927–934. doi: 10.1016/j.biopsych.2008.03.015
- Herpertz, S. C., Huebner, T., Marx, I., Vloet, T. D., Fink, G. R., Stoecker, T., … Herpertz-Dahlmann, B. (2008). Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry, 49, 781–791. doi: 10.1111/j.1469-7610.2008.01905.x
- Hubbard, J. A., Parker, E. H., Ramsden, S. R., Flanagan, K. D., Relyea, N., Dearing, K. F., … Hyde, C. T. (2004). The relations among observational, physiological, and self-report measures of children’s anger. Social Development, 13, 14–39. doi: 10.1111/j.1467-9507.2004.00255.x
- Hubbard, J. A., Smithmyer, C. M., Ramsden, S. R., Parker, E. H., Flanagan, K. D., Dearing, K. F., … Simons, R. F. (2002). Observational, physiological, and self-report measures of children’s anger: Relations to reactive versus proactive aggression. Child Development, 73, 1101–1118. doi: 10.1111/1467-8624.00460
- Karmiloff-Smith, A. (1998). Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences, 2(10), 389–398. doi: 10.1016/S1364-6613(98)01230-3
- Krämer, U. M., Jansma, H., Tempelmann, C., & Mϋnte, T. F. (2008). Tit-for-tat: The neural basis of reactive aggression. Neuroimage, 38, 203–211. doi: 10.1016/j.neuroimage.2007.07.029
- Kruk, M. R. (1991). Ethology and pharmacology of hypothalamic aggression in the Rat. Neuroscience and Biobehavioral Reviews, 15, 527–538. doi: 10.1016/S0149-7634(05)80144-7
- Kruk, M. R., Van Der Laan, C. E., Mos, J., Van Der Poel, A., Meelis, W., & Olivier, B. (1984). Comparison of aggressive behaviour induced by electrical stimulation in the hypothalamus of male and female rats. Progress in Brain Research, 61, 303–314. doi: 10.1016/S0079-6123(08)64443-X
- Larson, R. W., Moneta, G., Richards, M. H., & Wilson, S. (2002). Continuity, stability, and change in daily emotional experience across adolescence. Child Development, 73(4), 1151–1165. doi: 10.1111/1467-8624.00464
- Leibenluft, E. (2017). Pediatric irritability: A systems neuroscience approach. Trends in Cognitive Sciences, 21(4), 277–289. doi: 10.1016/j.tics.2017.02.002
- Levy, B. J., & Wagner, A. D. (2011). Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224(1), 40–62. doi: 10.1111/j.1749-6632.2011.05958.x
- Lewis, M. D., Lamm, C., Segalowitz, S. J., Steiben, J., & Zelazo, D. (2006). Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience, 18(3), 430–443. doi: 10.1162/jocn.2006.18.3.430
- Lindquist, K. A., & Barrett, L. F. (2012). A functional architecture of the human brain: Insights from the science of emotion. Trends in Cognitive Sciences, 16, 533–540. doi: 10.1016/j.tics.2012.09.005
- Lipp, H. P., & Hunsperger, R. W. (1978). Threat, attack and flight elicited by electrical stimulation of the ventromedial hypothalamus of the Marmoset Monkey Callithrix Jacchus. Brain, Behavior and Evolution, 15(4), 276–293. doi: 10.1159/000123783
- Lloyd, S. A., & Dixson, A. (1988). Effects of hypothalamic lesions upon the sexual and social behaviour of the male common marmoset (Callithrix jacchus). Brain Research, 463(2), 317–329. doi: 10.1016/0006-8993(88)90405-2
- Lockwood, P. L., Sebastian, C. L., McCrory, E. J., Hyde, Z. H., Gu, X., De Brito, S. A., & Viding, E. (2013). Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Current Biology, 23, 901–905. doi: 10.1016/j.cub.2013.04.018
- Lotze, M., Veit, R., Anders, S., & Birbaumer, N. (2007). Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: An interactive fMRI study. Neuroimage, 34(1), 470–478. doi: 10.1016/j.neuroimage.2006.09.028
- Luciana, M., Wahlstrom, D., Porter, J. N., & Collins, P. F. (2012). Dopaminergic modulation of incentive motivation in adolescence: Age-related changes in signaling, individual differences, and implications for the development of self-regulation. Developmental Psychology, 48(3), 844–861. doi: 10.1037/a0027432
- Machado, C. J., & Bachevalier, J. (2006). The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience, 120(4), 761–786. doi: 10.1037/0735-7044.120.4.761
- Maughan, B., Rowe, R., Messer, J., Goodman, R., & Meltzer, H. (2004). Conduct disorder and oppositional defiant disorder in a national sample: Developmental epidemiology. Journal of Child Psychology and Psychiatry, 45(3), 609–621. doi: 10.1111/j.1469-7610.2004.00250.x
- Mills, K. L., Goddings, A.-L., Clasen, L. S., Giedd, J. N., & Blakemore, S.-J. (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience, 36(3–4), 147–160. doi: 10.1159/000362328
- Moffitt, T. E. (1993). Adolescence-limited and life-course persistent antisocial behavior: A developmental taxonomy. Psychological Review, 100, 674–701. doi: 10.1037/0033-295X.100.4.674
- Moffitt, T. E. (2003). Life-course persistent and adolescence-limited antisocial behavior: A 10-year research review and research agenda. In B. B. Lahey, T. E. Moffitt, & A. Caspi (Eds.), Causes of conduct disorder and juvenile delinquency (pp. 49–75). New York: Guilford Press.
- Moffitt, T. E., Caspi, A., Dickson, N., Silva, P., & Stanton, W. (1996). Childhood-onset versus adolescent-onset antisocial conduct problems in males: Natural history from ages 3 to 18 years. Development and Psychopathology, 8, 399–424. doi: 10.1017/S0954579400007161
- Moffitt, T. E., Caspi, A., Harrington, H., & Milne, B. J. (2002). Males on the life-course-persistent and adoles- cence-limited antisocial pathways: Follow-up at age 26 years. Development and Psychopathology, 14, 179–207. doi: 10.1017/S0954579402001104
- Moore, W. E., 3rd., Pfeifer, J. H., Masten, C. L., Mazziotta, J. C., Iacoboni, M., & Dapretto, M. (2012). Facing puberty: Associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience, 7, 35–43. doi: 10.1093/scan/nsr066
- Morris, J. S., Frith, C. D., Perrett, D. I., Rowland, D., Young, A. W., Calder, A. J., & Dolan, R. J. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383(6603), 812–815. doi: 10.1038/383812a0
- Morton, J., & Frith, U. (1995). Causal modelling: A structural approach to developmental psychopathology. In D. Cicchetti, & D. Cohen (Eds.), Developmental psychotherapy (1st ed., pp. 357–390). New York: John Wiley & Sons.
- Nelson, E. E., Jarcho, J. M., & Guyer, A. E. (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. doi: 10.1016/j.dcn.2015.12.008
- Nelson, E. E., Leibenluft, E., McClure, E. B., & Pine, D. S. (2005). The social-reorientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–174. doi: 10.1017/S0033291704003915
- Nelson, R. J., & Trainor, B. C. (2007). Neural mechanisms of aggression. Nature Reviews Neuroscience, 8, 536–546. doi: 10.1038/nrn2174
- Nomura, M., Ohira, H., Haneda, K., Iidaka, T., Sadato, N., Okada, T., & Yonekura, Y. (2004). Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: An event-related fMRI study. Neuroimage, 21, 352–363. doi: 10.1016/j.neuroimage.2003.09.021
- Panksepp, J. (2005). Affective neuroscience. Oxford: Oxford University Press.
- Passamonti, L., Fairchild, G., Goodyer, I. M., Hurford, G., Hagan, C. C., Rowe, J. B., & Calder, A. J. (2010). Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry, 67, 729–738. doi: 10.1001/archgenpsychiatry.2010.75
- Pfeifer, J. H., Masten, C. L., Moore, W. E., 3rd., Oswald, T. M., Mazziotta, J. C., Iacoboni, M., & Dapretto, M. (2011). Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69, 1029–1036. doi: 10.1016/j.neuron.2011.02.019
- Pincham, H. L., Wu, C., Killikelly, C., Vuillier, L., & Fearon, R. M. (2015). Social provocation modulates decision making and feedback processing: Examining the trajectory of development in adolescent participants. Developmental Cognitive Neuroscience, 15, 58–66. doi: 10.1016/j.dcn.2015.10.003
- Potegal, M., Hebert, M., DeCoster, M., & Meyerhoff, J. (1996). Brief, high-frequency stimulation of the corticomedial amygdala induces a delayed and prolonged increase of aggressiveness in male Syrian Golden Hamsters. Behavioural Neuroscience, 110(2), 401–412. doi: 10.1037/0735-7044.110.2.401
- Raine, A., Dodge, K., Loeber, R., Gatzke-Kopp, L., Lynam, D., Reynolds, C., … Liu, J. (2006). The reactive-proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior, 32(2), 159–171. doi: 10.1002/ab.20115
- Repple, J., Pawliczek, C. M., Voss, B., Siegel, S., Schneider, F., Kohn, N., & Habel, U. (2017). From provocation to aggression: The neural network. BMC Neuroscience, 18, 27. doi: 10.1186/s12868-017-0390-z
- Romeo, R., Knapp, M., & Scott, S. (2006). Economic cost of severe antisocial behaviour in children – and who pays it. British Journal of Psychiatry, 188, 547–553. doi: 10.1192/bjp.bp.104.007625
- Romer, D., & Hennessy, M. (2007). A biosocial-affect model of adolescent sensation seeking: The role of affect evaluation and peer-group influence in adolescent drug use. Prevention Science, 8(2), 89–101. doi: 10.1007/s11121-007-0064-7
- Romer, D., Reyna, V. F., & Satterthwaite, T. D. (2017). Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Developmental Cognitive Neuroscience, 27, 19–34. doi: 10.1016/j.dcn.2017.07.007
- Scherf, K. S., Smyth, J. M., & Delgado, M. R. (2013). The amygdala: An agent of change in adolescent neural networks. Hormones and Behavior, 64(2), 298–313. doi: 10.1016/j.yhbeh.2013.05.011
- Schienle, A., Stark, R., Walter, B., Blecker, C., Ott, U., Kirsch, P., … Vaitl, D. (2002). The insula is not specifically involved in disgust processing: An fMRI study. Neuroreport, 13(16), 2023–2026. doi: 10.1097/00001756-200211150-00006
- Sebastian, C. L., McCrory, E. J., Dadds, M. R., Cecil, C. A. M., Lockwood, P. L., Hyde, Z. H., … Viding, E. (2014). Neural responses to fearful eyes in children with conduct problems and varying levels of callous-unemotional traits. Psychological Medicine, 44, 99–109. doi: 10.1017/S0033291713000482
- Sebastian, C. L., Tan, G. C. Y., Roiser, J. P., Viding, E., Dumontheil, I., & Blakemore, S.-J. (2011). Developmental influences on the neural bases of responses to social rejection: Implications of social neuroscience for education. Neuroimage, 57(3), 686–694. doi: 10.1016/j.neuroimage.2010.09.063
- Sebastian, C., Viding, E., Williams, K. D., & Blakemore, S.-J. (2010). Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition, 72(1), 134–145. doi: 10.1016/j.bandc.2009.06.008
- Sharp, C., van Goozen, S., & Goodyer, I. (2006). Children’s subjective emotional reactivity to affective pictures: Gender differences and their antisocial correlates in an unselected sample of 7–11-year-olds. Journal of Child Psychology and Psychiatry, 47, 143–150. doi: 10.1111/j.1469-7610.2005.01464.x
- Sickmund, M., & Puzzanchera, C. (Eds.). (2014). Juvenile offenders and victims: 2014 National Report. Pittsburgh, PA: National Center for Juvenile Justice.
- Siegal, A., Roeling, T. A., Gregg, T. R., & Kruk, M. R. (1999). Neuropharmacology of brain-stimulation-evoked aggression. Neuroscience and Biobehavioral Reviews, 23(3), 359–389. doi: 10.1016/S0149-7634(98)00040-2
- Silvers, J. A., Insel, C., Powers, A., Franz, P., Helion, C., Martin, R. E., … Ochsner, K. N. (2017). vlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7), 3502–3514.
- Silvers, J. A., McRae, K., Gabrieli, J. D., Gross, J. J., Remy, K. A., & Ochsner, K. N. (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12, 1235–1247. doi: 10.1037/a0028297
- Slotter, E. B., & Finkel, E. J. (2011). I3 theory: Instigating, impelling, and inhibiting factors in aggression. In P. Shaver & M. Mikulincer (Eds.), Human aggression and violence: Causes, manifestations, and consequences. (pp. 35–52). Washington, DC: American Psychological Association.
- Somerville, L. H. (2016). Searching for signatures of brain maturity: What are we searching for? Neuron, 92(6), 1164–1167. doi: 10.1016/j.neuron.2016.10.059
- Steinberg, L. (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9(2), 69–74. doi: 10.1016/j.tics.2004.12.005
- Steinberg, L. (2008). A neurobehavioral perspective on adolescent risk-taking. Developmental Review, 28, 78–106. doi: 10.1016/j.dr.2007.08.002
- Steinberg, L. (2013). The influence of neuroscience on US Supreme Court decisions about adolescents’ criminal culpability. Nature Reviews Neuroscience, 14(7), 513–518. doi: 10.1038/nrn3509
- Steinberg, L., Icenogle, G., Shulman, E. P., Breiner, K., Chein, J., Bacchini, D., … Takash, H. M. (2017). Around the world, adolescence is a time of heightened sensation seeking and immature self-regulation. Developmental Science, 21, e12532. doi: 10.1111/desc.12532
- Steinberg, L., & Silverberg, S. B. (1986). The vicissitudes of autonomy in early adolescence. Child Development, 57(4), 841–851. doi: 10.2307/1130361
- Sterzer, P., Stadler, C., Krebs, A., Kleinschmidt, A., & Poustka, F. (2005). Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry, 57, 7–15. doi: 10.1016/j.biopsych.2004.10.008
- Stoddard, J., Wan-Ling, T., Kim, P., Chen, G., Yi, J., Donahue, L., … Leibenluft, E. (2017). Irritability, anxiety, and the neural processes of implicit face emotion processing in youth. JAMA Psychiatry, 74, 95–103. doi: 10.1001/jamapsychiatry.2016.3282
- Stroud, L. R., Foster, E., Papandonatos, G. D., Handwerger, K., Granger, D. A., Kivlighan, K. T., & Niaura, R. (2009). Stress response and the adolescent transition: Performance versus peer rejection stress. Development and Psychopathology, 21, 47–68. doi: 10.1017/S0954579409000042
- Taylor, S. P. (1967). Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. Journal of Personality, 35(2), 297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x
- Telzer, E. H. (2016). Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. doi: 10.1016/j.dcn.2015.10.010
- Thomas, L. A., Kim, P., Bones, B. L., Hinton, K. E., Milch, H. S., Reynolds, R. C., … Leibenluft, E. (2013). Elevated amygdala responses to emotional faces in youths with chronic irritability or bipolar disorder. Neuroimage Clinical., 2, 637–645. doi: 10.1016/j.nicl.2013.04.007
- Van Leijenhorst, L., Zanolie, K., Van Meel, C. S., Westenberg, P. M., Rombouts, S. A. R. B., & Crone, E. A. (2010). What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex, 20, 61–69. doi: 10.1093/cercor/bhp078
- Viding, E., Sebastian, C. L., Dadds, M. R., Lockwood, P. L., Cecil, C. A. M., De Brito, S. A., & McCrory, E. J. P. (2012). Amygdala response to pre-attentive masked fear is associated with callous-unemotional traits in children with conduct problems. American Journal of Psychiatry, 169(10), 1109–1116. doi: 10.1176/appi.ajp.2012.12020191
- Vitaro, F., Brendgen, M., & Barker, E. D. (2006). Subtypes of aggressive behaviors: A developmental perspective. International Journal of Behavioral Development, 30, 12–19. doi: 10.1177/0165025406059968
- Vochteloo, J. D., & Koolhaas, J. M. (1987). Medial amygdala lesions in male rats reduce aggressive behavior: Interference with experience. Psychology & Behaviour, 41, 99–102.
- Whalen, P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B., & Jenike, M. A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411–418.
- White, S. F., Van Tieghem, M., Brislin, S. J., Sypher, I., Sinclair, S., Pine, D. S., … Blair, R. J. (2016). Neural correlates of the propensity for retaliatory behavior in youths with disruptive behavior disorders. American Journal of Psychiatry, 173(3), 282–290. doi: 10.1176/appi.ajp.2015.15020250
- Yu, R., Mobbs, D., Seymour, B., Rowe, J. B., & Calder, A. J. (2014). The neural signature of escalating frustration in humans. Cortex, 54, 165–178. doi: 10.1016/j.cortex.2014.02.013
- Zimring, F. (1998). American youth violence. New York, NY: Oxford University Press.
