Abstract
Acute myocardial infarction is the leading cause of mortality in the industrialized world. While it is essential to attempt an early reperfusion of ischemic myocardial territories, reperfusion itself adds damage to the heart, the ischemia–reperfusion (I/R) injury. Particularly the injury resulting from the very first minutes of reperfusion remains incompletely understood. MicroRNAs (miRNAs) are dynamic regulators in I/R injury. Nitric oxide (•NO) signaling, in turn, interacts with miRNA signaling. Our previous investigations showed that •NO signaling in I/R could be modulated by nitrite. We therefore sought to investigate the role of miRNAs in nitrite cardioprotection with focus on the first few minutes of reperfusion. The study was conducted in mice in vivo with 30 min of ischemia and 5 min of reperfusion. Mice received a single-dose of nitrite or saline intracardially 5 min prior to reperfusion. We identified nine miRNAs to be up-regulated after 5 min of reperfusion. The up-regulation of almost half of those miRNAs (miR-125a-5p, miR-146b, miR-339-3p, miR-433) was inhibited by nitrite treatment, perpetuating baseline values. In silico analysis revealed the Irak-M gene to be a target of miR-146b and miR-339-3p. Correspondingly, a rise in Irak-M transcript and protein levels occurred by nitrite treatment within the early phase of reperfusion. The results demonstrate that already a very short phase of reperfusion is sufficient for significant dysregulation in cardiac miRNAs expression and that nitrite preserves baseline values of miRNAs in the scale of only a few minutes. These findings hint at a potential novel cardioprotective mechanism of nitrite signaling.
Introduction
Restoration of cardiac perfusion after myocardial infarction in an appropriate time-frame is essential for adequate outcomes of patients [Citation1]. Nevertheless, reperfusion itself promotes tissue damage, the so-called ischemia/reperfusion (I/R) injury [Citation2,Citation3]. Emerging evidence points to a central yet incompletely defined role for the first few minutes of reperfusion after ischemia [Citation4]. On a subcellular level, the development and progression of myocardial injury in the early phase of reperfusion is mainly originated in mitochondria [Citation5]. As a consequence of incomplete dioxygen reduction, mitochondria produce an excess of reactive oxygen species (ROS). These ROS in turn oxidize proteins and lipids [Citation6–9]. Destruction of mitochondrial membrane integrity by permeabilization or rupture results in the execution of cell death via caspase activation [Citation8].
MicroRNAs (miRNAs) are ∼22 nucleotides long and established as dynamic regulators in I/R injury [Citation10]. They play a decisive role in protein synthesis and contribute to pathophysiological processes by altering the translation of key signaling molecules. Acting post-transcriptionally, they either inhibit translation initiation or promote messenger RNAs (mRNA) destabilization or degradation [Citation11,Citation12]. Several miRNAs including miR-1, miR-146a, miR-214, and miR-320 exhibit an altered expression profile during ischemia and after 24 h of reperfusion with beneficial as well as detrimental consequences [Citation13–15]. MiR-499, however, has been shown to remain unchanged during I/R. A knockdown of miR-499 promotes myocardial apoptosis and broadens the infarct zone, while transgenic overexpression of miR-499 attenuates the infarct size [Citation16].
Significantly elevated miR-499 levels in mice, exposed to chronic ligation of the left coronary artery, were yielded by nitrite treatment before ischemia, resulting in improved heart function [Citation17]. However, it is still not known to which extent miRNAs are involved in nitrite-mediated cardioprotection, particularly in the first few minutes of reperfusion. Inorganic nitrite, a precursor of bioactive nitric oxide (•NO), mediates impressive cardioprotection in I/R injury, upon exogenous administration before reperfusion as well as endogenous generation by remote ischemic preconditioning [Citation18]. However, the underlying mechanisms are not completely solved, which we sought to investigate further. We previously identified that nitrite-derived •NO diminishes excessive ROS levels in the early phase of reperfusion via inhibition of complex I of the mitochondrial electron transport chain and enhanced thiolprotein oxidoreductase activity of macrophage migration inhibitory factor (MIF) [Citation19–21].
The aim of the study was to investigate whether nitrite treatment affects potential alteration in cardiac miRNA expression profiles within the early phase of reperfusion and to identify miRNA–mRNA interactions and functional pathways linked to nitrite treatment.
Methods
Mice and ethics statement
All experiments were approved by the responsible committee according to the “European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes” (Directive 2010/63/EU) and animal care was in accordance with institutional guidelines. All mice were male NMRI (Naval Medical Research Institute) mice with an average age of 12 ± 3 weeks and a weight of 42 ± 3 g. They were obtained from Janvier (Saint Berthevin, France) and kept one week in the local animal house for acclimatization.
In vivo I/R injury
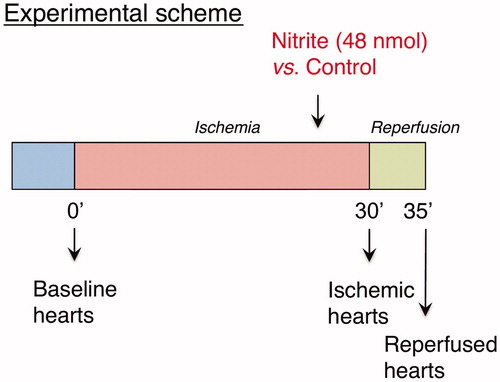
All mice underwent an open-chest in vivo myocardial I/R protocol as previously described [Citation18,Citation20]. The general protocol is provided in . Briefly, the left coronary artery was ligated with a 7-0 silk suture. After 30 min, the ligation was removed and the myocardium reperfused for 5 min. In treatment groups, mice received a single dose of sodium nitrite intracardially (1.67 μmol/kg body weight) 5 min before the end of ischemia, while control animals were challenged with an equal volume of sodium chloride 0.9%. Hearts were excised for miRNA and mRNA analyses at baseline, immediately upon completion of ischemia or after 5 min of reperfusion. Before surgical excision, hearts were perfused free of blood with sodium chloride 0.9% and hereafter snap frozen and stored at −80 °C until further preparation. The sample size was determined by an “A priori” power analysis using G*Power version 3.1 (Autenzell, Germany).
RNA isolation and quality control
Total RNA (miRNA and mRNA) was isolated from hearts using the miRVana™ microRNA Isolation Kit (Applied Biosystems/Ambion, Austin, TX). The extraction procedure was conducted in accordance with the manufacturer’s manual. Purified RNA was quantified using the Nanodrop system (ThermoScientific, Wilmington, DE). RNA and miRNA integrity were confirmed with RNA 6000 Nano LabChips and small RNA chips on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Samples exhibiting an RNA integrity number (RIN) > 8 were included.
miRNA expression analyses
For reverse transcription and quantification of expression levels of known miRNAs in mouse heart tissue, we used Rodent MegaplexTM RT Primer Pool A and TaqMan® Array miRNA Cards A version 2.0 according to the manufacturer’s specifications (ThermoFisher Scientific, Darmstadt, Germany). miRNA extracts were used to provide cDNA complementary constructs. The supplied master mix (4.5 μl) was added to 3 μl of the miRNA (300 ng miRNA) specimen. All samples were loaded to a master cycler to undergo a 167 min PCR reaction. The resulting cDNA was stored on −20 °C until further usage. Before addition of cDNA and TaqMan Universal Master Mix II no UNG (ThermoFisher Scientific, Darmstadt, Germany) to the miRNA array cards, all samples were equilibrated to room temperature. After plate loading and centrifugation, plates were sealed and run on the 7900HT Fast Real-Time PCR System (ABI PRISM® 7900HT, Applied Biosystems, Austin, TX). Raw data were analyzed with the manufacturer’s analysis tool (ExpressionSuite software v.1.0.3, ThermoFisher Scientific, Darmstadt, Germany). U6 snRNA, snoRNA-135, and snoRNA-202 were used as endogenous controls.
To validate the expression of selected differentially expressed miRNAs (miR-146b, miR-339-3p), we also conducted single tube real-time quantitative reverse transcription polymerase chain reaction (real-time qRT-PCR) analysis with TaqMan MicroRNA Assays containing miRNA-specific primer and TaqMan MGB probes (ThermoFisher Scientific, Darmstadt, Germany). The single tube analysis was performed according to the manufacturer’s specifications. Briefly, the supplied master mix (7 μl) was added to 5 μl of miRNA (15 ng) specimen and 3 μl of RT Primer. After amplification, 1.33 μl of all samples were immediately added to TaqMan Universal Master Mix II no UNG and 1 μl of commercially available TaqMan Assay Mix. Hereafter, the mixture was loaded on a 96-well Fast PCR Plate, sealed and transferred to the 7900HT Fast Real-Time PCR System (ABI PRISM® 7900HT, Applied Biosystems, Austin, TX). The expression of miRNA was normalized to snoRNA-135. The relative expression was calculated using the comparative Ct-method. Average threshold cycle (Ct) and SD values were assessed from triplicates of each sample using four biological replicates.
Gene expression analyses
Total RNA preparations were checked for RNA integrity by Agilent 2100 Bioanalyzer quality control as described above (Agilent Technologies, Palo Alto, CA). All samples in this study showed high quality RINs (mean 8.9). RNA was further analyzed by photometric Nanodrop measurement and quantified by fluorometric Qubit RNA assays (Life Technologies, Carlsbad, CA).
Synthesis of biotin labeled cDNA was performed on five replicates of each experimental group (reperfused myocardium with nitrite treatment and control) according to the manufacturers’ protocol (WT Plus Reagent Kit; Affymetrix, Inc., Santa Clara, CA). Briefly, 100 ng of total RNA were converted to cDNA. After amplification by in vitro transcription and 2nd cycle synthesis, cDNA was fragmented and biotin labeled by terminal transferase. Finally, end labeled cDNA was hybridized to Affymetrix Mouse Gene 2.0 ST Gene Expression Microarrays for 16 h at 45 °C, stained by streptavidin/phycoerythrin conjugate and scanned as described in the manufacturers’ protocol.
Data analyses on Affymetrix CEL files were conducted with GeneSpring GX software (Vers. 12.5; Agilent Technologies, Palo Alto, CA). Probes within each probeset were summarized by GeneSprings’ ExonRMA16 algorithm after quantile normalization of probe level signal intensities across all samples to reduce inter-array variability [Citation22]. Input data pre-processing was concluded by baseline transformation to the median of all samples.
To improve signal-to-noise ratio, a given probeset had to be expressed above background (i.e. fluorescence signal of a probeset was detected within the 20th and 100th percentiles of the raw signal distribution of a given array) in all five replicates in at least one of two, or both conditions to be further analyzed in pairwise comparison. Differential gene expression was statistically determined by moderated t-test. The significance threshold was set to p = 0.01.
Bioinformatics analyses
Functional related categories of genes were identified using GeneSpring GX and Database for Annotation, Visualization, and Integrated Discovery (DAVID Bioinformatics database) as described previously [Citation23]. Differentially expressed genes (p < 0.01) with an additional fold change >1.2 were considered relevant and were imported into DAVID Bioinformatics database [Citation24] and gene ontology (GO) terms and advanced pathway analysis were performed [Citation25]. The whole mouse (DAVID default) was chosen as a background reference. A modified Fisher’s exact test was used to assess the significance of the association between the observed data and the data in the GO and canonical pathway. Each generated pathway was assigned a significance score, according to the number of differentially regulated focus genes in the data set. This score was the negative logarithm of the p value, indicative of the likelihood that focus genes were found together in a pathway randomly. miRNA–mRNA interactions were explored using the TargetScan website (Release 7.0).
Immunoblotting
Hearts were collected in lysis buffer containing 50 mM Tris–HCl, 150 mM NaCl, 0.5 mM EDTA, 1% NP-40 and protease- and phosphatase inhibitor (pH 7.4). Protein concentration was measured using DC Protein Assay (Bio-Rad, Munic, Germany). Equivalent amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), followed by the transfer onto nitrocellulose membranes and immunoblotted with primary antibody against IRAK-M and tubulin (Abcam, Cambridge, UK). After incubation with the secondary antibody, visualization of bound secondary antibody was by enhanced chemiluminescence (ThermoFisher, Darmstadt, Germany) and imaged on an Imager 600 (GE Healthcare, Freiburg, Germany).
Statistical methods
ExpressionSuite software v.1.0.3 (ThermoFisher Scientific, Darmstadt, Germany) was used to compute changes in levels of known miRNAs in mouse hearts. The respective reference group was set as indicated in the manuscript. A p value of <0.05 was considered significant. MiRNAs detected in only one specimen were omitted from the analysis. MiRNAs with Ct values below 35 were considered expressed. Data are presented as volcano plots displaying the change in expression as log2 and the significance levels p as − log10 or as indicated. Unpaired Student’s t-test was used to compare two groups. Data are presented as means ± standard deviation (SD). The data were transferred to Graph Pad Prism (GraphPad, San Diego, CA) for the generation of bar graphs.
Results
miRNAs are differentially expressed in the early phase of reperfusion after acute myocardial infarction
To gain an overview of changes in miRNA expression particularly within the first 5 min of reperfusion after ischemia, we performed the in vivo I/R model depicted in . Using TaqMan Array miRNA Cards A, we investigated the miRNA expression profiles in whole mouse hearts (controls) at baseline, after 30 min of ischemia and after 5 min of reperfusion. Out of 336 unique murine miRNAs analyzed, we found six differentially expressed miRNAs exhibiting a significantly down-regulation after 30 min of ischemia (n = 4, p < 0.05). These miRNAs corresponded to let-7a, let-7b, miR-23b, miR-181c, miR-208, miR-301b (). Within the 5 min of reperfusion after ischemia, nine miRNAs were exclusively up-regulated. We observed a significant increase in expression levels of conserved miR-98, miR-125a-5p, miR-146b, miR-191, miR-200c, miR-339-3p, miR-351, miR-433, miR-484 with fold-changes ranging from 2.55 to 4.745 (n = 4, p < 0.05; , ).
Figure 2. Volcano plots show differential miRNA expression. Depicted miRNAs are differentially regulated in 5 min of reperfusion vs. ischemia control group (A) and in nitrite-treated reperfusion vs. reperfusion control group (B) (ischemia n = 3, reperfusion control n = 4, reperfusion + nitrite n = 4; p < 0.05).
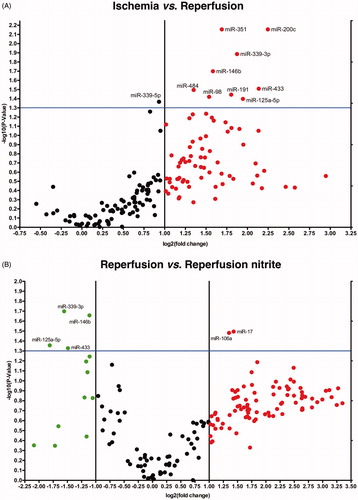
Table 1. Six miRNAs Identified to be down-regulated during 30 min of ischemia.
Table 2. miRNAs identified to be regulated in ischemia/reperfusion injury.
Nitrite modulates miRNA expression in the reperfused myocardium
Nitrite, when administered before reperfusion in the nanomolar range, has demonstrated impressive cardioprotective effects linked to posttranslational modification of various proteins after its reduction to •NO, as shown by us and others [Citation19,Citation20]. To evaluate whether nitrite treatment affects the altered expression profile of cardiac miRNAs in early reperfusion, we investigated mouse hearts 5 min after reperfusion as shown in . A total of six miRNAs was found to be significantly different between the reperfused control and the reperfused nitrite groups, namely miR-17, miR-106a, miR-125a-5p, miR-146b, miR-339-3p, and miR-433 (p < 0.05) (). MiR-17 and miR-106a were up-regulated after nitrite treatment. As mentioned above, miR-125a-5p, miR-146b, miR-339-3p, and miR-433 were significantly up-regulated within the first 5 min of reperfusion by holding baseline levels during ischemia (), pointing to a reperfusion-bound mechanism. These miRNAs were found to be significantly down-regulated in mouse hearts receiving nitrite before reperfusion compared to the reperfusion control group with fold-changes in the range of 2.2–3.5 (n = 4, p < 0.05; ).
Nitrite modulates gene expression in the reperfused myocardium
To date mRNA expression profiling of nitrite treated mouse hearts undergoing myocardial ischemia and 5 min of reperfusion in comparison to control reperfused mouse hearts has not been performed yet. To examine an effect of nitrite on mRNA expression profiling, we performed gene expression microarray experiments on reperfused hearts of control and nitrite treated mice (experimental protocol, ). For detecting differential gene expression, we chose a statistical significance threshold of p = 0.01. After data processing and application of the filtering criteria, 25,436 expressed transcripts could be detected. Of those, we identified 356 differentially expressed genes in nitrite-treated reperfused hearts with 193 transcripts being up- and 163 transcripts down-regulated, respectively (). To gain a deeper understanding of these transcriptional changes with respect to biological processes, GO and pathway analysis were performed through DAVID Bioinformatics database. DAVID functional annotations tool was used to categorize the differentially expressed transcripts with p < 0.01 according to biological processes, molecular functions and cellular components ().
Figure 3. mRNA analysis and pathway analysis using DAVID-software. (A) Reperfused mice with nitrite treatment vs. control group show differentially expressed gene transcripts. (B) DAVID analysis shows three enriched pathways in reperfused mice with nitrite treatment (p < 0.01).
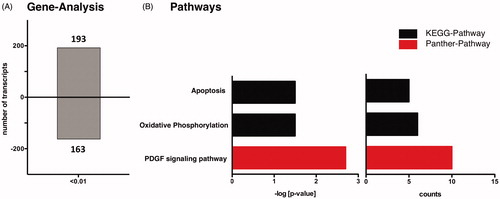
Table 3. Nitrite-induced enriched gene ontology (GO) terms according to biological processes, cellular components and molecular functions within the heart. GO terms are ordered by Fisher’s exact test –log [p value]. Differentially expressed transcripts involved in the term (count) with p < 0.01 were included. FDR, false discovery rate.
Affected signaling pathways in the reperfused myocardium
To evaluate regulatory and functional clusters in which targets were significantly altered by nitrite treatment in reperfused mouse hearts, we additionally performed DAVID pathway analysis [Citation24,Citation26]. Relevant pathways with a significance level of p < 0.01 and a fold enrichment cut-off >3 pertained to KEGG (11 genes) and Panther pathway (10 genes) (). The highest enriched KEGG pathway corresponded to “apoptosis”. We here identified five differentially expressed genes related to the apoptotic signaling in nitrite-treated mouse hearts (), namely the Interleukin-1 receptor associated kinase 3 (Irak-M), inhibitor of apoptosis protein (Birc3), tumor necrosis factor receptor 1 (Tnfrsf1a), interleukin-3 receptor (Csf2rb2), and protein kinase A (Prkacb). These transcripts were found to be significantly up-regulated (). The second highest enriched KEGG pathway corresponded to “oxidative phosphorylation” and contained six down-regulated genes in nitrite-treated mouse hearts within the first 5 min of reperfusion, namely the NADH dehydrogenase (ubiquinone) 1 beta subcomplex 6 (Ndufb6), NADH dehydrogenase (ubiquinone) 1 (Ndufc1), NADH dehydrogenase (ubiquinone) flavoprotein 3 (Ndufv3), cytochrome c oxidase, subunit VIIIb (Cox8b), ATPase, H+ transporting, lysosomal V0 subunit D1 (Atp6v0d1) and ATPase, H+ transporting, lysosomal V0 subunit E2 (Atp6v0e2) ().
Figure 4. Apoptosis pathway in nitrite treated mice after 5 min of reperfusion. Stars indicate differentially regulated genes in reperfusion after nitrite treatment vs. untreated control group (p < 0.01).
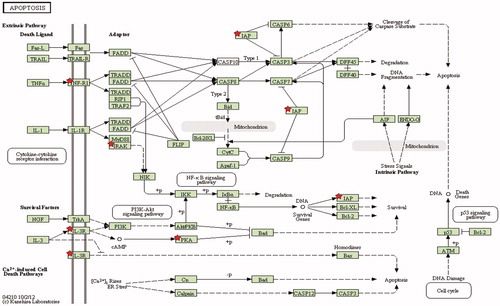
Table 4. Differentially expressed genes related to apoptosis pathway in reperfusion in nitrite treated mice vs. control group.
Table 5. Differentially expressed genes related to oxidative phosphorylation pathway in reperfusion in nitrite treated mice vs. control group.
Putative miRNA–mRNA interactions in thenitrite-treated reperfused myocardium
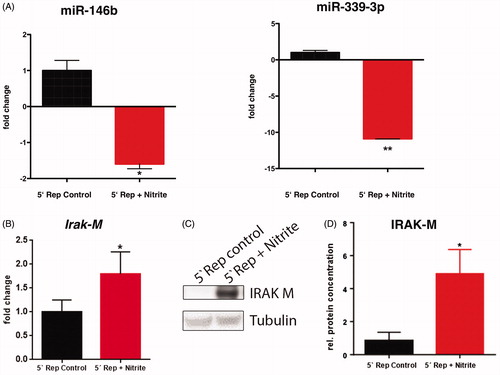
Following the bioinformatics analyses, we focused our attention on putative interactions of the identified miRNAs displaying an altered expression in the nitrite-treated reperfused mouse hearts, namely miR-17, miR-106, miR-125a, miR-146b, miR-339-3p, and miR-433 () and differentially expressed genes ( and ). Using the TargetScan database 7.0 for mouse species, we scanned the above-mentioned differentially expressed genes related to the apoptotic pathway eliciting possible miRNA–mRNA interactions. This analysis yielded the miRNAs miR-146b, miR-339-3p, and miR-433 to be predicted to target the 3′UTR binding site of Irak-M, which was 1.3-fold up-regulated after only 5 min of reperfusion in the nitrite treated group (). Increased levels of Irak-M are consistent with decreased levels of the above-mentioned miRNAs, due to less inhibition of their target gene Irak-M. IRAK-M is established to be an important negative regulator of pro-inflammatory signaling pathways [Citation27,Citation28]. To validate the expression levels of these three miRNAs as well as the transcript level of Irak-M, we performed single tube real-time qRT-PCRs as well as immunoblotting for IRAK-M protein level. As shown in , we could confirm the reduced expression levels of miR-146b (1.6-fold; n = 5; p < 0.05) and miR-339-3p (11.1-fold; n = 5, p < 0.05) as well as the increase of Irak-M transcript level (1.8-fold; n = 5; p < 0.05) and protein level (five-fold; n = 5; p < 0.05). The expression level of miR-433 was unchanged (n = 5, p = ns). Exploring possible miRNA interactions with genes differentially expressed and related to the oxidative phosphorylation pathway using TargetScan database 7.0 revealed no interaction with the above-mentioned miRNAs.
Figure 5. Nitrite treatment is associated with reduced miR-146b and miR-339-3p expression and increased Irak-M transcript and expression level. (A) The levels of miR-146b and miR-339-3p expression were evaluated by real-time quantitative reverse transcription polymerase chain reaction (real-time qRT-PCR) and normalized to sno135. (B) Irak-M transcript level was detected by real time qRT-PCR and normalized to GAPDH transcripts. (C) The protein level of IRAK-M was examined by immunoblotting and tubulin was set as internal control for sample loading. (D) Intensities of IRAK-M bands were normalized to tubulin. Data are presented as mean ± SD (A, B) and SEM (C); n = 4–7; *p < 0.05, **p < 0.01.
Discussion
Here, we demonstrate that (i) a very short phase of perfusion is sufficient for a significant dysregulation of cardiac miRNAs, (ii) nitrite is in part capable of preventing this dysregulation, in particular of miR146b and miR-339-3p, and (iii) the nitrite-related preservation of miR146b and miR-339-3p baseline values corresponds with elevated levels of IRAK-M.
A number of miRNAs have been shown to regulate important processes involved in the pathophysiology of acute myocardial infarction, in particular in cardiac remodeling leading to the development of chronic heart failure [Citation29]. The timely reperfusion after ischemia causes substantial myocardial damage. However, the early phase of reperfusion has only been investigated poorly in the setting of miRNA regulation processes so far. We observed that expression levels of conserved miR-98, miR-125a-5p, miR-146b, miR-191, miR200c, miR-339-3p, miR-351, miR-433, and miR-484 were increased after only 5 min of reperfusion, while holding baseline levels during ischemia. Some of the above mentioned dysregulated miRNAs have been identified as biomarkers for cardiac disease. However, others have been linked to specific signal transduction pathways. A previous experimental study has associated dysregulated miR-98 signaling, which is a member of the let-7 family, with augmented cardiac hypertrophy in response to circulatory stimuli [Citation30]. This regulatory effect was under control of thioredoxin, which in conjunction with MIF, plays a central role in the regulation of I/R injury. However, a connection between MIF signaling and miRNA in I/R injury has not been studied. Also we have found miR-125a-5p to be significantly up-regulated in early phase of reperfusion among the nine other miRNAs mentioned above. MiR-125a-5p has recently been associated with heart failure. This was evaluated in the serum of patients [Citation31]. It remains to be investigated whether our cardiomyocyte levels contribute to the increase in the circulatory compartment once heart failure progresses. The miR-146 family plays an important role in the regulation of myocardial processes: Augmentation as detected by us in the early phase of reperfusion has previously been associated with an increase in cell death in simulated I/R injury in cardiac cell cultures [Citation32]. In addition, they play a role in chronic cardiac remodeling and immunogenic responses [Citation33]. It is tempting to speculate a specific miR-146b reducing therapy might contribute to cardioprotection from I/R injury.
Nitrite has previously been shown to exert indirect and direct effects on cardiomyocytes. The indirect effects result from the bio-reduction of nitrite to •NO under hypoxic and ischemic conditions [Citation18–20,Citation34]. In the heart, this relates to the reductase function of myoglobin [Citation20,Citation35,Citation36]. The direct effects of nitrite are less known. However, changes of nitrite levels have been associated with alterations in gene, protein and miRNA expression levels [Citation37]. The exact mechanisms of the interaction between nitrite and miRNAs remain unclear. Nitrite effects could range from nitration of RNA molecules to oxidation or posttranslational S-nitrosation of regulatory molecules. Future studies are needed to investigate the direct signaling pathway, which cannot be deduced from the present investigation. Here we associated nitrite with an increase in miR-17 and miR-106a in early reperfusion. Particularly miR-17 is involved in cardiac remodeling through matrix metalloproteinases [Citation38] and in the prevention of cardiomyocyte apoptosis [Citation39]. Therefore a protection effect of nitrite through miR-17 signaling can be assumed. Strikingly, miR-125a-5p, miR-146b, miR-339-3p, and miR-433 were significantly down-regulated by nitrite treatment. As outlined in the previous section, miR-146b is related to many cardiac disease processes and nitrite appears to reduce deleterious miR-146b signaling at least in the very first minutes of reperfusion. According to miRNA target prediction, the Irak-M gene is one of the possible targets of miR-146b and miR-339-3p. IRAK-M is an important negative regulator of pro-inflammatory signaling pathways by inhibiting cytokine expression [Citation27,Citation28]. In the scope of acute myocardial infarction, IRAK-M was previously shown to be up-regulated in macrophages and fibroblasts after 6 h of reperfusion leading to a diminished adverse post-infarction remodeling and limited fibroblasts-mediated matrix degradation [Citation27,Citation28]. A timely activation of the anti-inflammatory response within the “late” phase of reperfusion is crucial for cardiac repair. Thereby, timely repression of this response is critical for effective healing [Citation3]. Our data revealed that the levels of miR-146b and miR-339-3p were significantly lower in nitrite-treated reperfused mice in comparison to control reperfused mice, which obviously augmented both Irak-M transcript and IRAK-M protein levels already within the early phase of reperfusion, indicating the negative relationship between the miRNAs miR-146b and miR-339-3p and IRAK-M. Interestingly, we did not detect a significant change in miR-21 expression levels, which has previously been introduced as a master regulator in I/R injury. This may be related to the very early time point in reperfusion, while previous investigation was conducted after longer time frames [Citation14,Citation40].
Conclusions
Nitrite rapidly modulates miRNA-signaling bi-directionally in the early phase of reperfusion with an increase of potentially protective miRNAs and a reduction of miRNAs previously associated with myocardial injury. These findings hint at a potential novel cardioprotective mechanism of nitrite signaling. Among these miRNAs, miR-146b, and miR-339-3p may deconvolve their role in nitrite cardioprotection by targeting Irak-M. Nitrite preserves baseline values of miRNAs in particular of miR-146b and miR-339-3p accompanied by increased Irak-M transcript and protein levels. It should be noted that these changes in both, the cardiac transcriptome and proteome take place rapidly within only the first 5 min of reperfusion. This is consistent with the well-established role of miRNAs being molecular switches rapidly influencing the transcriptome profiling of a cell. Future studies must identify the exact signaling pathway and underlying mechanism related to miRNAs that mediate the nitrite cardioprotection and demonstrate whether the observed changes in miRNA and gene expression levels relate to effects from nitrite or •NO after reduction of nitrite.
Acknowledgements
We thank Pia Stock and Andrea Odersky for expert technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) (RA 969/4-2 to TR). All the data supporting the findings are included within the manuscript.
Additional information
Funding
References
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest 1985;76:1713–1719.
- Eltzschig HK, Eckle T. Ischemia and reperfusion-from mechanism to translation. Nat Med 2011;17:1391–1401.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–1135.
- Hausenloy DJ, Yellon DM. Myocardial ischemia–reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100.
- de Lima Portella R, Lynn Bickta J, Shiva S. Nitrite confers preconditioning and cytoprotection after ischemia/reperfusion injury through the modulation of mitochondrial function. Antioxid Redox Signal 2015;23:307–327.
- Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundler SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014;515:431–435.
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 2006;319:1405–1412.
- Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail Rev 2007;12:249–260.
- Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res 2004;94:53–59.
- Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest 2013;123:11–18.
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008;455:58–63.
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010;466:835–840.
- Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest 2012;122:1222–1232.
- Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009;119:2357–2366.
- Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J 2009;50:377–387.
- Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 2011;17:71–78.
- Qipshidze-Kelm N, Piell KM, Solinger JC, Cole MP. Co-treatment with conjugated linoleic acid and nitrite protects against myocardial infarction. Redox Biol 2013;2:1–7.
- Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 2014;114:1601–1610.
- Luedike P, Hendgen-Cotta UB, Sobierajski J, Totzeck M, Reeh M, Dewor M, et al. Cardioprotection through S-nitros(yl)ation of macrophage migration inhibitory factor. Circulation 2012;125:1880–1889.
- Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia–reperfusion injury. Proc Natl Acad Sci USA 2008;105:10256–10261.
- Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 2007;204:2089–2102.
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003;19:185–193.
- Rammos C, Totzeck M, Deenen R, Köhrer K, Kelm M, Rassaf T, et al. Dietary nitrate is a modifier of vascular gene expression in old male mice. Oxid Med Cell Longev 2015;2015:658264.
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57.
- Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 2012;40:D109–D114.
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000;25:25–29.
- Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002;110:191–202.
- Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, et al. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol 2012;32:2598–2608.
- Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol 2015;12:135–142.
- Yang Y, Ago T, Zhai P, Abdellatif M, Sadoshima J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ Res 2011;108:305–313.
- Wong LL, Armugam A, Sepramaniam S, Karolina DS, Lim KY, Chong JP, et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 2015;17:393–404.
- Li JW, He SY, Feng ZZ, Zhao L, Jia WK, Liu P, et al. MicroRNA-146b inhibition augments hypoxia-induced cardiomyocyte apoptosis. Mol Med Rep 2015;12:6903–6910.
- Wang J, Wang Y, Han J, Li Y, Xie C, Xie L, et al. Integrated analysis of microRNA and mRNA expression profiles in the left atrium of patients with nonvalvular paroxysmal atrial fibrillation: role of miR-146b-5p in atrial fibrosis. Heart Rhythm 2015;12:1018–1026.
- Totzeck M, Schicho A, Stock P, Kelm M, Rassaf T, Hendgen-Cotta UB. Nitrite circumvents canonical cGMP signaling to enhance proliferation of myocyte precursor cells. Mol Cell Biochem 2015;401:175–183.
- Totzeck M, Hendgen-Cotta UB, Rammos C, Petrescu AM, Meyer C, Balzer J, et al. Assessment of the functional diversity of human myoglobin. Nitric Oxide 2012;26:211–216.
- Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res 2007;100:1749–1754.
- Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 2005;1:290–297.
- Li SH, Guo J, Wu J, Sun Z, Han M, Shan SW, et al. miR-17 targets tissue inhibitor of metalloproteinase 1 and 2 to modulate cardiac matrix remodeling. FASEB J 2013;27:4254–4265.
- Song S, Seo HH, Lee SY, Lee CY, Yoo KJ, Yoon C, et al. MicroRNA-17-mediated down-regulation of apoptotic protease activating factor 1 attenuates apoptosome formation and subsequent apoptosis of cardiomyocytes. Biochem Biophys Res Commun 2015;465:299–304.
- Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res 2009;82:21–29.