Abstract
The development of mucoadhesive formulations of buprenorphine for intended sublingual usage in the treatment of drug addiction is described. The formulations include mucoadhesive polymer films, with or without plasticizers, and mucoadhesive polymer tablets, with or without excipients that enhance drug release and/or improve tablet compaction properties. The mucoadhesive polymers studied include carbomers such as Carbopol 934P, Carbopol 974P, and the polycarbophil Noveon AA-1, with excipients chosen from pregelatinized starch, lactose, glycerol, propylene glycol, and various molecular weights of polyethylene glycol. The development of plasticizer-containing mucoadhesive polymer films was feasible; however, these films failed to release their entire drug content within a reasonable period. Thus, they were not determined suitable for sublingual usage because of possible loss by ingestion during routine meal intakes. The mucoadhesive strength of tablet formulations containing Noveon AA-1 appears to be slightly superior to the Carbopol-containing tablets. However, the Carbopol 974P formulations exhibited superior drug dissolution profiles while providing adequate mucoadhesive strength. The tablet formulations containing Carbopol 974P as mucoadhesive polymer, lactose as drug release enhancer, and PEG 3350 as compaction enhancer exhibited the best results. Overall, the mucoadhesive tablet formulations exhibited superior results compared with the mucoadhesive film formulations.
Therapies to prevent and/or treat drug abuse need careful consideration of the biopharmaceutical aspects of the treatment drugs and suitable delivery systems that can provide an ideal therapeutic profile and improve patient compliance. Ideally, drugs for the treatment of abuse must possess sufficiently long half-lives that allow reduction in frequency of administration, slow metabolism to inactive metabolites, thus requiring less drug to be administered, and lack of addiction potential of their own. Buprenorphine has gained much interest in recent years in the treatment of opioid-type drug addiction. It has strong analgesic and narcotic antagonist activity and is 25–50 times more potent than morphine (Gutstein and Akil Citation2001). Pharmacologically, buprenorphine, a highly lipophilic semisynthetic derivative of the opioid alkaloid thebaine, is a partial opiate agonist. It has agonistic effect on the mu and antagonistic effect on the kappa receptors, with the agonist properties predominating at low doses and antagonist properties predominating at higher doses (Cowan, Lewis, and Macfarlane Citation1977). A partial agonist is less likely to cause respiratory depression, which is the major toxic effect of opiate drugs, compared with full agonists such as heroin and methadone. Buprenorphine hydrochloride, the water-soluble salt form of buprenorphine, has a mean plasma half-life of 3.21 hr (Kuhlman et al. Citation1996) and is highly metabolized in the intestinal wall and liver to norbuprenorphine, which is a weakly active metabolite with half-life of 57 hr (Kuhlman et al. Citation1998). Both buprenorphine and norbuprenorphine form inactive glucuronides (Iribarne et al. Citation1997).
Compared with the potential of buprenorphine as a first- or second-line agent in the treatment of opiate addiction, studies on buprenorphine drug delivery systems are relatively few. A subcutaneously implanted system utilizing a cholesterol-glyceryl tristearate matrix produced sustained analgesic effect in rats for 12 weeks or more (Pontani and Misra Citation1983). In an early study on noncrystalline prodrugs of buprenorphine, synthesized for transdermal delivery, success was limited because the lipophilic form was sequestered in the lipid-rich skin layers (Stinchcomb et al. Citation1996). A matrix-type transdermal patch of buprenorphine (Transtec®, Napp Pharmaceuticals) was recently introduced in the European market for the management of stable cancer and noncancer pain, and early clinical efficacy reports are fairly promising (Radbruch Citation2003). Eriksen et al. (Citation1989) reported that the systemic bioavailability of buprenorphine administered by nasal spray is greater than 40%, which is comparable to the 30–40% bioavailability via the intramuscular and subcutaneous routes. Addition of 30% polyethylene glycol (PEG) 300 as a co-solvent to a nasal formulation of buprenorphine does not enhance bioavailability of the drug any further (Lindhardt et al. Citation2001). Buprenorphine has been studied in a microcapsule system intended for parenteral use and produced a steady in vitro release for 45 days (Mandal Citation1999). Concerns over residual organic solvents used in most microparticle preparations have restricted FDA approval of parenteral microparticulate systems, in general, and further studies are needed to evaluate their efficacy and safety in vivo.
Intravenous buprenorphine has been used in pain management for many years. The oral route of administration produces poor bioavailability of approximately 15% (McQuay, Moore, and Bullingham Citation1986) and lacks commercial potential. Systemic bioavailability following sublingual administration, which bypasses first pass metabolism, is much superior and has been reported to be up to 58% (Bullingham et al. Citation1982). The sublingual region offers a nonkeratinized epithelium with high permeability and a smooth and relatively immobile surface with easy accessibility. For the treatment of drug abuse, an immediate release sublingual tablet of buprenorphine, Subutex™ (manufactured by Reckitt Benckiser), was recently introduced in the U.S. market. This delivery system for buprenorphine has been available in Europe for nearly a decade and is widely used as an alternative to methadone in the treatment of opiate addiction (Gasquet, Lancon, and Parquet Citation1999). Literature on bioavailability of sublingual buprenorphine presents variable numbers ranging from 19–58% of the administered dose. Although sublingual delivery of buprenorphine has been proven effective, bioavailability by this route can be erratic because of salivary washout and involuntary swallowing.
We hypothesize that increasing the contact time with the sublingual mucosa with a mucoadhesive delivery system could improve sublingual bioavailability and result in more predictable plasma levels of the drug, leading to better therapeutic efficacy and reproducibility. No study has been published to date on mucoadhesive sublingual delivery of buprenorphine aimed at the treatment of drug addiction. These dosage forms would adhere to the sublingual mucosa and withstand tongue movement for a significant period, potentially decreasing the chances of involuntary swallowing of the dosage form. A sustained release effect also may be expected from the dosage form, which would make delivery of higher doses of buprenorphine for the preferred 3-times/week dosing regimen feasible with minimal side effects. With easy accessibility to the sublingual area, the delivery systems can be self-administered by the patient with minimal or no supervision that in turn can reduce health care costs involved in the treatment of drug addiction.
In this article, we discuss the development of mucoadhesive polymer films and tablets of buprenorphine and evaluation of their physical properties and drug release characteristics. The effect of plasticizers on the film properties was studied, as well as the effect of excipients on “tabletability” and drug release properties from the compressed tablets. The polymeric dosage forms described are hydrogels that swell on coming in contact with water and do not allow prompt dissolution like an immediate release tablet; therefore, we anticipate that potential for diversion of these dosage forms as a street drug for intravenous use would be limited if applied in the clinical arena in the future.
MATERIALS AND METHODS
The carbomers Carbopol 934P, 974P and Noveon AA-1 were obtained by the courtesy of Noveon Inc. (OH, USA). Starch 1500 (pregelatinized maize starch) was obtained by the courtesy of Colorcon Inc. (PA, USA). Lactose monohydrate, glycerol, propylene glycol, PEG (MW 400, 1000, 3350, and 8000), mucin, and buprenorphine were obtained from Sigma Chemical Co. (MO, USA).
Preparation of Mucoadhesive Polymer Films
Considering the comfort issue involved with a drug delivery system designed to adhere to a sensitive and mobile area, we adjudged that a thin, flexible polymer film would be ideal for sublingual use. A general protocol used in several literature references describing polymer films was adopted. Double-filtered deionized water was degassed under vacuum before adding the polymers to minimize the formation of air bubbles within the gel. Each of the following polymers in 200–500 mg quantities, Carbopol 934P, Carbopol 974P, and Noveon AA-1, were solubilized in water or 95% ethanol using a paddle stirrer at 1000 rpm for 10 min to result in 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, and 5.0% w/w gels. Homogeneous gel formation for the higher concentrations (4.5 and 5% w/w) proved difficult by stirring and was achieved by placing the mixtures in plastic bags and kneading by hand to prevent formation of poorly wetted polymer agglomerates. Amounts higher than 5.0% w/w could not be homogeneously solubilized. All gels were kept overnight at 4°C to allow complete hydration, following which they were centrifuged at 5000 rpm for 30 min to remove air bubbles before film casting. Two techniques were used to cast the polymer films: (a) gels poured on Teflon® plates and placed in the oven at 40°C for 24 hr or until dry to the touch; and (b) gels placed between two Teflon® plates separated with 1 mm thick spacers at the edges and dried in a desiccator under vacuum for 48–72 hr.
Preparation of Plasticizer Containing Mucoadhesive Polymer Films
Plasticizers were added to the aqueous gel systems described above to reduce brittleness, improve flexibility, and improve surface texture and smoothness of the films. PEG has been described in the literature to also improve mucoadhesion properties of certain polymers. Glycerol, propylene glycol, or PEG 400, 1000, 3350, or 8000 were each added to the aqueous gel systems to result in final concentrations of 0.5, 1.0, 5.0, or 10.0% w/w plasticizer in the system and stored overnight under refrigeration. Gels were centrifuged and films were cast on Teflon® plates using the method (a) described previously.
Preparation of Mucoadhesive Tablets
Flat-faced core tablets were prepared by direct compression of various combinations of the polymers Carbopol 934P, 941, 971P, or 974P or Noveon AA-1, with or without starch, lactose, PEG 3350 and 8000, using a hydraulic laboratory pellet press (Carver Inc., IN, USA). After testing for physical characteristics and performing thermal analysis to study polymer-excipient interactions, select formulations were chosen and 8 mg buprenorphine incorporated into each unit dose. The total weight of components per compact was kept constant at 200 mg, and a constant force of 1 ton was applied for 60 sec. The diameter of the die used was 13 mm, providing a potential surface contact area of 1.33 cm2 for the tablets. Formulations were designed so that the tablet thickness would not exceed 2 mm and preferably be close to 1.5 mm.
Physical Characterization and In Vitro Disintegration and Dissolution Tests
The physical characteristics of the compacts such as thickness and hardness were evaluated using a micrometer (Central Tool Co., RI, USA) and a manual hardness tester (Pfizer, NY, USA), respectively. Visual observations were noted for surface smoothness. Thermal analysis was done using a differential scanning calorimeter (MDSC 2920, TA Instruments, DE, USA) by placing 2–4 mg samples in sealed aluminum pans and ramp heating from room temperature to 300°C at a scan rate of 10°C/min. All components were scanned in their pure state and compared with thermograms of the compressed tablets to observe changes in peak position or any characteristics that would indicate interactions between the drug and excipients. In vitro disintegration tests were done using a single-station USP disintegration apparatus (Erweka, NJ, USA) and double-distilled deionized water (ddH2O) as the medium. In vitro dissolution tests were done using a USP dissolution apparatus (Vankel, NJ, USA) with the basket rotating at 50 rpm in 500 mL ddH2O at 37°C. Then 5-mL samples were withdrawn at selected time intervals until the gel matrix completely dissolved.
The amount of buprenorphine in the dissolution samples was estimated by UV spectroscopy (Lambda-Bio, Perkin-Elmer, MA, USA). The reference medium for UV spectroscopy was obtained by dissolving a drug-free tablet in 500 ml ddH2O. Mucoadhesive strength was estimated by using a manually operated surface tension apparatus (DuNuoy Tensiometer, Cenco, IL, USA) modified for this purpose and similar to a design reported by Robert, Buri, and Peppas (1988). A schematic sketch of the instrument is presented in . We replaced the suspension ring (meant for measuring surface/interfacial tension) with an A-shaped wire affixed to an aluminum plate (weight 456 mg, thickness 0.5 mm, and diameter 2 cm) at the open ends. One face of the mucoadhesive tablet was immobilized on the aluminum plate using glue and the system was suspended. Next 30% (w/w) mucin gel was placed on the metal station at the base of the tensiometer. The tablet was brought into contact with the mucin gel, manually, and held in place for 10 sec. The dial on the tensiometer was then gently twisted until separation occurred and the reading at the point of separation was noted.
RESULTS AND DISCUSSION
Prolonging drug delivery is well recognized as an advantage as it increases the therapeutic value of many drugs. Nevertheless, for mucoadhesive sublingual delivery, even if the system were capable of staying in place for prolonged periods, it would still be impractical to design a delivery system that would be retained in place beyond 2–3 hr. If not disturbed by drinking fluids, it is difficult to imagine that a sublingual drug delivery system would not be lost while eating regular meals, which would invariably lead to loss of drug from first-pass metabolism. Therefore, our goal was to develop a formulation that would localize buprenorphine in the sublingual area and prolong the release better than the currently marketed immediate release dosage form, while releasing the entire drug content within approximately 120 min. Additionally, the formulation must lack any burst release effects, must not exhibit drug-excipient interactions, must possess sufficient mucoadhesive strength, and possess suitable physical properties such as tablet hardness, thickness, and surface smoothness for proper adhesion.
Mucoadhesive Polymer Film Formulations
Films with No Plasticizers
The films prepared by method (a) were brittle, with variable thicknesses and uneven surfaces. The films prepared by method (b), which was hoped to improve on method (a) by controlling film thickness, were comparably brittle and difficult to remove from the sandwiched Teflon® casting surfaces without distorting the structure of the film. The nature of solvent (water or ethanol) used to solubilize the polymers did not appear to cause a significant difference in the gel formation process or in the end products, except that ethanol dried faster and produced a more uneven surface in method (a). We concluded that these formulations, produced using either of these methods, would not be suitable for industrial scale-up.
Films with Plasticizers
In general, the plasticizer containing polymer films were easier to fabricate and handle. The films containing either glycerol or propylene glycol as plasticizers took 48 hr or longer to set and tended to stretch irreversibly during removal from the casting surface. We determined that these plasticizers are capable of yielding films with excellent flexibility, but for ease of handling they must be cast on an impervious flexible backing, with the latter incorporated as part of the formulation design as manipulations to the film must be done in conjunction to the backing material to prevent distortion. Hence, these plasticizers may be more suitable for designing transdermal films compared with dosage forms that need to be placed in the oral cavity, which preferably must be devoid of nonedible material.
2 Dependence of the polymer film thickness on volume of gel cast and drying conditions. The drug-free formulation represented here contained 2.5% w/w Carbopol 974P and 0.5% w/w PEG 400.
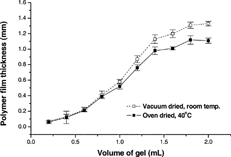
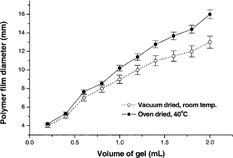
In formulations containing PEG as plasticizer, the films with 5.0 and 10.0% w/w plasticizer content did not set properly and were too stretchy to be removed intact from the casting surface. The 0.5 and 1.0% w/w PEG 400-containing films exhibited the best physical characteristics, as the surface was smooth and the films were flexible. Once the formulations were optimized, we added buprenorphine to the gel during the preparation process, filled the prepared gels in a hypodermic glass syringe, and placed 0.2–2 ml quantities on wax paper such that each unit dose contained 8 mg of buprenorphine. The drug-containing gels were allowed to spread and set into circular discs that were formed naturally. As expected, an inverse relationship was noted between film diameter and thickness. and illustrate the effect of drying conditions on film thickness and diameter produced by various quantities of 0.5% w/w PEG 400-containing gels. The vacuum dried films showed greater thickness and smaller diameters compared with oven dried films. The 40°C temperature in the oven likely allows for the spread of the films more than occurs at room temperature in the desiccator. This phenomenon is more evident when the volume of gel to be dried is larger.
Mucoadhesive Tablet Formulations
All tablets were observed to possess a smooth, shiny surface, regardless of the composition; therefore, the entire surface area would potentially be available for adhesion. shows the dependence of tablet thickness and hardness on the composition of some representative formulations.
Physical characteristics of mucoadhesive tablet formulations
3 Dependence of the film diameter on volume of gel cast and drying conditions. The drug-free formulation represented here contained 2.5% w/w Carbopol 974P and 0.5% w/w PEG 400.
Tablets Prepared with Mucoadhesive Polymer Only
In vitro disintegration tests indicated that all pure polymer compacts, containing no other excipients, required over 8 hr to swell and erode. We incorporated buprenorphine in the polymer Carbopol 974P (8 mg buprenorphine +192 mg polymer) and studied the tablet for in vitro dissolution using a USP apparatus; the amount of buprenorphine released over 12 hr was very small when the absorbance was recorded in the UV spectrophotometer. This observation corroborates the observations of McQuinn et al. (Citation1995) who found that mucoadhesive polymer discs containing Carbopol 934P and 2.9 mg buprenorphine free base, when placed on the gum of human volunteers, released 0.42 ± 0.18 mg of the drug over 12 hr, which translates to approximately 14% drug release. This led us to conclude that the pure polymer compacts would not be capable of releasing therapeutically effective quantities of buprenorphine in the sublingual environment in vivo within a reasonable usage period.
Tablets Prepared with Mucoadhesive Polymer and PEG
Addition of up to 10% w/w PEG 3350 improved the dissolution profile compared with the pure polymer compacts, but further increase in PEG content did not make additional improvements in the dissolution rate or extent. As shown in , the addition of PEG 3350 slightly increased the hardness of the tablets; however, it did not make any difference to the tablet thickness.
Tablets Prepared with Mucoadhesive Polymer and Starch
We hypothesized that the addition of pregelatinized starch would improve the disintegration and dissolution profiles of the compacts and increase the porosity of the compressed polymer matrix. Amounts of pregelatinized starch between 5 and 70% w/w were added to the formulation. But contrary to our expectations, all concentrations of starch increased the tablet hardness beyond 12 kg and increased disintegration time beyond 12 hr. There was no improvement in the dissolution profiles of the starch-polymer compacts compared with the pure polymer compacts; hence, the results for this group of formulations are not discussed further.
Tablets Prepared with Mucoadhesive Polymer and Lactose, with or Without PEG
Lactose was the second excipient that we expected would increase porosity of the swelling polymer gel matrix. Various amounts of lactose, between 5–80% w/w, were added to the formulations. As shown in , an increase in lactose content was inversely related to tablet hardness and disintegration time for all polymers, and the tablet thickness was directly related to lactose content. At lactose content 70% w/w and above, the tablets become increasingly friable and chip easily during removal from the die. However, tablets with relatively high lactose content and low mucoadhesive polymer concentrations provided the most desirable disintegration and dissolution profiles, while providing adequate mucoadhesive force. We added various amounts of PEG 3350 to the high lactose–containing formulations to investigate whether it improves “tabletability.” The addition of PEG 3350 indeed increased the hardness, reduced friability, and improved the surface smoothness of the tablets, while maintaining the desirable disintegration and dissolution profiles provided by the large amount of lactose in the formulations. All formulations were tested for their mucoadhesive (tensile) strength and the results are presented in . From our experiments and similar reports in literature, we concluded that formulations containing Carbopol 974P and Noveon AA-1 were comparable in their mucoadhesive capability and were superior to the other polymers we tested in our formulations.
4 Differential scanning calorimetry scans for Carbopol 974P, lactose PEG 3350, and buprenorphine, compared with a tablet compact made from these ingredients.
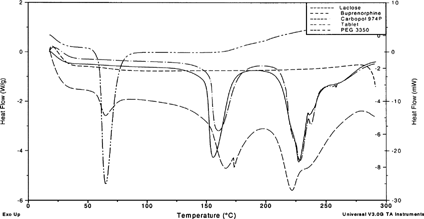
Differential scanning calorimetry (DSC) revealed no interactions between buprenorphine and the formulation excipients. shows the DSC thermograms for pure Carbopol 974P, lactose, PEG 3350, and buprenorphine, and compares them with the scan of a compressed tablet containing all these materials. There was no shift in peak position, or appearance of new or disappearance of existing peaks, indicating the ingredients are stable and do not interact with each other physically or chemically as a result of compacting stress.
5 Release profiles of buprenorphine from various formulations, each containing 8 mg buprenorphine; ▪ = tablet formulation containing 60% lactose, 10% PEG 3350, and 30% Carbopol 974P; • = tablet formulation containing 60% lactose, 10% PEG 3350, and 30% Noveon AA-1; ▴ = Carbopol 974P polymer film containing 0.5% PEG 400 as plasticizer.
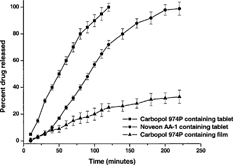
There was good correlation between disintegration times and drug dissolution profiles for these formulations. As the lactose content increased, disintegration time decreased and the drug dissolution was faster. compares the dissolution profile of buprenorphine from tablets containing 8 mg buprenorphine, 60% w/w lactose, 10% w/w PEG 3350, and 30% w/w Carbopol 974P, and tablets containing 8 mg buprenorphine, 60% w/w lactose, 10% w/w PEG 3350, and 30% w/w Noveon AA-1. All features were identical for the two formulations except for the mucoadhesive polymer, which was either Carbopol 974P or Noveon AA-1. As evidenced from the graph, the Noveon AA-1–based formulation showed a gradual but slow rate of release, which extended the dissolution time well beyond 2 hr. Similarly, drug release from the Carbopol 974P–based formulation was gradual; however, this tablet was capable of releasing the total drug content completely within the first 2 hr. Based on previously published literature evidence, the mucoadhesive force generated by either formulation in vitro was adjudged to be adequate for prolonged adhesion.
For the sake of comparison, we also included in the dissolution profile of a polymer film containing 8 mg buprenorphine and 0.5% w/w PEG 400, with the balance made up of Carbopol 974P. As shown in the graph, the film released only about 30% of its drug content over the period of 220 min with the release rate tapering off gradually with time.
CONCLUSIONS
The mucoadhesive tablet formulations produced overall superior results compared with the mucoadhesive film formulations. The high lactose, low mucoadhesive polymer Carbopol 974P and PEG 3350–containing tablet formulations exhibited the best overall results. These formulations provide a sustained release profile of the drug without any burst release effects and exhibited no drug-excipient interactions. They were capable of releasing their entire drug content within 2 hr, which provides a reasonable period for sublingual usage in vivo. Based on existing literature reports, the mucoadhesive strength of these formulations was judged adequate for localization on the sublingual mucosal surface during the anticipated 2 hr usage period.
The authors acknowledge Peter Willeitner for his technical assistance during the preliminary formulation phase of the dosage forms.
REFERENCES
- Bullingham R. E. S., McQuay H. J., Porter E. J. B., Allen M. C., Moore R. A.. 1982. Sublingual buprenorphine used postoperatively: ten hour plasma drug concentration. Br. J. Clin. Pharmacol.. 13: 665–673. [PUBMED], [INFOTRIEVE]
- Cowan A., Lewis J. W., Macfarlane I. R.. 1977. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br. J. Pharmacol.. 60: 537–545. [PUBMED], [INFOTRIEVE]
- Eriksen J., Jensen N. H., Kamp-Jensen M., Bjarno H., Friis P., Brewster D.. 1989. The systemic availability of buprenorphine administered by nasal spray. J. Pharm. Pharmacol.. 41: 803–805. [PUBMED], [INFOTRIEVE]
- Gasquet I., Lancon C., Parquet P.. 1999. Predictive factors for patient maintenance on buprenorphine high dosage treatment: A naturalistic study in primary care. Encephale.. 25: 645–651. [PUBMED], [INFOTRIEVE]
- Gutstein H. B., Akil H.. 2001. Opioid Analgesic. The Pharmacological Basis of Therapeutics, Hardman L. E., Limbird, A. G., Gilman, pp. 569–619, New York, McGraw Hill Publishing
- Iribarne C., Picart D., Dreano Y., Bail J. P., Berthou F.. 1997. Involvement of cytochrome P450 3A4 in N-dealkylation of buprenorphine in human liver microsomes. Life Sci.. 60: 1953–1964. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Kuhlman J. J., Jr., Lalani S., Magluilo J., Jr., Levine B., Darwin W. D., Johnson R. E., Cone E. J.. 1996. Human pharmacokinetics of intravenous, sublingual and buccal buprenorphine. J. Anal. Toxicol.. 20: 369–378. [PUBMED], [INFOTRIEVE]
- Kuhlman J. J., Jr., Levine B., Johnson R. E., Fudala P. J., Cone E. J.. 1998. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 93: 549–559. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Lindhardt K., Bagger M., Andreasen K. H., Bechgaard E.. 2001. Intranasal bioavailability of buprenorphine in rabbit correlated to sheep and man. Int. J. Pharm.. 217: 121–126. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Mandal T. K.. 1999. Development of biodegradable drug delivery system to treat drug addiction. Drug Dev. Ind. Pharm.. 25: 773–779. [CROSSREF], [PUBMED], [INFOTRIEVE]
- McQuay H. J., Moore R. A., Bullingham R. E. S.. 1986. Buprenorphine kinetics. Advances in Pain Research and Therapy, K. M., Foley, C. E., Inturrisi, New York, Raven Press
- McQuinn R. L., Kvam D. C., Maser M. J., Mller A. L., Oliver S.. 1995. Sustained oral mucosal delivery in human volunteers of buprenorphine from a thin non-eroding mucoadhesive polymeric disk. J. Control Rel.. 34: 243–250. [CROSSREF]
- Pontani R. B., Misra A. L.. 1983. A long-acting buprenorphine delivery system. Pharmacol. Biochem. Behav.. 18: 471–474. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Radbruch L.. 2003. Buprenorphine TDS: Use in daily practice, benefits for patients. Int. J. Clin. Pract. Suppl.. 133: 19–22; discussion 23–24
- Robert C., Buri P., Peppas N. A.. 1988. Experimental method for bioadhesive testing of various polymers. Acta Pharm. Technol.. 34: 95–98
- Stinchcomb A. L., Paliwal A., Dua R., Imoto H., Woodard R. W., Flynn G. L.. 1996. Permeation of buprenorphine and its 3-alkyl-ester prodrugs through human skin. Pharm. Res.. 13: 1519–1523. [PUBMED], [INFOTRIEVE]