Abstract
We evaluated the ability of antibody-derived delivery vehicles (DVs) to direct synthetic biotinylated oligodeoxynucleotides (ODNs) to prostate tumors in vitro. A monoclonal mouse antibody raised against prostate-specific antigen (PSA) was modified by two approaches to produce either: (1) a bispecific delivery vehicle recognizing both PSA and biotin (DVHc), or (2) an avidin-conjugated anti-PSA delivery vehicle (DVPSA). Immunoreactivity of the DVs and their ability to form complexes were tested in vitro. Although both DVs retained immunoreactivity and were able to form complexes with biotinylated ODNs, the avidin modification method was more efficient. This method may be useful for selective delivery of antisense ODNs to prostate tumors.
Keywords :
INTRODUCTION
Specific delivery of therapeutic agents, such as antisense ODNs, to tumor sites remains a problem. Once targeted, a variety of methods have been used to enhance the uptake of ODNs into tumor cells. These have included the use of liposomes, polycationic compounds, vectors, microinjections, and electroporation (Gewirtz and Glazer Citation1996; Bennett et al. Citation1992; Bergan et al. Citation1993; Kang et al. Citation1999). All of these methods improve cellular uptake but lack cell specificity, and they have largely limited in vivo application of antisense-based therapeutics to either intratumoral inoculation or ocular instillation. Unlike liposomes or polycationic agents, antibodies are specific and can target the unique proteins that exist only on tumor cells. Immunoconjugates consisting of various toxins (i.e., ricin, diphtheria toxin, saporin) bound with specific directing antibodies have been evaluated for efficacy in both in vitro and in animal models. Some have even reached the status of human clinical trials (reviewed in Ghetie and Vitetta Citation1994). It has been reported that a bifunctional antibody (reactive with both ricin and PSA) produced a 50% reduction in protein synthesis in the PC-3 cell line in vitro (Webb et al. Citation1985). Another bispecific antibody, recognizing both vinblastine and carcinoembrionic antigen, when administered to nude mice, significantly improved the inhibitory effect of this drug on tumor growth (Corvalan et al. Citation1988). Although antibodies have been employed as protein carriers for different types of drugs (Nolan and O'Kennedy Citation1990; Bera et al. Citation1999), they have not found wide utilization for ODN delivery (Leonetti et al. Citation1990; Walker Citation1995).
Previously, we utilized a bispecific antibody (reactive with PSA and biotin) for targeting biotinylated antisense ODNs to prostate tumor cells expressing PSA. A heteroconjugate antibody was constructed from anti-PSA and antibiotin mouse IgG1 monoclonals. The heteroconjugate directed biotinylated-horseradish peroxidase to glandular epithelial cell formations expressing PSA in paraffin blocks of prostatic tissue (Rubenstein et al. Citation1997). However, this design has limited binding capacity (one ODN molecule per molecule of delivery vehicle), and more efficient binding of ODN to the carrier antibody is required. This can be achieved by utilizing an avidin–biotin system. Avidin can be easily conjugated to the Fc portion of IgG antibody via carbohydrate moieties, and, unlike modification of amino-groups or disulfide bonds of the IgG, this modification does not directly affect antigen-binding sites. In addition, an avidin molecule has four identical subunits, each able to bind a single molecule of biotin. Therefore, a single IgG molecule conjugated to avidin can bind several biotinylated ODN molecules. In this study, we compared the ability of the previously constructed bispecific heteroconjugate (DV)Hc with the avidin-modified monoclonal IgG1 (DVPSA) to bind biotinylated ODNs and direct them to prostate tissue.
MATERIALS AND METHODS
Delivery Vehicles Preparation
Bispecific DVHc
Bispecific (anti-PSA/antibiotin) antibodies were prepared as previously described (Brennan et al. Citation1985). Briefly, anti-PSA and antibiotin specific mouse IgG1 monoclonals (DAKO Corp., Carpinteria, CA, USA) were reduced overnight at room temperature with 1mM 2-mercaptoethylamine in 0.1 M phosphate buffer containing 1 mM EDTA and 10 mM sodium arsenite. Reduced antibodies were then converted into stable thionitrobenzoate (TNB) derivatives by treatment with 5 mM Ellman's reagent. After 3 hr, excess reagent was removed on dextran chromatography columns (Pierce Chemical Co., Rockford, IL, USA). Fractions containing IgG derivatives were collected and TNB derivatives of anti-PSA were stored at 4°C. The TNB derivatives of antibiotin were treated with 10 mM 2-mercaptoethylamine for 30 min at room temperature to regenerate thiol derivatives. Equimolar amounts of TNB (anti-PSA) and thiol (antibiotin) derivatives were mixed and dialyzed overnight against phosphate buffer to allow the reassociation of IgG. The sample was then concentrated in Microcon−10 (Millipore Corp., Bedford, MA, USA) and IgG content was measured by radial immunodifussion (Mouse IgG LL kit, The Binding Site Inc., San Diego, CA, USA).
Avidin Conjugated DVPSA
Anti-PSA monoclonal mouse IgG1 (DAKO) was oxidized with 10 mM sodium periodate in phosphate buffered saline (pH, 6.0) for 1.5 hr in the dark. Excess reagent was removed on a dextran chromatography column (Pierce) and fractions containing IgG were incubated overnight with avidin-hydrazide (Pierce). After unconjugated avidin was removed by microcentrifugation in Microcon 100 (Millipore Corp.), the antibody concentration was determined by radial immunodiffusion (Mouse IgG LL kit, The Binding Site, Inc., San Diego, CA). All steps were carried out at room temperature. Antibody specific for Aspergillus niger glucose oxidase enzyme (DAKO) was utilized as a negative control (DVIgG), since its antigen is neither present nor inducible in mammalian tissues.
Immunohistochemistry
Paraffin-embedded sections of human prostate carcinoma (DAKO) were deparaffinized and rehydrated. To retrieve antigen, sections were treated with 10 mM citrate buffer, pH 6.0 for 6 min in a microwave oven. As a reference for PSA-specific staining, sections were incubated with unmodified monoclonal mouse anti-PSA antibody (DAKO) and immunostained utilizing a BioGenex detection system (BioGenex Laboratories Inc., San Ramon, CA, USA). The sequence of reagent application was streptavidin-conjugated goat antimouse antibody, biotinylated alkaline phosphatase, and chromogenic substrate (Fast Red). For DVHC the procedure was different. DVHC was preincubated with biotin-conjugated HRP and anti-HRP–rabbit antibody (1:250, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), then applied onto the sections. The immune complex was detected with streptavidin-conjugated goat antirabbit antibody, followed by biotinylated alkaline phosphatase, and Fast Red dye.
Delivery vehicles (DVIgG, DVPSA) were preincubated for 2 hr at 37°C with biotin-conjugated HRP (dilution 1:250 in PBS, Pierce Chemical Co.). The mixture was then applied over the sections, which were pretreated with hydrogen peroxide for 20 min to block endogenous peroxidase. After a 1 hr incubation at 37°C, sections were thoroughly washed in PBS, stained with 3-amino-9-ethylcarbazol substrate (AEC), and counterstained with hematoxylin.
Antisense ODNs
Two HPLC-purified antisense ODNs were utilized in this study: a 39 mer ODN with random sequence and a 39 mer ODN with a sequence complementary to the translation initiation site of mRNA encoding human epidermal growth factor receptor (EGFR), a factor frequently overexpressed in prostate tumors. The ODNs were modified to have phosphorothioate at three bases at both ends and labeled with biotin at 5′-end. For fluorescence experiment ODN was also labeled with FITC. ODN's synthesis and all modifications were performed by Operon Technologies Inc. (Alameda, CA, USA).
ODN 3′-End Labeling
Synthetic ODNs were labeled with 35S-ddATP (NEG-057H, DuPont/NEN Life Science Products Inc., Boston, MA, USA) according to a protocol provided with the oligonucleotide 3′ end labeling system (NEP-100, DuPont/NEN Products). Briefly, 15 nM of ODN was incubated with 25 μ Ci of 35S-ddATP (specific activity 1000 Ci/mMol) in the presence of terminal deoxynucleotidyl transferase (36 units per reaction) in sodium cacodylate buffer (pH 7.2) containing dithiothreitol and CoCl2. After 2 hr at 37°C, the reaction was stopped with NENSORB Reagent A, and labeled ODN was separated from unincorporated radioactive nucleotides using Nucleic Acid Purification Cartridges (NLP-022X, DuPont/NEN Products). Labeled ODN was typically recovered in 500 μ l of elution buffer (provided with cartridges). Three μ l of purified ODN was reserved for determination of specific radioactivity in a liquid scintillation counter (LSC).
Mobility Shift Assay
Constant and uniform amounts of biotinylated 35S-ODN with random or EGFR specific sequence (130000 cpm/9 nM/reaction) were incubated with approximately 6 μ g of delivery vehicle (DVIgG, DVHC, or DVPSA). After 1.5 hr incubation at 37°C, the mixture containing ODN-DV complex was subjected to electrophoresis on native 12% PAGE. Samples were run at a constant current of 30 mA in a Mini-Protean II Dual Slab Cell (BioRad Laboratories, Hercules, CA, USA). To detect 35S-ODNs the resulting gel was dried and placed onto Kodak X-Omat imaging film (Eastman Kodak, Rochester, NY, USA) in a cassette. After a 4-day exposure at −50°C the film was developed and analyzed using SigmaScan Software (SPSS Inc., Chicago, IL, USA).
Immunofluorescence
For immunofluorescence, DVIgG and DVPSA were preincubated with an excess of biotinylated ODN carrying FITC label to allow ODN-DV complex formation. Tissue sections of paraffin-embedded prostate carcinoma were processed as described for immunohistochemistry and incubated for 1 hr in the solution containing ODN-DV complexes. Sections were washed in PBS, mounted in VECTASHIELD Mounting Media (Vector Lab, Inc., Burlingame, CA) and viewed under a fluorescent microscope. Control sections were incubated in the solution that contained ODN only.
RESULTS
Construction of Prostate-Specific Delivery Vehicles
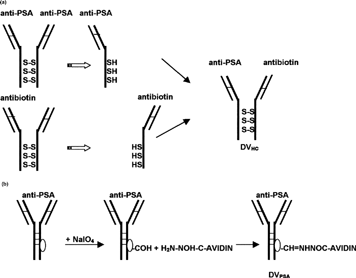
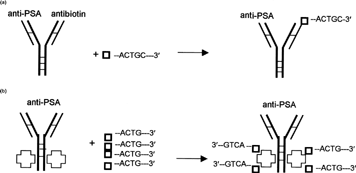
Two types of delivery vehicles specific for human PSA were constructed for this study: DVHC and DVPSA (, respectively). These DVs were comprised of PSA-specific antibody modified to bind biotinylated molecules. DVHC was prepared from antihuman PSA and antibiotin-specific monoclonal antibody (DAKO), as shown in . DVPSA was prepared from the same anti-PSA antibody (DAKO) utilized for DVHC preparation by conjugation of an avidin-hydrazide to the carbohydrate moieties present on the Fc portion (). As specified by the manufacturer (DAKO), the initial anti-PSA monoclonal antibody reacts with secretory and ductal epithelium of both normal and neoplastic prostatic tissue. The antibiotin antibody reacts with both free biotin and biotin conjugated to other molecules such as DNA probes, ODNs, or proteins. All antibodies utilized for this study were monoclonal IgG1, of mouse origin, possessing κ light chains.
1 Diagram of delivery vehicles preparation. (a) Preparation of bispecific delivery vehicle (DVHc) from anti-PSA and antibiotin monoclonal mouse IgG1: Antibodies were reduced to dissociate and then were allowed to reform SH bonds. (b) Preparation of avidin-conjugated delivery vehicle (DVPSA) from monoclonal mouse IgG1. Carbohydrate residue on the Fc portion of antibodies was oxidized to generate aldehyde, which in turn reacted with avidin-hydrazide.
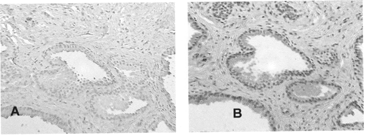
Immunoreactivity of Constructed DVs
The results for both unmodified anti-PSA antibody and DVHC, are shown in , respectively. Red staining of the glandular epithelium and secretions observed in sections treated with HRP-DVHC complex () correspond to staining demonstrated by unmodified anti-PSA antibodies (). These sections also displayed intense stromal staining. The results for avidin-conjugated DVIgG and DVPSA are presented in . No staining was observed in negative control sections treated with HRP-DVIgG (); however, as expected, those treated with HRP-DVPSA demonstrated glandular staining ().
2 Immunoreactivity of heteroconjugate delivery vehicle (DVHc). (A) Tissue sections of paraffin-embedded human prostate carcinoma were treated with unmodified anti-PSA primary antibody followed by streptavidin-conjugated secondary antibody, biotinylated alkaline phosphatase and chromogenic substrate (Fast Red). Staining obtained from these sections served as a reference for PSA expression. (B) DVHC was preincubated with biotinylated HRP and applied to the sections. A three-step amplification system was utilized to detect complex localization as described in the Materials and Methods section of this article. Complexes were visualized with Fast Red. Red staining indicates PSA.
ODN-DV Complex Formation
All constructed DVs should complex with biotinylated ODN: DVHC via its antibiotin-specific combining site, DVIgG, and DVPSA via avidin conjugated to carbohydrates on the Fc portion (, respectively). To evaluate complex formation between ODN and DV we studied changes in ODN's mobility in native 12% polyacrylamide gels. To confirm that ODN-DV complex formation was independent of the ODN's specific complementary sequence, two different 35S-ODNs were utilized, (1) one with a random sequence and (2) another with a sequence specific to EGFR mRNA.
4 Diagram of complex formation between ODN and delivery vehicles. (a) Complex between bispecific delivery vehicle (DVHC) and biotinylated ODN. (b) Complex between avidin conjugated delivery vehicle (DVPSA) and biotinylated ODN (□–ACTG-3′—Biotinylated ODN, —Avidin).
As revealed by autoradiography, all DVs altered the mobility of both ODNs (). 35S-ODNs applied to the gel alone moved rapidly and formed bands at the bottom of the gel. These bands are seen on the autoradiogram and represent unbound biotinylated ODN (lane 4, Random; lane 5, EGFR specific). The addition of either DV (DVPSA, DVIgG, or DVHc) to one of the ODNs resulted in changes in the bands position (lane 1–3, Random; lane 6–8, EGFR specific). While DVPSA and DVIgG yielded strong shifted bands at the top of the gel with traces of unbound ODN at the bottom of the gel (lanes 1 and 2, Random; lanes 6 and 7, EGFR), DVHC resolved into two distinct bands: (1) a noticeable but very weak band corresponding to the ODN-DV complex and (2) a very strong band corresponding to unbound ODN (lane 3, random; lane 8, EGFR).
5 Mobility shift assay. Biotinylated ODNs were radioactively labeled with 35S-ddATP and mixed with unlabeled ODN to adjust concentration. A constant amount of ODN (130000 cpm/0.9 nM/binding reaction) was incubated with DVPSA, DVIgG, or DVHC (∼5 μ g). Incubation was carried out at 37°C for 1.5 hr. Twelve percent nondenaturing polyacrylamide gel was run at constant current (100A). The gel was dried and exposed to X-OMAT film (Kodak) for 4 days. As a control ODN alone (Lane 4, Random; [ R], lane 5, EGFR specific) were included in the gel. Lanes 1–3: 35S-Random ODN incubated with DVs. Lanes 6–8: 35S-EGFR-specific ODN incubated with DVs.
![5 Mobility shift assay. Biotinylated ODNs were radioactively labeled with 35S-ddATP and mixed with unlabeled ODN to adjust concentration. A constant amount of ODN (130000 cpm/0.9 nM/binding reaction) was incubated with DVPSA, DVIgG, or DVHC (∼5 μ g). Incubation was carried out at 37°C for 1.5 hr. Twelve percent nondenaturing polyacrylamide gel was run at constant current (100A). The gel was dried and exposed to X-OMAT film (Kodak) for 4 days. As a control ODN alone (Lane 4, Random; [ R], lane 5, EGFR specific) were included in the gel. Lanes 1–3: 35S-Random ODN incubated with DVs. Lanes 6–8: 35S-EGFR-specific ODN incubated with DVs.](/cms/asset/51c7ae95-87ff-457b-8153-d4bf7358db90/idrd_a_16453_f0005_b.gif)
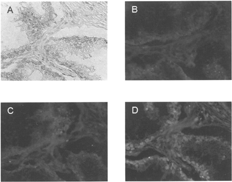
In Vitro Localization of ODN-DV Complexes
In this experiment only avidin-modified delivery vehicles (DVIgG and DVPSA) were employed. Light microscopy illustrates morphology of the neoplastic glandular epithelium and stromal connective tissue (). Fluorescence was observed only in sections treated with ODN-DVPSA complexes and was localized to the prostatic epithelial cells of acinar glands (). Neither biotinylated ODN alone nor ODN-DVIgG complex demonstrated specific fluorescence of epithelial cells ().
DISCUSSION
Immunostaining of glandular epithelium of human prostate carcinoma indicated that prostate-specific delivery vehicles DVPSA and DVHC retained immunoreactivity and were able to localize biotinylated HRP to the antigen-expressing tissue, while negative control DVIgG demonstrated no staining. However, a 3-step amplification system was required for the detection of biotinylated HRP localized to the paraffin-embedded tissue sections by DVHC. These sections also demonstrated intense staining of stromal tissue, which could be nonspecific due to the amplification system utilized in this assay. Alternatively the sensitivity of the amplification system may allow detection of epithelial cancer cells infiltrating the stroma.
In the mobility shift experiment, the shifted bands were observed in all samples containing ODN preincubated with DV, suggesting a formation of the ODN-DV complex. However, DVPSA and DVIgG were more efficient in binding biotinylated ODNs (, lanes 1–2 and 6–7) and produced more stable complexes than the DVHC (, lanes 3 and 8). It is likely that a greater quantity of DVHC is required to bind to the applied amount of ODN, since this delivery vehicle has only one immunoreactive site against biotin () and is able to bind only one molecule of biotinylated ODN. Furthermore, ODN–DVHC complexes could be less stable under these experimental conditions, and a dissociation of complex may have occurred during electrophoresis. Lastly, the specificity of the applied ODN did not affect its binding to the DV since there was no difference in band position between random sequence and EGFR-specific ODN. Although unbound random and EGFR-specific ODNs produced slightly different patterns on the gel (, lanes 4 and 5, respectively), this is more likely due to differences in the ODN's molecular weight. Analysis of human prostate carcinoma sections treated with fluorescently labeled ODN-DV complexes further suggests that DVPSA is able to localize biotinylated ODN to PSA-expressing prostate epithelial cells.
In summary, both modifications produced delivery vehicles, which retained immunoreactivity and were able to target biotinylated HRP to prostate carcinoma tissue. Also, as appeared from mobility shift assay, all DVs were capable of forming complexes with biotinylated ODNs. However, as theorized, avidin-modified antibodies (DVPSA and DVIgG) were more efficient. In addition, ODN–DVPSA complexes demonstrated specific in vitro localization to PSA-expressing prostatic epithelial cells.
This approach may be useful for targeting ODNs directly to the tumor site in vivo, particularly to prostate tumors. The choice between prostate-tumor–specific targets becomes very important to the future development of antibody-derived DVs. PSA is a unique organ-specific protein produced by the prostatic epithelium and is normally found in low concentrations in male plasma. Plasma PSA levels are usually elevated in conjunction with benign prostatic hyperplasia (BPH), malignancy (adenocarcinoma), and also with surgical trauma. This 33kDa glycoprotein, which belongs to the kallikrein family and exhibits serine protease activity, is recognized as a marker for prostate cancer and can be used as a screening agent. PSA was suggested as a target to improve selective delivery of drugs to the prostate tumor cells and was successfully tested on human prostatectomy specimens (Sinha et al. Citation1996). However, in vivo utilization of this target might not be very efficient since PSA is a secretory protein.
The more recently discovered and characterized prostate-specific membrane antigen (PSMA) is also produced by prostatic epithelium and might present a better target (reviewed in Pinto and Heston Citation1999). Its expression is enhanced in higher grade prostate cancers, metastatic tumors, and particularly in hormone-refractory prostate carcinoma (Wright et al. Citation1995; Israeli et al. Citation1994; Diamond et al. Citation1995). More importantly, in contrast to PSA, PSMA is an integral protein and recently has been identified as a receptor that can undergo constitutive and antibody-mediated internalization (Liu et al. Citation1998). Furthermore, a monoclonal antibody specific for human PSMA has been generated, which recognizes both normal and malignant prostatic epithelium (Horoszewich et al. Citation1987). The modification of this antibody with 111In (CYT-356) is utilized in a diagnostic test (ProstaScint) developed by Cytogen Corporation, which identifies prostate cancer recurrence and metastatic disseminations in patients (Elgamal et al. Citation1998; Petronis et al. Citation1998; Khan et al. Citation1998). These facts make PSMA a very attractive target for the localization of toxins, drugs, or ODNs directly to the prostate tumor site. Compounds (in our case antisense ODN) may be directly localized by an antibody specific for PSMA and subsequently be internalized with this receptor-like protein. In a similar manner a recent study reported that an antibody to the transferrin receptor improved delivery of antisense ODN to human glioblastoma cells in vitro (11). Further in vivo studies are under way to evaluate tissue distribution and tumor uptake of ODNs targeted to prostate tumor by PSA- and PSMA-specific DVs.
This research was supported by the Blum Kovler Foundation, the Cancer Federation, the Sternfeld family, and contributions made to the Maurice Rubenstein memorial fund at the Hektoen Institute.
This work was submitted by Yelena Mirochnik in partial fulfillment for the PhD from Rush University, Chicago, IL.
REFERENCES
- Bennett C. F., Chiang M. Y., Chan H., Shoemaker J. E., Mirabelli C. K.. 1992. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol. Pharmacol.. 41: 1023–1033. [PUBMED], [INFOTRIEVE]
- Bera T. K., Viner J., Brinkmann E., Pastan I.. 1999. Pharmacokinetics and antitumor activity of a bivalent disulfide-stabilized Fv immunotoxin with improved antigen binding to erbB2. Cancer Res.. 59: 4018–4022. [PUBMED], [INFOTRIEVE]
- Bergan R., Connel Y., Fahmy B., Neckers L.. 1993. Electroporation enhances c-myc antisense oligonucleotide efficacy. Nucleic Acids Res.. 21: 3567–3573. [PUBMED], [INFOTRIEVE]
- Brennan M., Davison P. F., Paulus H.. 1985. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science. 229: 81–83. [PUBMED], [INFOTRIEVE]
- Corvalan J. R. F., Smith W., Gore V. A.. 1988. Tumor therapy with vinca alkaloids targeted by a hybrid-hybrid monoclonal antibody recognizing both CEA and vinca alkaloids. Int. J. Cancer. 2(S): 22–25
- Diamond S. M., Fair W. R., Heston W. D. W.. 1995. Modulation of prostate-specific membrane antigen (PSM) expression in vitro by cytokines and growth factors. Proc. Am. Assoc. Cancer Res.. 36: 643a
- Elgamal A. A., Troychak M. J., Murphy G. P.. 1998. ProstaScint scan may enhance identification of prostate cancer recurrences after prostatectomy, radiation, or hormone therapy: analysis of 136 scans of 100 patients. Prostate. 37: 261–269. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Gewirtz A. M., Glazer P. M.. 1996. Facilitating oligonucleotide delivery: helping antisense deliver on its promise. Proc. Natl. Acad. Sci. USA. 93: 3161–3163. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Ghetie V., Vitetta E.. 1994. Immunotoxins in the therapy of cancer: from bench to clinic. Pharmac. Ther.. 63: 209–234. [CROSSREF]
- Horoszewich J. S., Kawinski E., Murphy G. P.. 1987. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res.. 7: 927–936
- Israeli R. S., Powell C. T., Corr J. G., Fair W. R., Heston W. D. W.. 1994. Expression of the prostate-specific membrane antigen. Cancer Res.. 54: 1807–1811. [PUBMED], [INFOTRIEVE]
- Kang S. H., Zirbes E. L., Kole R.. 1999. Delivery of antisense oligonucleotides and plasmid DNA with various carrier agents. Antisense Nucleic Acid Drug Dev.. 9: 497–505. [PUBMED], [INFOTRIEVE]
- Khan D., Williams R. D., Manyak M. J., Haseman M. K., Seldin D. W., Libertino J. A., Maguire R. T.. 1998. 111-indium capromab pendetide in the evaluation of patients with residual or recurrent prostate cancer after radical prostatectomy. The Prosta Scint Study Group. J. Urology. 159: 2041–2046. [CROSSREF]
- Leonetti J. P., Machy P., Degols G., Lebleu B., Leserman L.. 1990. Antibody-targeted liposomes containing oligodeoxynucleotides complementary to viral RNA selectively inhibit viral replication. Proc. Natl. Acad. Sci. USA. 87: 2448–2451. [PUBMED], [INFOTRIEVE]
- Liu H., Rajasekaran A. K., Moy P., Xia Y., Kim S., Navarro V., Rahmati R., Bander N. H.. 1998. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res.. 58: 4055–4060. [PUBMED], [INFOTRIEVE]
- Nolan O., O'Kennedy R.. 1990. Bifunctional antibodies: concept, production and applications. Biochim. Biophys. Acta. 1040: 1–11. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Petronis J. D., Regan F., Lin K.. 1998. Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clin. Nucl. Med.. 23: 672–677. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Pinto J. T., Heston W. D. W.. 1999. Prostate specific membrane antigen, a unique glutamate carboxypeptidase: a review of recent findings. Prostate. 1: 15–26
- Rubenstein M., Mirochnik Y., Guinan P.. 1997. Delivery of biotinylated agents to prostate cancer cells utilizing hybrid monoclonal antibodies. In Oligonucleotide and Gene Therapy-Based Antisense Therapeutics with New Applications to Genomics, W., Hori. IBC Library Series, Southborough, MA, 4.2.1–4.2.10
- Sinha A. A., Sackrison J. L., DeLeon O. F., Wilson M. J., Gleason D. F.. 1996. Antibody Immunoglobulin G (IgG) against human prostatic specific antigen (PSA) as a carrier protein for chemotherapeutic drugs to human prostate tumors: Part 1. A double immunofluorescence analysis. Anat. Rec.. 245: 652–661. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Walker I., Irwin W. J., Akhtar S.. 1995. Improved cellular delivery of antisense oligonucleotides using transferrin receptor antibody-oligonucleotide conjugates. Pharmaceut. Res.. 12: 1548–1553. [CROSSREF]
- Webb K. S., Ware J. L., Parks S. F., Walther P. J., Paulson D. F.. 1985. Evidence for a novel hybrid immunotoxin recognizing ricin A-chain by one antigen-combining site and a prostate restricted antigen by the remaining antigen-combining site: potential for immunotherapy. Cancer. Treat. Rev.. 69: 663–672
- Wright G. L., Jr., Haley C., Beckett M. L., Schellhammer P. F.. 1995. Expression of prostate specific membrane antigen in normal, benign and malignant prostate tissues. Urol. Oncol.. 1: 18–28. [CROSSREF]