Abstract
The objective of our study was to incorporate and evaluate Silymarin, a chemically defined natural hepatoprotective agent, in lipid microstructured systems. Various constituents of lipid microspheres—namely, internal oily core; surfactant such as soyabean lecithin; and cosurfactants such as span 20, tween 20, tween 80, and propylene glycol—were tried in different concentrations to optimize the final formulation characteristics such as globule size range, structural integrity, sustainability, and percent drug-holding capacity. The final formulation (formulation A) was characterized with respect to size and morphology using transmission electron microscopy and laser diffraction technique. The enhanced mean percent release of 56.70 ± 2.03% was observed in 36 hr from silymarin-loaded lipid microspheres (formulation A), as compared to 18.67 ± 0.192% with silymarin solution (formulation B). Thus, a stable delivery system having synergistic hepatoprotective effect of silymarin and soyabean lecithin could successively be produced for passive targeting to the liver.
Keywords :
INTRODUCTION
It has been recognized that the real problem with the existing therapeutic molecules is not the molecules themselves but the mismanagement in their transport or distribution in the biological system. Work in this area has been going on for a long time to find a solution, which resulted in a series of varied systems along with improvization in their delivery tactics. In recent times, lipidic supramolecular systems have emerged as one of the favorites. The liposomes, niosomes, solid particulate systems, and lipid microspheres are some in the class that have found a wide range of applications. Recently, the lipid emulsions, which until now have been popular for total parenteral nutrition (TPN), have drawn attention towards delivering the drugs carried within membranous microstructures to the desired site and for effective interaction with the receptor present there (Yamaguchi Citation1994; CitationMizushima et al. 1982, 1983). Lipid emulsions are phospholipid-based structures, also referred to as lipid microspheres. They are considered a good potential substitute for the much-hyped closed bilayered, vesicular carriers (liposomes, niosomes, ufasomes, etc.) because they resemble them in many ways and could mimic their in vivo behavior. Lipid emulsion preparation of corticosteroids, nonsteroidal anti-inflammatory drugs, and prostaglandins have shown more potent activity than free drugs (CitationMizushima et al. 1982, 1983). Colloidal nature and foreignness of lipid microspheres enable them to be dispatched to the liver as soon as they get into systemic circulation. Lipid emulsions mainly composed of phospholipids can themselves serve as hepatoprotectants. Essential phospholipids are known heptatoprotectives and are available in the market for this purpose (e.g., Essentiale™, Rhone Poulenc) (Jaeschke et al. Citation1987). Equipped with desired delivery potential to hepatic sites, they are considered here to strengthen the hepatoprotective action of silymarin. Silymarin, a group of flavonoid compounds obtained from Silybum marianum, is the chemically defined mixture of 3 isomers; silybin (major isomer), silychristin, and silydianin. The drug is a unique hepatoprotective agent that has a positive effect on metabolism and physiology of liver cells, influencing their regenerative capacity due to two main actions, antioxidant and protein-restoring activities. The drug also prevents toxic and foreign substances from penetrating liver cells by stabilizing the outer membranes of liver cells (Wellington and Jarvis Citation2001; Miguez et al. Citation1994).
The present study includes the incorporation of silymarin into lipid microspheres (with most appropriate characteristics so that it acts as a suitable carrier system to transport silymarin effectively to liver), in vitro characterization, and stability studies.
MATERIALS AND METHODS
Materials
Silymarin was gift from Ranbaxy Laboratories Ltd. (Gurgaon, India), soyabean lecithin was generous sample from Natterman Phospholipids (Germany), and span 20, tween 20, tween 80, and propylene glycol were purchased from S.D. Fine Chemicals (Mumbai, India). Soyabean oil, castor oil, and olive oil were obtained from Protina (India). All other materials used in the study were of analytical grade.
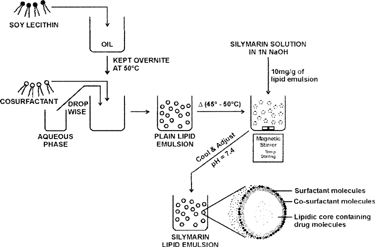
General Technique for Preparation
Soyabean lecithin was dispersed well in soyabean oil and allowed to dissolve completely by keeping it overnight at 50°C. The cosurfactants (hydrophilic/lipophilic) were added to the oily or aqueous phase as per their solubility. The aqueous phase was added to the oily phase dropwise with simple agitation to obtain lipid emulsion. Silymarin solution (10% w/v in 1 M sodium hydroxide) was added to the prepared emulsion so as to obtain the final concentration of 10 mg/g of lipid emulsion at a temperature of 50°C under stirring with a magnetic stirrer. After 10–15 min, the pH was adjusted to 7.4 with 1 N orthophosphoric acid () (Moreno Citation2001).
Formulation Component Selection
First, the selection of oil was made by taking various oils (soyabean oil, castor oil, and olive oil) in combination with surfactant (soyabean lecithin) and cosurfactant (tween 80) to prepare plain lipid emulsion (). Then the surfactant (soyabean lecithin) level was optimized by preparing different formulations () while keeping the amount of drug, oil, and cosurfactant constant. Further various cosurfactants at their different concentrations were tried in order to select the suitable cosurfactant and its optimum concentration while keeping the amount of drug, oil, and lecithin constant (). Finally, the drug level in the formulation was optimized (). The various constituents of lipid emulsion, namely internal oily core, lecithin as surfactant, and cosurfactants were tried in different concentrations to optimize the final formulation characteristics such as globule size range, structural integrity, sustainability, and percent drug-holding capacity. However, the emphasis was given to emulsion sustainability and drug-holding capacity. The lipid emulsion that gave maximum sustainability and percent drug-holding capacity (Formulation A) was subjected to further studies.
Effect of various oils on the plain lipid emulsion formation and stability characteristics at room temperature (20 ± 5°C)
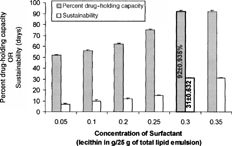
2 Effect of varying concentrations of surfactant (lecithin) on percent drug-holding capacity and sustainability of silymarin lipid emulsion.
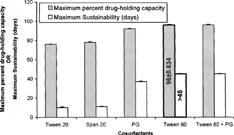
3 Effect of different cosurfactants on maximum percent drug-holding capacity and maximum sustainability of silymarin lipid emulsion.
Structure and Morphology
Morphology and structure of lipid microspheres was determined using Transmission electron microscopy (TEM), and photomicrographs were taken at suitable magnifications ().
Size and Size Distribution Measurements
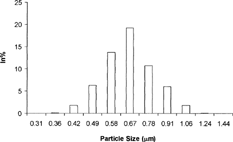
Size of the globules was determined for formulation A with the help of Master sizer (Malvern instruments Ltd., UK) using Laser light-scattering technique ().
Stability Studies
The stability of prepared emulsion was tested by exposing them to different storage conditions, i.e., at room temperature (20 ± 5°C), in refrigerator (4°C), and at higher temperature (40 ± 5°C) for 45 days () and also subjecting to repeated centrifugation (15, 30, 45, 60, 120, 180, and 240 min) at 4000 rpm (). Sustainability of the formulations A at different pH (2–12) was also determined (). Drug leakage behavior of the formulation A has been tested by measuring the percent drug remaining entrapped in the oily phase at various intervals of up to 35 days (). The entrapped drug within the microdroplets was determined by preparing a suitable dilution of the lipid emulsion in methanol, and the initial drug content was determined spectrophotometrically. Then the same emulsion was passed through membrane filter (0.45 μ) to separate the unentrapped drug, and after suitable dilution drug content was again determined spectrophotometrically. Difference between two values gave us the amount of unentrapped drug or the drug leaked.
Oil phase separation studies of formulation A at room temperature (20 ± 5°C), at higher temperature (40 ± 5°C), and at lower temperature (4°C)
Effect of centrifugation (4000 rpm) on oil phase separation
Effect of different pH on formulation A (at 20 ± 5°C)
Drug leakage studies of formulation A at room temperature (20 ± 5°C) during storage period
In Vitro Release Rate Studies
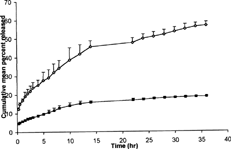
These studies were carried out through dialysis bag using dissolution apparatus USP 1. The treated dialysis membrane was soaked in diffusion medium (Phosphate buffer pH = 7.4) up to 48 hr and refrigerated till use. The membrane was washed with distilled water before use. The formulations A and B (), each equivalent to 20 mg of silymarin, were filled in different dialysis bags and placed in basket of dissolution apparatus USP 1. The study was run at 50 rpm, and the dissolution vessel was maintained at 37 ± 0.5°C. An aliquot of 5 ml of samples was withdrawn at suitable time intervals till 36 hr and replaced with same amount of medium to maintain the volume of dissolution medium as 900 ml. The samples were quantitated by UV spectrophotometer at 287 nm ().
Composition of silymarin formulations
RESULTS AND DISCUSSION
Formulation Considerations and Process Conditions
The various process parameters, namely stirring time, stirring speed, and method of addition of one phase to another, were optimized to prepare silymarin lipid emulsion. The optimal time for stirring at 3000 rpm was found to be 2 hr, and the rate of addition of aqueous phase to the oily phase was noted to be the crucial step in preparing lipid emulsion. The initial 25% of the total aqueous phase should be added dropwise. Next drop is added only when the previous one is homogenously mixed.
Formulation Component Variables
Among the various oils (castor oil, olive oil, and soyabean oil), the soyabean oil in the phase volume ratio of 8.8:1 with respect to aqueous and nonaqueous phase, has shown maximum stability of the formed emulsion ().
The suitability of soyabean oil for the formation of emulsion may be attributed to its constituents long-chain fatty acids, (i.e., arachidonic acid and linoleic acid) in combination with phospholipids, which would have helped in forming efficient interfacial barrier in the emulsion. In the formulation made up of different amounts of lecithin as surfactant while keeping other components, i.e., drug (1%) and soyabean oil (5%), constant, the most stable (i.e., sustainability of 31 days ± 0.632) with maximum percentage of drug-holding capacity of 92 ± 0.935 was one wherein the lecithin level was kept at 0.3 g/25 g of total lipid emulsion. The enhanced stability in this case may be related to the complete covering and layering of phospholipids around the oily core, which would have sufficed the coalescence barrier properties. Beyond this level sustainability remains unchanged ().
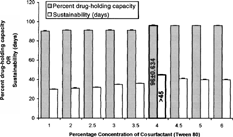
The formulations with different types of cosurfactants with their variable concentration levels, while keeping the other components constant (), exhibited different stability and drug-holding capacity. The maximum sustainability of 15 ± 0.684 days and 15 ± 0.329 days and percent drug-holding capacity of 76 ± 0.424% and 78 ± 0.954% were observed at 4% concentration of tween 20 and span 20, respectively.
Propylene glycol and tween 80 were found to improve the emulsification efficacy of lecithin. Propylene glycol showed maximum sustainability of 37 ± 0.452 days and percent drug-holding capacity of 92 ± 0.835 at 10% concentration. Due to its short chain length, propylene glycol can diffuse rapidly between the different phases, which ensures the flexibility of interfacial film enough to deform readily around the oily droplets (Miyata et al. Citation1996).
The optimal level of tween 80 was found to be 4% of the total lipid emulsion, which could produce the sustainability of more than 45 days and percent drug-holding capacity of 96 ± 0.634. The critical level of tween 80 may be justified for its role as auxuillary surfactant around the oily core, which forms mixed interfacial film with lecithin around the oil core, making its surface hydrophilic (by virtue of its long chain of polyoxyethylene glycols) and consequently preventing the coalescence (Karison Citation1984; Heller and Pugh Citation1960). The enhanced percent drug-holding capacity (i.e., 96 ± 0.628) with tween 80 may be accounted to its own drug-accommodating domains. At the same time, increasing the amount of tween 80 above this level brings the sustainability a little low, i.e., (40 days ± 0.628), which may be due to leaching out of the excess tween 80 that led to the phase separation (). The combination of the selected concentrations of tween 80 (4%) and propylene glycol (10%) also improved the emulsifying efficacy of lecithin, but to the same extent as with tween 80 (4%) alone, which was chosen as the best cosurfactant out of all other cosurfactants studied.
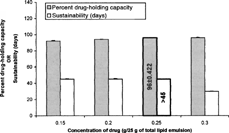
Based on the results of the above studies, the lipid, surfactant, and cosurfactant levels of this formulation were fixed, and the effect of drug level in these microspheres was studied for sustainability and percent drug-holding capacity. Various formulations were prepared with different amounts of the drug, i.e., 0.15/25 g (0.6%), 0.20/25 g (0.8%), 0.25/25 g (1%), and 0.3/25 g (1.2%), respectively (). It was noted that with the increase in amount of the drug till 1%, percent drug-holding capacity of lipid emulsion was increased. The enhancement in the drug loading may be justified to the solubilizing and drug-accommodating capacity of the lipidic core along with mixed surfactant and cosurfactant domains. But on further increasing the drug level, the sustainability of the prepared emulsion was lowered to 30 days ± 2.84, which may be due to the saturation of the oily and aqueous domains, and thus the drug in excess of the holding capacity of the lipid emulsion could not be accommodated. Based on the results of sustainability and percent drug-holding capacity, the formulation A was found to be optimum and used for further characterization studies.
Shape, Size, and Size Distribution Measurements
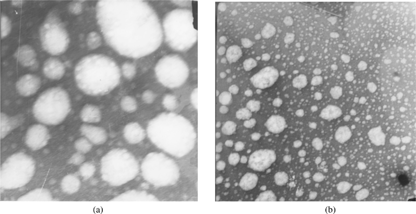
Results of transmission electron microscopy (as can be seen from the photomicrographs in ) revealed that the lipid emulsion microdroplets were almost spherical in shape, having the appearance of a coated surface. A coated surface can be identified as flexible interfacial film formed with the emulsifier and coemulsifier.
The size distribution range was from 0.31–1.24 μm, with the most frequent size of 0.632–0.732 μm. The median diameter (the diameter for which 50% of the particles measured were less than that size) was found to be 0.46 μm. 10% of the measured particles were less than 0.33 μm, while 90% of the measured particles were less than 0.89 μm. The specific surface was found to be 10.7592 sq. m/g.
The submicron range and a good uniformity in the microdroplets size may be ascribed to the composition of the prepared lipid emulsion in which the optimum level of cosurfactant (tween 80) intercalates with that of surfactant (lecithin) to provide the microdroplets of appropriately chosen oily core with interfacial barrier of desired strength and efficiency.
Stability Studies
Formulation A was found to be appreciably stable at room temperature (20 ± 5°C) and at lower temperature (4°C). At higher temperature (40 ± 5°C), the lipid emulsion was stable for 35 days. After that a little bit of phase separation, i.e., 0.8 and 1.3%, was seen on the fortieth day and forty fifth day of the storage period, respectively (). This observation may well be accounted for by the fact that after 40 days the interfacial film might have become destabilized at higher temperature due to the thermal energy effect on the fluid state. Hence, possible conformational changes in the lecithin layer at the surface of oily droplets might have led to the demixing of the oil phase with aqueous phase, resulting in phase separation.
The emulsion stability was adjudged to be acceptable, as there was only a small loss of oily phase after 60 min of centrifugation at 4000 rpm. And at repeated centrifugation up to 240 min there was only minimal additional loss of oily phase ().
All the formulations were stable in the pH range of 6–8 (). Below pH 6 and above pH 8, the lipids present in the emulsion might have been affected and thus were not able to form the effective interfacial film around the oil globules.
It was observed that in formulation A, at room temperature (20 ± 5°C), the drug remained entrapped within oily phase, and only 3% of the drug was found to be leaked out after the span of 35 days. ().
In Vitro Release Rate Studies
The prepared silymarin lipid emulsion (Formulations A) was compared with the silymarin solution (Formulation B) with respect to their drug release behavior.
The mean percent release of 56.70 ± 2.039 was observed in case of formulation A after 36 hours, in which time period the mean percent release was only 18.67 ± 0.192 for the silymarin solution ().
The faster and the better drug release from the silymarin lipid emulsion as compared with silymarin solution might be due to the fact that the surfacial presence of the surfactants in the prepared silymarin lipid emulsions help to carry the drug in the solubilized form towards the outer domain of oily sphere and thus can better transport it from the surface of the system.
This suggests that the prepared silymarin lipid microspheres in submicron range can carry the drug in a way to better increase the bioavailability of the drug so that it can reach the system in the required concentration.
CONCLUSION
From this study, enhanced release of silymarin from silymarin-loaded microspheres was observed as compared to silymarin solution. It was concluded that silymarin can be incorporated successfully in lipid microspheres having the desired characteristics of size, shape, and entrapment with reasonable stability. The twin effect from phospholipids and silymarin, while they are placed and structured into a suitable carrier system, is foreseen, which could passively target silymarin to the liver with lesser drug loss en route.
REFERENCES
- Heller W., Pugh T. L.. 1960. Steric stabilization of collidal solutions by adsorption of flexible macromolecules. J. Polym. Sci.. 203–217
- Jaeschke H., Werner C., Wendel A.. 1987. Disposition and Hepatoprotection by phosphatidyl Choline liposomes in mouse liver. Chem. Biol. Interact.. 64: 127–137. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Karison P.. 1984. Kurzes Lehrbuch der Biochemic für Mediziner and Naturwissenschaftler. Thieme Verlag. 134–141
- Miguez M. P., Anundi I., Sainz-Pardo L. A., Lindros K. O.. 1994. Hepatoprotective mechanism of Silymarin: No evidence for involvement of Cytochrome P450 2E1. Chem. Biol. Interact.. 91: 51–63. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Miyata I., Miyamoto H., Yonese M.. 1996. Effect of chain lengths of n-Alcohol on the formation of Single-phase microemulsions in n-Heptane/n-Alcohol/sodium Dodecyl Sulfate/water systems. Chem. Pharm. Bull.. 1049–1055
- Mizushima Y., Hamano T., Yokoyama K.. 1982. Use of lipid emulsions as a novel carrier for consticosteroids. J. Pharm. Pharmacol.. 34: 49–50. [PUBMED], [INFOTRIEVE]
- Mizushima Y., Wada Y., Etoh Y., Watanabe K.. 1983. Antiinflammatory effect of indomethacin ester incorporated in lipid microsphere. J. Pharm. Pharmacol. 35: 398–399. [PUBMED], [INFOTRIEVE]
- Moreno M. A., Frutos P., Ballesteros M. P.. 2001. Lyophilized lecithin based oil-in-water microemulsions as new and low toxic delivery system for Amphotericin B. Pharm. Res.. 18: 344–351. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Wellington K., Jarvis B.. 2001. Silymarin: A review of its clinical properties in the management of hepatic disorders. Bio. Drugs.. 15: 465–489
- Yamaguchi T.. 1994. Lipid microspheres for drug delivery from the pharmaceutical viewpoint. Crit. Rev. Ther. Drug Carrier Sys.. 11: 215–229