Abstract
Aquasomes, a new drug delivery system comprised of surface-modified nanocrystalline ceramic carbohydrate composites, was developed to serve as haemoglobin carrier for oxygen delivery. The hydroxy-apatite ceramic core was prepared by coprecipitation and self-precipitation and coated with various sugars like cellobiose, maltose, sucrose, and trehalose. The effect of drying methods, i.e., air drying, vacuum drying, and lyophilization, on the degree of binding was studied by concanavalin-induced aggregation method. Haemoglobin was adsorbed over the sugar-coated ceramics, and percent loading was estimated by benzidine method. The adsorption of sugars on calcium hydro-apatite powder and haemoglobin adsorption on sugar-adsorbed ceramic followed both Freundlich and Langmuir isotherm. The haemoglobin aquasome formulations (equivalent to 7.5% Hb) were suspended in a phosphate buffer containing 7.5% w/v albumin and 0.01% w/v lecithin, and they were evaluated for oxygen-carrying capacity, which was found to be similar to fresh blood. The Hill coefficients were found to be fairly good for its use as oxygen carrier. The haemoglobin aquasome formulations did not induce haemolysis of red blood cells nor alter the blood coagulation time. The haemoglobin content of the formulation remained unchanged on storage for 30 days. The haemoglobin desorption was fairly low under shear conditions, indicating good stability of formulation in biological system. During in vivo study in rats the survivals were monitored as function of hematocrit in rats receiving isovolemic exchange transfusion. Arterial blood pressure and heart rate did not change significantly in animals transfused with aquasomal suspension on 50% exchange transfusion.
INTRODUCTION
Colloidal drug delivery systems (CDDS) are particulate or vesicular dosage form in nanometer size range. They include liposomes (Tsuchida et al. Citation1985; Yuasa et al. Citation1986), niosomes (Namdeo and Jain Citation1996), nanospheres (Kreuter Citation1991), multiple emulsion (Lieberman et al. Citation1988) and ceramics (Kossovsky et al. Citation1996). CDDS are essentially required for effective transportation of loaded drug to the target site.
The blood serves a number of important functions such as delivery of oxygen to tissues, host defense, transport of hormones and nutrients, hemostatis, disposal of metabolic wastes, etc. The most critical function of blood is oxygen transfer because in deprived oxygen supply, the cells, tissues, and organs may be irreversibly damaged and lead to death (Tortora and Anagnostakos Citation1989). The limitations of whole human blood are that prior to blood transfusion the donor blood must be crosschecked and typed for every patient, resulting in transfusion delay. Moreover, the extended storage life of blood at refrigerated condition is about 42 days, which also limits the use of blood transfusion (Goldstein Citation1991).
A number of approaches have been developed that can be used to transport and deliver oxygen effectively and safely. These include haemoglobin (Goldstein Citation1991; Perutz et al. Citation1978), crosslinked haemoglobin (Arnone Citation1974; Perutz et al. Citation1972), and perfluorochemicals. The use of haemoglobin (Hb) was restricted due to nephrotoxity, autooxidation, and dissociation of tetramer to dimer. In the present study attempts were made to deliver haemoglobin using colloidal ceramic carbohydrate composites termed aquasomes. These delivery vehicles are composed of natural molecular stabilizers, hence they protect biochemically active molecules from both degradation and dehydration. The advantages of this system are adequate residence time in circulation, absence of other pharmacological actions, no interference with typing and cross-matching of blood, and good stability at wide temperature ranges.
MATERIALS AND METHODS
Cellobiose and trehalose dihydrate (Himedia Lab. Pvt. Ltd., Mumbai, India), haemoglobin, maltose and sucrose (Loba chemie, Mumbai, India, dextran (40,000 MW), and concanavalin (Sigma Inc., New York, NY, USA) were of analytical grade, and all other chemicals used were of laboratory reagent grade.
Aquasomes are prepared by a three-step method that involves production of ceramic cores, adsorption of sugars on ceramic core, and immobilization/adsorption of bioactive molecule on sugar-coated ceramic.
Preparation of Core
Hydroxy-apatite ceramic core was prepared by two different methods, i.e., coprecipitation (Correia et al. Citation1996) and self-precipitation (Li et al. Citation1997).
In coprecipitation method 0.19 N diammonium hydrogen phosphate solution was added dropwise with continous stirring to 0.32 M calcium nitrate solution maintained at 75°C in a flask bearing one charge funnel, a thermometer, and a reflux condenser fitted with a CO2 trap. During the addition, the pH of calcium nitrate was maintained between 8–10 using concentrated aqueous ammonia solution. The mixture was then stirred for 4–6 days at the same temperature and pH. The precipitate was filtered, washed thoroughly with triple distilled water, and finally air-dried overnight at 100°C. The powder was then sintered by heating to 800–900°C.
In self-precipitation method the simulated body fluid of pH 7.2 containing NaCl (134.8 mM), KCl (5.0 mM), MgCl2 (1.5 mM), CaCl2 (2.5 mM), NaHCO3 (4.2 mM), Na2HPO4 (1.0 mM), and Na2SO4 (0.5 mM) was used. The pH of the solution was adjusted to 7.26 every day with HCl. One hundred milliliters of this solution was transferred to a series of polystyrene bottles of 100 ml capacity. The bottles were tightly sealed and kept at 37 ± 1°C for one week. The formation of precipitate was then observed on the inner surface of the bottles. The precipitate was filtered, washed thoroughly with triple-distilled water, and finally air-dried overnight at 100°C. The powder was then sintered by heating to 800–900°C.
Adsorption of Sugar on Ceramic
The ceramic cores of calcium hydroxy apatite were coated with sugar by adsorption method (Martin Citation1994). The freshly prepared, sonicated, and washed calcium hydroxy apatite (< 1 μm in size) was weighed accurately (approximately 500 mg) and placed into well-cleaned and dried iodine flasks. The flasks were arranged in four series and labeled 1 to 10. By means of a burette 1 to 10 ml of sugar solution (5 mg/ml of trehalose, cellobiose, maltose, and sucrose) was transferred in iodine flasks of each series and volume made up to 50 ml with triple-distilled water. These flasks were stoppered and shaken vigorously for about 20 min and then suspended in a trough containing water at room temperature and left for about 1 h with intermittent shaking. The suspension was then centrifuged at 2000 rpm for 5 min. One milliliter aliquots of the supernatant of each centrifuge tube were taken, and residual sugar was estimated by developing color with anthrone reagent and measuring absorbance at 620 nm on Shimadzu UV 1601 spectrophotometer. The adsorption isotherms were calculated from the plots and are shown in . The sugar-coated ceramics were later dried by three different processes, i.e., air drying, vacuum drying in desiccator, and lyophilization. The excess poorly bound sugar on ceramic was removed by washing with fresh ultrapure distilled water using dialysis method. The water was replaced every 6 h for 48 h.
Langmuir and Freundlich adsorption parameters of sugars on hydroxy apatite
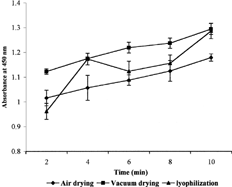
In order to estimate the extent of sugar coating, the suspension of sugar-coated ceramics (100 μg/ml) was taken in a cuvette and turbidity was recorded at 450 nm. One mililiter of concanavalin A solution (10 μg/ml) was added, and absorption was noted at time intervals of 2, 4, 6, 8, and 10 min. The absorbance was plotted against time () and slop was taken as a measure of the rate of aggregation that indicates the extent of sugar coating.
Coating of Haemoglobin
One percent solution of haemoglobin (250 ml) was prepared in 0.01 N ammonium hydroxide. By means of a burette, aliquots of 1, 2, 3, … to 10 ml of 1% haemoglobin were added to each of the 10 flasks containing sugar-coated ceramics. The flasks were stoppered and shaken vigorously for 20 min and left for about 1 h with intermittent shaking to produce three-layered haemoglobin-adsorbed aquasomes. In order to determine the extent of haemoglobin adsorption, the suspensions were centrifuged and supernatant analyzed for haemoglobin content spectrophotometrically at 624 nm by benzidine method (Crosby and Furth, Citation1956) at 624 nm. The adsorption isotherm parameters were calculated from plots and are shown in .
Langmuir and Freundlich adsorption parameters of haemoglobin on sugar coated hydroxy apatites
Haemoglobin Aquasome Formulation
Phosphate buffer saline (0.01 mM, pH 7.4) was used for washing the haemoglobin aquasomes. The haemoglobin aquasome formulation (HAF) was prepared by suspending haemoglobin-adsorbed aquasomes in Phosphate buffer saline (pH 7.4) in such a way that it was equivalent to 7.5% haemoglobin. The buffer solution used was a low salt buffer, which contained 0.7 mM Na2HPO4, 0.13 mM NaH2PO4, 12.1 mM NaCl, and 0.012 mM KCl. The suspending medium was prepared by adding 7.5% w/v albumin and 0.01% w/v lecithin in PBS.
Size, Viscosity, and Hb Loading
The particle size of aquasomes was determined using scanning electron microscopy (SEM), in which the stubs were prepared using adhesive tapes and coated with gold and observed under suitable magnification of × 2000 K and × 10000 K ().The viscosity of the haemoglobin aquasome formulation was determined by Brookfield viscometer at constant sheer rate (100 rpm) using spindle number 3. For the determination of Hb loading, 100 mg of HAF was taken in a flask and sufficient quantity of dilute HCl was added to dissolve the hydroxy-apatite coat. After suitable dilution the dissolved haemoglobin was estimated by benzidine method, and the results are shown in .
Physical characteristics of haemoglobin adsorbed formulations
Oxygen Carrying Capacity and Cooperativity
The oxygen carrying capacity was determined using Van-Slyke apparatus. The oxygenated blood sample was introduced into the extraction chamber over the mercury. The gases were extracted from solution by shaking extraction chamber for 2 to 3 min. Gas volume was reduced to 0.5 ml by admission of mercury in extraction chamber upwards with the help of a leveling bulb. Stopcock was closed and the pressure (p1) was read on the manometer. Sodium hydrosulfide solution 0.4 ml (42.5 mg of sodium hydrosulfide in 5 ml 1N NaOH solution) was introduced into the extraction chamber, which was then shaken to absorb the liberated oxygen. The volume in extraction chamber was readjusted to 0.5 ml and the pressure (p2) was read on the manometer. The partial pressure of oxygen (pO2) is given by equation (1),
(1)
This gives pO2 of oxygenated blood. The partially oxygenated blood sample was prepared by mixing 1 ml oxygenated blood with 0.02, 0.04, 0.06, … 0.4 ml of sodium hydrosulfide solution, and its pO2 was obtained by similar procedure. The percentage of oxygen saturation was calculated by equation (2),
(2)
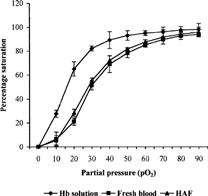
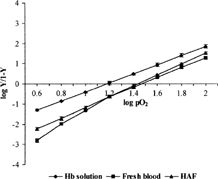
The same procedure was adopted for determination of pO2 and percent oxygen saturation of Hb solution and haemoglobin aquasome formulation. The graph was plotted between pO2 and percent oxygen saturation to give oxygen dissociation curve (). The Hill Coefficients were calculated on the basis of equation (3) and Hill's plots were obtained ():
(3)
Haemolysis and Coagulation Time
In order to check the hemolysis, HAF was mixed with citrated whole blood and shaken for 2.5 h at 37°C. After incubation, the solutions were centrifuged and supernatants analyzed for the free haemoglobin. For determining the effect of HAF on coagulation time, the whole blood drawn from rat was placed in four clean glass test tubes and HAF was mixed. Two test tubes were placed in water bath at 37°C and the other two were kept at 20°C. Each tube was gently tilted in every 30 s, and the time required to form a clot was noted.
Stability Studies
The HAF formulation was kept at room temperature for storage stability study. The haemoglobin desorbed, total haemoglobin, and methaemoglobin formed were estimated on 1, 7, 15, and 30 days (). The desorption of haemoglobin from formulations was determined by shearing the sample using mechanical stirrer at shear rates upto 500−1. Samples were incubated at 37°C for 24 h in PBS and sheared for 30 min. The sheared samples were centrifuged, and the concentration of total haemoglobin in the supernatant of the sheared samples was measured using benzidine method. In order to study the effect of serum on Hb desorption, the HAF was kept in 10% serum solution for 24 h, and haemoglobin desorption was estimated by the benzidine method in the supernatant after centrifugation.
Stability studies data of formulations
Isovolumic Exchange Transfusion
The experimental animal used in these studies were Spargue dawley albino rats of both sexes weighing 250 ± 12 g. Rats were entered into exchange transfusion studies in pairs (one control and one experimental). On the day of exchange, rats were anaesthesized by anaesthetic ether and surgically implanted with an intraarterial cannula in the jugular vein for administration of red blood cell substitute, second cannula in femoral vein for isovolemic blood removal, and third cannula in the carotid artery to monitor blood pressure and heart rate.
The efficacy of HAF as a red blood cell substitute was determined using a model in which survival was monitored as a function of hematocrit in rats receiving isovolumic exchange transfusion of either HAF or dextran. Sterile solution of 5.2% w/v dextran in PBS served as a control nontoxic volume expander without oxygen-carrying capacity. PBS without dextran was also used as a control in the 50% isovolumic exchange transfusion runs.
Flow rates in the blood removal and replacement cannulas were controlled between 0.20 to 0.30 ml/min. Exchange transfusion was continuous, thus providing an isovolumic exchange of a test or control material. Total intravascular volume was maintained at a constant level, and no abrupt changes in the composition of the intravascular fluid occurred during the exchange. The hematocrit dilution data is reported in .
Hematocrit dilution curve for continuous isovolemic exchange transfusion in rats
RESULTS AND DISCUSSION
Thermodynamically, hydroxy-apatite is the most stable calcium phosphate at pH, temperature, and composition of physiological fluid. The morphology of the final product was influenced by the nature of reagents, pH of solution, aging time, and temperature. The reproducibility of the process could be achieved by close control of these parameters. The coprecipitation method with uncontrolled pH produced crystalline ceramics in micrometer size range, while under the controlled pH conditions elongated rounded particles in nanometer-to-micrometer size range were produced, which on sintering at 800 to 900°C gave nearly spherical particles in nanometer size range. The self-precipitation method produced spherical particles in the size range of 1 to 5 μm, but the yield was very less because it formed monolayer precipitate on the surface of the container. The seeding increased the rate of crystallization, but the particles so produced were irregular in size and shape.
The adsorption of sugar on calcium hydroxy-apatite was found to follow both Freundlich and Langmuir adsorption isotherm satisfactorily. The correlation coefficient of Freundlich isotherm (log C versus log x/m) was found to be in the range of 0.937 to 0.983, while that of Langmuir isotherm (C versus C/x/m) was found to be in the range of 0.980 to 0.999. The Freundlich and Langmuir adsorption parameters, i.e., binding constant b, sugar adsorbed per gram of hydroxy-apatite Ym, and Log K and n (both determined from the graphical data), are shown in . The binding constant ‘b’ of different sugars followed the order cellobiose > maltose > sucrose > trehalose, while the sugar adsorbed per gram of hydroxy-apatite Ym followed the order trehalose > maltose > sucrose > cellobiose. These can be explained on the basis of epitaxial arrangement of sugars adsorbed or the packing of adjacent sugar molecules adsorbed on hydroxy-apatite. Packing of cellobiose and maltose is comparatively greater than sucrose and trehalose, while the sucrose and trehalose try to arrange themselves in a manner in which lowest energy of adsorption is achieved (Patil et al. Citation1999). This packing involves adsorbed as well as intercalated molecules. The intercalated molecules may be removed easily under stress conditions, which accounts for their low binding constants.
The adsorption parameters of haemoglobin on sugar-adsorbed hydroxy-apatite powder were also studied. The binding constants were found to be in the order trehalose > sucrose > maltose > cellobiose. This can be explained on the basis of interaction between the sugars and haemoglobin due to cavity portion on the sugar layer adsorbed on hydroxy-apatite. Haemoglobin may closely fit into cavities, thereby achieving better adsorption. Cellobiose, which showed the closest packing during adsorption, has the lowest binding constant; however, the amount of haemoglobin adsorbed was higher than trehalose. The value for sucrose and maltose lies between the value of cellobiose and trehalose. The Freundlich adsorption parameters were highest for trehalose and lowest for cellobiose, as shown in .
The adsorption of haemoglobin on sugar-coated ceramics showed correlation coefficient in the range of 0.951 to 0.986 for Freundlich isotherm and from 0.874 to 0.999 for Langmuir isotherm.
The extent of sugar coating was studied by concanavalin-mediated aggregation of sugar-coated ceramics. Concanavalin is known to bind glucose units of sugars and therefore causes aggregation. The rate of aggregation as indicated by the slope of absorbance versus time plot was substantially faster for the ceramics coated with sugar and dried by lyophilization as compared to air- or vacuum-dried ceramics, as shown in .
The aquasome-adsorbed haemoglobin was resuspended in buffer containing 0.01% lecithin and 7.5% albumin to obtain homogeneously dispersed aquasome formulation equivalent to approximately 7.5% haemoglobin. At this concentration level of albumin the colloid osmotic pressure of this “artificial plasma” approximately balances with that of the blood plasma and is iso-oncotic with respect to whole blood. The lecithin facilitates dispersion of formulation.
The haemoglobin aquasome formulation showed large-size aggregates of about 10 to 15 μm that were highly porous in nature. The close-up view of the aggregate surface showed the nanometric size of individual particles (). The aquasome formulation showed non-Newtonian behavior, as about 50–55% w/w slurries were required to produce 7.5% concentration of Hb. The non-Newtonian behavior can be attributed to an increasing difficulty for the particles to orient in the direction of shear stress at this powder concentration. It is reported that powder concentration lower than 70% w/w exhibit Newtonian behavior, but the presence of albumin, a polymeric material, in our formulation might have contributed to non-Newtonian flow. Haemoglobin loading was found to be 7.53%, 7.29%, 7.36%, and 7.42% for different sugars—i.e., sucrose, cellobiose, maltose, and trehalose, respectively—as shown in . This shows that all these sugars are suitable for preparation of haemoglobin aquasome formulation.
The oxygen carrying capacity and oxygen cooperativity was studied by plotting the oxygen dissociation curves between pO2 and percent oxygen saturation for Hb solution, fresh blood, and HAF. The mean saturation of haemoglobin with oxygen for Hb solution (P50 = 15.25) was lower than that of HAF (P50 = 28.44), which was almost similar to fresh blood (P50 = 29.11), as shown in . This indicates that aquasomal formulation has good oxygen carrying capacity. The oxygen cooperativity, Hill coefficients for Hb solution, fresh blood, and HAF were 2.2, 2.4, and 2.6, respectively, which are fairly good for its use as oxygen carrier ().
The study of hemolytic effect on RBCs revealed that there was no free Hb content in the supernatant, indicating that HAF did not induce haemolysis of red cells. The coagulation time of whole blood of rat did not change on addition of HAF.
The stability studies indicated that the total haemoglobin content remained unchanged during storage for 30 days while the haemoglobin desorbed ranged between 0.1 to 1.64% during this period. The methaemoglobin concentration increased from 9.6 to 12.4% of total haemoglobin. This showed that hemoglobin was tightly adsorbed over ceramics, and desorption was very low during relatively long periods of storage, as shown in .
Total desorption of haemoglobin from HAF over a period of 24 h was very small and remained at level of 0.55 ± 0.15%, independent of the rate of shear. The lower percentage of desorption demonstrated the integrity of HAF. The desorption of haemoglobin from HAF in 10% serum in 24 h was found to be much less, i.e., 0.70 ± 0.20%. This indicated the good stability of HAF in biological fluid system.
The isovolemic exchange transfusion studies were performed on three pairs of rats. Mortality was observed at a mean hematocrit of 10.2 (±1.8%) in control rats exchanged with dextran, but the rats exchanged with HAF survived to a mean hematocrit of 23 (±3%) achieved after 100 min of exchange transfusion, as shown in .
Data demonstrating the effect of 50% exchange transfusion with HAF and 5.5% w/w solution of dextran in PBS (isotonic and isooncotic) on arterial blood pressure and heart rate are presented in . Arterial blood pressure did not change significantly in animals transfused with HAF or dextran solution, and 50% exchange transfusion had no effect on heart rate.
The effect of 50% isovolemic exchange transfusion on arterial systolic blood pressure and heart rate
CONCLUSION
This study demonstrates that the haemoglobin-adsorbed aquasomes can carry the oxygen satisfactorily, and it also establishes the superiority of haemoglobin aquasomal formulation over the other methods acting as artificial blood substitute. The self-assembling surface modified nanocrystalline ceramic core capable of nondenaturing attachment can be used for various applications like delivery of bioactive molecules as well as viruses.
Shailendra Patil expresses his thanks to UGC for awarding Junior Research Fellowship during M. Pharm course when the work was carried out. The authors also thank to the Head of Department of Pharm. Sciences, for providing necessary facilities in which to carry out these investigations.
REFERENCES
- Arnone A., Perutz M.. 1974. Structure of inositol hexaphosphate-human deoxyhaemoglobin complex.. Nature. 237: 146–149
- Correia P. N., Magalhaes M. C. F., Maeques P. A. A. P., Senos A. M. R.. 1996. Characterization of modified hydroxyapatite powders.. J. Mater. Sci. Mater. In. Med.. 7: 501–505
- Crosby W. H., Furth F. W.. 1956. A modification of the benzidine method for measurement of hemoglobin in plasma and urine.. Blood. 11: 380–383. [PUBMED], [INFOTRIEVE]
- Goldstein J., 1991; Biotechnology of Blood.. Boston, Butterworth Heinemann
- Kossovsky N., Gelman A., Rajguru S., Nguych R., Sponsler E. E., Hantyszyn H. J., Chaw K., Chung A., Torres M., Zemanovich J., Crowder J., Bamajikn P., Ly K., Philipsose J., Ammns D., Anderson S., Goodwin C., Soliemanzadeh P., Yao G., Wei K.. 1996. Control of molecular polymorphisms by a structured carbohydrate/ceramic delivery vehicle—aquasomes.. J. Control. Release. 39: 383–388. [CROSSREF]
- Kreuter J.. 1991. Nanoparticle-based dmg delivery systems.. J. Control. Release. 16: 169–176. [CROSSREF]
- Lieberman H. A., Rieger M. M., Banker G. S., 1988. Pharmaceutical Dosage. Forms Disperse System. Vol. 1, New York, Marcel Dekker Inc.
- Li J., Liao H., Sjostrom M.. 1997. Characterization of calcium phosphates precipitated from simulated body fluid of different buffering capacities.. Biomaterials. 18: 743–747. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Martin A., 1994; Physical Pharmacy, 4th ed, New DelhiIndia, B. I. Waverly Pvt. Ltd
- Namdeo A., Jain N. K.. 1996. Niosomes as drug carriers. Ind. J. Pharm. Sci.. 58(2)41–46
- Patil S., Khopade A. J., Nagaich S., Agrawal G. P., Jain N. K.. 1999. Adsorption studies of sugars on calcium hydroxy apatite ceramics and haemoglobin on sugar coated ceramics.. Pharmazie. 54: 310–311
- Perutz M. F., Pulsinelli P. D., Ranney H. M.. 1972. Structure and subunit interaction of haemoglobin.. Nature New Biology. 237: 259–264. [PUBMED], [INFOTRIEVE]
- Perutz M. F.. 1978. Hemoglobin structure and respiratory transport.. Sci. Am.. 239: 92–125. [PUBMED], [INFOTRIEVE]
- Tortora G. J., Anagnostakos M. P., 1989; Principles of Anatomy and Physiology, 6th ed, New York, Harper and Row.
- Tsuchida E., Hasegawa E., Matsushita Y., Eshima K., Yuasa M., Nishide H.. 1985. Polymerized liposome as the carrier of heme. A physiological stable oxygen carrier under physiological conditions.. Chem. Lett.. 969–972
- Yuasa M., Aiba K., Ogata Y., Nishide H., Tsuchida E.. 1986. Structure of the liposome composed of lipid-heme and phospholipids: The lipid-heme liposome as a red blood cell model.. Biochem. Biophys. Acta. 860: 558–565. [CROSSREF]