Abstract
The objective of our study was to prepare and evaluate osmotic matrix (OM) tablets of diclofenac sodium (DS). In vitro studies were done on USPXXIV dissolution apparatus II in different release medium. Surface characteristics of coating films and osmotic contribution of OM tablets also were studied. In vivo evaluation was carried out in 6 healthy human volunteers using HPLC method to assay plasma samples, and the results were compared with the performance of fabricated matrix and two commercial tablets of DS. Through in vitro drug release kinetics, using regression coefficient analysis and Peppas equation, different pharmacokinetic parameters and relative bioavailability were determined. OM tablets were found to provide more prolonged and controlled therapeutic plasma DS levels and also showed improved bioavailability in comparison to fabricated matrix and commercial tablets studied.
Diclofenac sodium (DS), a nonsteroidal anti-inflammatory drug, is used in the treatment of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis (Hui et al. Citation1998). Because of its short biological half-life and the hazards of adverse gastrointestinal (GI) reactions, the development of an oral sustained-release formulations of this drug is highly desirable (Garcia-Encina et al. Citation1995; Rani and Mishra Citation2001a and b), to achieve improved therapeutic effect with negligible side effects and improved patient compliance. Many efforts have been made toward achieving sustained-release formulations of DS (Nishihata Citation1987; Liu et al. Citation1995; Hosny, Al-Helw, and Al-Dardiri Citation1997; Mishra, Panyam, and Sharma Citation1999; Rani et al. Citation2001a and Citation2001b; Sajeev et al. Citation2002; Rani and Mishra Citation2001a and Citation2001b), especially matrix formulations. But the reliability of the drug dissolution rate in biological fluids from matrices, which are diffusion-controlled systems, is affected by the variability of pH and hydrodynamic conditions of the GI tract (Lee and Robinson Citation1987). When swellable matrices were coated with an impermeable film on one base and the lateral surface (leaving only the surface of the other side available for swelling and drug release), it led to development of osmotic matrix (OM) systems (Catellani et al. Citation1998). Their release kinetic behavior was found closer to linear (Bettini, Catellani, and Colombo Citation1996). Therefore, an effort was made in our study to prepare and evaluate OM tablets of DS to achieve much more prolonged and controlled delivery of DS for better therapeutic effect and patient compliance.
MATERIALS AND METHODS
Materials
DS was obtained as a gift sample from Win-Medicare Ltd., India. Commercial tablets of DS [Batches C10 (Voveran-SR®-100 mg) and C11 (Voveran®-2× 50 mg conventional tablets)] were purchased from an Indian market. Hydroxypropyl methylcellulose (HPMC-K4M) and cellulose acetate (CA) were obtained from Dow Chemicals (France) and Thomas Baker (Chemicals) (India) respectively. Polyethylene glycol (PEG 400) and Triacetin were obtained from Glaxo Lab Ltd. and Loba Chemie in India, respectively. All other chemicals/reagents used were of analytical grade except those used in HPLC analysis were of HPLC grade.
Preparation of OM Tablets of DS
Swellable Matrix Tablets
Swellable matrix tablets (FM1) were prepared by direct compression technique using 33.3% w/w of DS, 66.7% w/w of polymer (HPMC), and 1.0% w/w of magnesium stearate, in each tablet. All the ingredients were passed through sieve No. 85 (aperture size 180 μm, British Standard), blended uniformly, and compressed on a Manesty E2 tabletting machine using 10-mm deep concave punches at a pressure that gave Monsanto hardness of 8 Kg/cm2.
Partial Coating of Matrix Tablets
The matrix tablets were partially coated using an aspirator (pipette connected with vaccum pump holding tablet from one side) on their base and lateral surface by carefully dipping them in an organic solution of film-forming polymer. The solution was prepared by dissolving 2, 5, or 6 gm of CA and different concentrations (0%, 5%, 33%, and 66% w/w of CA) of PEG 400 or Triacetin, as porosigenic agents, in a mixture solvent (methylene chloride:ethyl acetate:cellosolve in 3:1:1 v/v) with volume made up to 100 ml. The dipping volume and time, including time of rotation, remained constant until complete film coating was achieved and solvent evaporation was allowed at 37 ± 0.1°C for 48 hr. The composition of all fabricated OM formulations are shown in . All fabricated formulations were evaluated for various physical parameters using standard methods. The film coatings were evaluated using scanning electron microscope (SEM)-Olympus polarizing microscope B × 50P (model 840A) with SC35 camera, Japan.
Composition of coating solutions used for partial coatings of different batches of fabricated osmotic matrix (OM) tablets of DS
In Vitro Studies
In vitro studies, in triplicate, were done on USPXXIV (USP 24, 2001) dissolution apparatus II in different release media [pH 7.4, distilled water (DW), 0.02M sodium chloride (SCL) in DW, and 0.2M SCL in DW] maintained at 37 ± 0.2°C and at 100 rpm. Withdrawn samples were analyzed on Jasco UV/VIS Spectrophotometer (model 7800, Japan) at 275 nm. In vitro studies were conducted to investigate the effect of the following factors on DS release from different formulations of OM tablets:
DS release from OM versus FM tablets.
Effect of polymer concentration in coating film on DS release from OM tablets.
Effect of the type and concentration of porosigenic agents on DS release from OM tablets.
Effect of osmotic contribution on DS release from OM tablets.
Effect of pH of release medium on DS release from FM and OM tablets.
In vitro drug release kinetics also were studied for different formulations.
In Vivo Studies
In vivo studies were performed following standard protocols in 6 healthy human volunteers of either sex weighing 55–75 Kg and 24–29 years old in a crossover design. Volunteers agreed in writing to participate in the study after explanation of the experimental protocol. All subjects were in good health on the basis of their medical history and complete physical examination. The volunteers were neither smoking nor on any kind of medication before and during the experiment. The modified HPLC method (Giagoudakis and Markantonis Citation1998) was used to analyze plasma samples (stored frozen at -20°C until analysis) collected at different time intervals up to 24 hrs following oral administration of formulated tablets (FM and OM tablets) as well as two commercial tablets (C1 and C2) to human subjects. HPLC procedure was carried out our methods: Novapak C-18 column, 4 μm (150 × 3.9 mm) (Shimadzu, Japan); a mixture of 40 volumes of Acetonitrile and 60 volumes of 0.025 M ammonium acetate solution as the isocratic elution mobile phase with a flow rate of 1 ml/min; injection volume of 75 μl; and a detection wavelength of 275 nm for detection in a Shimadzu UV detector.
The plasma drug concentrations versus time profiles and the calculated bioavailability and pharmacokinetic parameters for different formulations were compared.
Statistical Analysis of Data
Experimental results were expressed as mean ± SD values. Student t test also was performed to determine the level of significance. Difference was considered to be statistically significant at p<.05 and nonsignificant at p >. 05.
RESULTS AND DISCUSSION
The various physical parameters evaluated for all fabricated formulations were found within the official limits (data not shown).
DS Release from OM versus FM Tablets
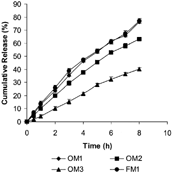
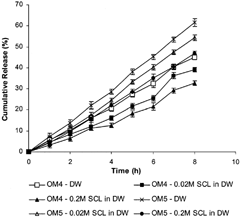
The cumulative percent drug release versus time profiles shown in clearly indicate that all the fabricated OM tablets of DS showed more controlled and prolonged DS release compared with FM1 matrix tablets.
Polymer (CA) Concentration in Coating Film
We observed () that the increase in polymer (CA) concentration incorporated within coating film from 2% (OM1) to 5% (OM2) to 6%w/v (OM3) resulted in more prolonged and controlled drug release. The possible mechanism of controlled and prolonged drug release from OM tablets as compared with matrix (FM1) tablet is attributed to controlled diffusion of the drug through the swelled gel polymeric matrix available on one side only in OM tablets (having no porosigenic agents), where otherwise the coated membrane was present on base and lateral surface, as in OM1, OM2, and OM3. However, in FM1 matrix tablets drug diffusion occurred through swelled polymeric matrix available over all the sides of the matrix tablets and resulted in more drug release than OM tablets.
Type and Concentration of Porosigenic Agents
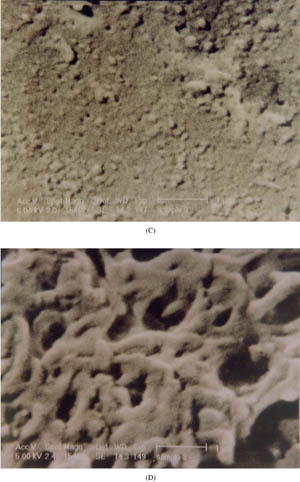
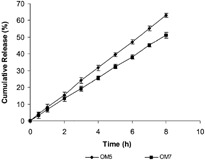
The drug release also was affected by the type of porosigenic agent used in coating films. The batch OM7 containing 33% Triacetin in coating solution exhibited a significantly (p<.05) decreased rate and extent of DS release compared with batch OM5 having 33% PEG 400 (). This may be attributed to more hydrophilicity of PEG 400, which led to formation of more pores during dissolution resulting in higher DS release. This is evidenced by the SEM analyzed microphotographs shown in . From SEM analysis, it was observed that before dissolution, the surface of the coating film containing 33% Triacetin (OM7) was smoother than that containing 33% PEG 400 (OM5) which had nonuniform surface with shear marks. After the dissolution, the coating films lost their integrity and the pores were clearly evident. More pores were observed in coating films with PEG 400 (OM5) compared with those with Triacetin (OM7), possibly a result of more hydrophilic nature of PEG 400 than Triacetin.
2 In vitro release profiles showing the effect of porosigenic agents, 33% w/w PEG 400 (OM5) and 33% w/w Triacetin (OM7) used in coating, on DS release from OM tablets in pH 7.4 buffer. Error bars represent ±S.D (n=3).
3 SEM of the film coatings having Triacetin and PEG 400 before and after dissolution. (A) Triacetin and (B) PEG 400 before dissolution. (C) Triacetin and (D) PEG 400 after dissolution. (Continued)

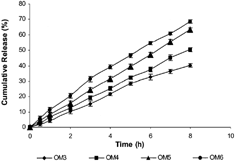
Further, it we observed () that with an increase in the concentration of porosigenic agent (PEG 400) in OM tablets (OM3 – 0%, OM4 – 5%, OM5 – 33%, and OM6 – 66% w/w PEG 400), a significant increase in rate and extent of drug release was found. The DS release profiles from all the OM tablets were much linear than the matrix (FM1) tablets. The presence of porosigenic agents in the coating film of OM tablets resulted in double control and more linearity of drug release: one by controlled drug diffusion through swelled gel polymeric matrix from one side of the tablets and the second by controlled drug permeation from the other side of the tablets where the micropores formed in the coating membrane due to dissolution of porosigenic agents (PEG 400 or Triacetin) in the release medium. With an increase in concentration of porosigenic agents (PEG 400) in the OM tablets (OM3, OM4, OM5, and OM6), subsequent increase in DS release was observed (). It is attributed to formation of more micropores in the coating film.
Osmotic Contribution
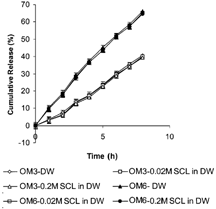
To study the osmotic contribution of osmotic matrix on DS release, the in vitro release investigations on OM3, OM4, OM5, and OM6 were done in release media with increasing osmolarity (0.02M SCL in DW and 0.2M SCL in DW). The in vitro drug release profiles shown in indicate that batch OM3, containing no porosigenic agent in coating film, and batch OM6, containing highest amount (66% w/w) of PEG 400 as porosigenic agent in coating film, exhibited insignificant (p >. 05) differences in rate and extent of drug release in release media of increasing osmolarity. This indicated no effects of increased osmotic pressure of release media on DS delivery from OM3 and OM6 tablets. Since OM3 did not contain any porosigenic agent, drug release occurred by diffusion process through the gel formed around uncoated side of the tablet, without any osmotic contribution of the system. However, a greater amount of PEG 400 (water soluble porosigenic agent) resulted in formation of large numbers of micropores in the coating film during drug release study, and the drug release from batch OM6 was expected to be controlled by the diffusion of drug through gel around uncoated part of the tablet and also by permeation through large numbers of micropores formed in the membrane without any osmotic contribution of the systems (Catellani et al. Citation1998). But, as shown in , batches OM4 and OM5 exhibited a decrease in rate and extent of DS release with an increase in the osmolarity of the release medium indicating that osmotic contribution also played a role in drug release from these two batches. This behavior was observed because the coating membrane in these two cases probably behaved as a semipermeable membrane with comparatively less microporous character than that in OM6 and thus resulted in osmotically controlled DS release from such systems.
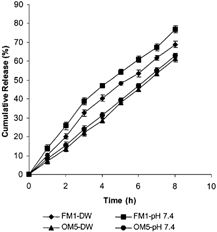
Effect Release Medium pH on DS Release
To further verify whether drug delivery from osmotically contributed systems remain independent of environmental pH, batches FM1 and OM5 were evaluated to study pH effect (effect of different release media) on DS release from them. We observed () that the matrix tablet FM1 exhibited significant difference in DS release in two different release media (pH 7.4 and DW) whereas osmotic matrix tablet (OM5) exhibited no significant difference in DS release in these two release media. Thus, the results clearly suggest that variation of pH does not affect the DS release from OM tablets, compared with FM tablets.
In Vitro Drug Release Kinetics
The in vitro drug release kinetics and mechanisms from different batches of OM tablets and FM1 was studied by regression coefficient analysis (Sankar et al. Citation2001) and the Peppas equation (Ritger and Peppas Citation1987). and , show that all OM tablets exhibited drug release with zero-order kinetics, FM1 showed Higuchi kinetics of drug release, and more linearity was observed among OM tablets as determined by ‘n’ values using Peppas equation.
In vitro release kinetics (analyzed by regression coefficient method) of DS from different batches of OM tablets
In vitro release kinetics (analyzed by Peppas equation) of DS from different batches of OM tablets
In Vivo Studies
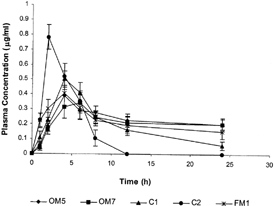
From in vivo data () and plasma profiles (), it was observed that OM tablets provided more controlled and prolonged plasma DS levels compared with commercial tablets. We found that the fabricated OM tablets exhibited lower Cmax but within therapeutic range (Reiss et al. Citation1978) and higher Tmax values than the commercial tablets (C1 and C2). This clearly indicate that the fabricated OM tablets evaluated in vivo were able to deliver DS within therapeutic range, slowly with more controlled rate and for a much longer duration. Significantly higher values of AUC0 - 24, relative bioavailability, and mean residence time (MRT) of fabricated OM formulations in comparison to C1 and C2 further indicated the superiority of fabricated OM formulations over commercial SR (C1) and conventional (C2) tablets. Thus, the developed OM tablets of DS are expected to perform therapeutically better as a potential prolonged and controlled release dosage form.
Bioavailability and pharmacokinetic parameters (mean ±SD [n=6]) for different fabricated and commercial tablets
The authors acknowledge University Grants Commission, New Delhi (India), for financial assistance. They also thank V.K. Arora and Tausif of Ranbaxy Research Laboratory, Gurgaon (India), for their help in providing facilities for the analysis of plasma samples on HPLC.
REFERENCES
- Bettini R., Catellani P. L., Colombo P.. 1996. Osmotic matrix for controlled drug delivery. Proc. Int. Symp. Contr. Rel. Bioact. Mater.. 23: 55–56
- Catellani P. L., Colombo P., Peppas N. A., Santi P., Bettini R.. 1998. Partial permselective coating adds an osmotic contribution to drug release from swellable matrix. J. Pharm. Sci.. 87: 726–730. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Garcia-Encina G., Torres D., Seijo B., Vila, Jato J. L.. 1995. Formulation and in vitro evaluation of HPMCP-microencapsulated drug-resin complexes for sustained release of diclofenac. Int. J. Pharm.. 121: 239–243
- Giagoudakis G., Markantonis S. L.. 1998. An alternative high-performance liquid chromatographic method for the determination of diclofenac and flurbiprofen in plasma. J. Pharm. Biomed. Anal.. 17: 897–901. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Hosny E. A., Al-Helw A. R. M., Al-Dardiri M. A.. 1997. Comparative study of in vitro release and bioavailability of sustained release diclofenac sodium from certain hydrophilic polymers and commercial tablets in beagle dogs. Pharm. Acta Helv.. 72: 159–164. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Hui X., Hewitt P. G., Poblete N., Maibach H. I., Shainhouse J. Z., Wester R. C.. 1998. In vivo bioavailability and metabolism of topical diclofenac lotion in human volunteers. Pharm. Res.. 15: 1589–1595. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Lee V. H. L., Robinson J. R.. 1987; Controlled Drug Delivery: Fundamentals and Applications, 2nd ed., New York, Marcel Dekker
- Liu C. H., Kao Y. H., Chen S. C., Sokoloski T. D., Sheu M. T.. 1995. In vitro and in vivo studies of the diclofenac sodium controlled-release matrix tablets. J. Pharm. Pharmacol. 47: 360–364. [PUBMED], [INFOTRIEVE]
- Mishra B., Panyam J., Sharma A. V.. 1999. In vitro release of diclofenac sodium from multiple emulsions; effect of location of drug and pH of the aqueous phase. Acta Pharm. Turc.. 41: 42–45
- Nishihata T.. 1987. Simple formulation of sustained-release tablets of sodium diclofenac and examination in humans. Int. J. Pharm.. 40: 125–128
- Rani M., Mishra B.. 2001a. Comparative evaluation of in vitro performance of commercial and fabricated sustained release diclofenac sodium tablets. Ind. J. Pharm. Sci.. 63: 247–250
- Rani M., Mishra B.. 2001b. Effect of admixed polymers on diclofenac sodium release from matrix tablets. Pharm. Pharmacol. Lett.. 11: 76–78
- Rani M., Mishra B.. 2003. Development and evaluation of carbomer based matrices for the controlled delivery of diclofenac sodium. Acta Pharm. Turc.. 45: 5–10
- Reiss W., Sterlin H., Degen P., Faigle J. W., Gerardin A., Moppert J., Sallman A., Schmin A., Schweizer A., Sule M., Thesbald W., Wagner J.. 1978. Pharmacokinetics and metabolism of the anti-inflammatory agent voltaren. Scan. J. Rheumatol.. 22: 17–29
- Ritger P. L., Peppas N. A.. 1987. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J. Contr. Rel.. 5: 37–42
- Sajeev C., Vinay G., Archna R., Saha R. N.. 2002. Oral controlled release formulation of diclofenac sodium by microencapsulation with ethyl cellulose. J. Microencap.. 19: 753–760
- Sankar C., Rani M., Srivastava A. K., Mishra B.. 2001. Chitosan based Pentazocine microspheres for intranasal systemic delivery: development and biopharmaceutical evaluation. Pharmazie. 56: 223–226. [PUBMED], [INFOTRIEVE]
- United States Pharmacopoeia 24. 2001, Rockville, MD, United States Pharmacopoeial Convention. p. 2051