Abstract
Cisplatin an antineoplastic medicine, was incorporated into a silica gel received by the sol-gel method. Various techniques of trapping an active substance in gel were applied: at the stage of sol creation—by the predoping method—and already upon receipt of hard xerogel—the postdoping method. Further, the research aimed at the determination of the dynamics of cisplatin release from sol-gel processed silica xerogel to the water phase. Based on the achieved results, we may state that the sol-gel method is useful for trapping a medicine like cisplatin in silica gel and gives repeatable results with regard to medicine release from the pores of the so-established matrix. The postdoping method appeared to be more beneficial, because the gained gel's active substance is released almost entirely (94–96%). The time of release was 3 days.
The sol-gel process is a method of preparation of amorphous oxide gels and xerogels. This is a simple and efficacious preparative method enabling the attainment of multicomponent solid bodies of exceptional purity and homogeneity at a low temperature, applicable as carriers of numerous chemical compounds, including medicines. By using the sol-gel process, amorphous materials are obtained from the stage of solution through sol to gel (Schmidt Citation1988).
The basis for the sol-gel method is the reactions of hydrolysis and condensation of alkoxides: M(OR)n where R is an alkyl and M is a metal or a semimetal like silica, titanium, aluminium, boron, and zirconium. Both liquid and solid alkoxy-compounds are used in the form of alcohol solutions, where the solvent is most often ethanol, and rarely such higher alcohols as propanol or butanol (Livage and Sanchez Citation1994). The reactions lead to obtaining wet polymers, which after drying produce hard xerogels.
The hydrolysis and condensation of alkoxides are accelerated by catalysts: the acid one and the basic one, which also determine the shape of polymer structures. The acid catalyst leads to the formation of a monolithic polymer with a small field of the surface proper, whereas the basic catalyst facilitates the production of complex agglomerate consisting of branched colloidal particles. This results in a delivery of a porous material, with high adsorptive capabilities (Schmidt Citation1988; Buckley and Greenblatt Citation1994).
Xerogels obtained by using the sol-gel method possess all the attributes of a good carrier for triggering a substance with therapeutic properties. The properties include above all biocompatibility, which makes them an attractive material for applications in medicine. In addition, such a form of medicine could bring about improved therapeutic activity, protection against degradation, change in pharmacokinetics, as well as control of biodistribution and decrease in toxicity (Buckley and Greenblatt Citation1994; Ahola et al. Citation2001; Radin et al. Citation2002; Nguyen et al. Citation2002).
Additives with therapeutic properties may be introduced to xerogels in the sol-gel process either at the formation of sol or upon the formation of porous xerogel. In the former (known as predoping), as a result of polycondensation, respective molecules are trapped in the xerogel lattice. In case of the latter (postdoping, impregnation), xerogel becomes saturated with an appropriate substance, after being immersed in the substance solution. The substance molecules penetrate pores in the diffusion process dependent on the diameter of the pores and the solution viscosity (Klein Citation1993).
The purpose of the present study was to evaluate the suitability of sol-gel produced silica xerogels as the carrier material for the controlled release of cisplatin, an antineoplastic medicine used in oncological chemotherapy.
The properties of the polymer and the interaction between the medicine and the polymer are the factors conditioning the success of the conducted experiment. Its use, beside the adsorptive characteristics, is further supported by the simplicity of its production in addition to mechanical, chemical, and temperature resistance guaranteeing the possibility of material sterilization, desirable in the production of a parenteral medicine.
MATERIALS AND METHODS
The materials and reagents included tetraethoxysilane (TEOS) a.p., Fluka A.G.; ethanol 96% a.p., POCh Gliwice; cisplatin (PLATIDIAM), lyophilizate for intravenous infusion (in the mix with mannitol, sodium chloride, 35% hydrochloric acid); Lachema-Brno, Czech Republic; redistilled water; 25% a.p. ammonium hydroxide, Fluka A.G.; and 30% hydrochloric acid, Fluka A.G.
Our apparatus included a spectrophotometer UV-VIS by Hewlett Packard 8452A, an electric centrifuge MPW type 211, and a magnetic mixer.
Methods of Obtaining Various Forms of Medicine
Using Acid Catalyst by Predoping Method
Silica gel was prepared by the hydrolysis and polycondensation of tetraethoxysilane (TEOS) with water and hydrochloric acid in a mole ratio of TEOS : H2O : HCl = 1 : 4 : 0.6 in ethanol solution, at room temperature (Livage and Sanchez Citation1994). Then, 400 mg PLATIDIAM preparation, containing 20 mg cisplatin, was dissolved in redistilled water and added into the silica solution after 1 h hydrolysis. The beaker content was further mixed for 30 min, then it was covered with a parafilm and left for gelation. The final content of cisplatin in the materials was 10.83 mg/g xerogel. Each sample contained 3.8 mg of cisplatin in 0.5 g xerogel.
Using Acid Catalyst by Postdoping Method
Silica gel with the acid catalyst was obtained in the manner as stated above. Hard xerogel that was obtained was dried in a dryer at the temperature of 120°C for 3 h, and then inserted in the desiccator over P2O5. Then 100 mg PLATIDIAM, which accounts for 5 mg cisplatin, was dissolved in 5 ml redistilled water. The concentration of the obtained solution of cisplatin was 1 mg/ml. Then, 1 g gel was poured with 5 ml cisplatin solution and left covered in a dark room for 5 days. After that time, the absorbance of the cisplatin solution over gel was measured, the capacity of gel remaining over the cisplatin solution was measured, and the quantity of cisplatin adsorbed in gel was determined. Next, gel was dried on the filter paper, upon which it was left for complete drying and then weighed. The final content of cisplatin in the materials was 1.79 mg/g xerogel (35.8%). Each sample contained 0.89 mg of cisplatin in 0.5 g xerogel.
Using Basic Catalyst by Postdoping Method
Silica gel with cisplatin using basic catalyst was obtained in the manner stated above, with the difference that the catalyst was 25% ammonia solution (a mole ratio of TEOS : H2O : NH4OH = 1 : 4 : 0.4). The final content of cisplatin in the materials was 4.16 mg/g xerogel (83.2%). Each sample contained 2.08 mg of cisplatin in 0.5 g xerogel.
Study of In Vitro Release
The in vitro study of cisplatin release was determined by ultraviolet spectroscopy, using a Hewlett Packard 8452A UV-VIS spectrophotometer. A silica gel sample was placed in a polyethylene bottle containing 20 ml redistilled water. The bottle was placed in the magnetic mixer for the purpose of gentle mixing of the water solution. The concentration of cisplatin in water was measured by taking 8 ml water solution of the examined therapeutic substance released from silica gel into water, at selected time intervals. Before each measurement of absorbance, the cisplatin water solution from over gel was rotated in the electric centrifuge at 3000 rotations for 15 min.
The analysis was carried out measuring the absorbance values at the maximum absorbance of cisplatin at a wavelength λ = 301 nm. The experiment was finished when the difference between the subsequent values of the solution absorbance was smaller than 0,01 absorbance units. The measurements of each sample were repeated 3 times.
RESULTS
presents the parameters of the process of cisplatin release trapped in silica gel obtained by using the predoping technique with the application of the acid catalyst: released substance quantity (in %); a, b, R2 ratios. Based on the time of the process observation and the released substance quantity, we ascertained that the process dynamics is best described with the following logarithmic equation: y = a 1n t + b.
Juxtaposition of process parameters of cisplatin release from silica gel obtained by predoping method
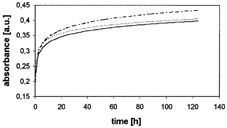
depicts courses of the processes of cisplatin release from silica gel obtained by the predoping technique in samples 1, 2, and 3.
1 Courses of processes of cisplatin release from silica gel obtained by predoping technique in samples 1, 2, and 3.
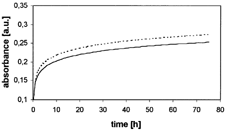
presents the data regarding the parameters of cisplatin release from silica gel obtained by the postdoping method using the basic catalyst. shows the courses of the processes of cisplatin release from silica gel to the water phase in samples 1 and 2. We ascertained that the active substance had been released regularly, indicating a logarithmic dependence of absorbance (and hence also concentration) on release time. The substance is released almost entirely (94–96%), whereas release time is about 3 days. The measurements of cisplatin release from silica gel obtained by the postdoping method with the application of the acid catalyst demonstrated that the active substance was not released from that form of silica gel.
2 Courses of processes of cisplatin release from silica gel obtained by postdoping technique in samples 1 and 2.
Juxtaposition of process parameters of cisplatin release from silica gel obtained by postdoping method
DISCUSSION
The conducted studies were the first attempt to examine the sol-gel method with respect to its use in trapping cisplatin in silica gel. The studies further aimed at the determination of the dynamics of cisplatin release from silica xerogel obtained by the sol-gel method. The manner of cisplatin release could be used while preparing an appropriate form of the drug. Assuming that the pace of cisplatin release is conditioned by the diffusion process depending on the diameter of pores, gels were prepared with different catalysts that facilitate the formation of either a monolithic material (acid catalyst) or a porous material having considerable adsorptive capabilities (basic catalyst). We also applied various techniques of trapping an active substance in gel: at the stage of sol creation (by the predoping method) and already upon obtaining hard xerogel (the postdoping method).
Based on the accomplished results, we may state that the sol-gel method is useful for trapping a medicine like cisplatin in silica gel and produces repeatable results regarding medicine release from pores of the matrix obtained in this way. Comparing the two techniques of receiving a form of the medicine, because of the postdoping method (impregnation). We obtained gel from which the active substance is almost totally released (94–96%). This complies with the assumptions, because gel produced in this way (using the basic catalyst) is characteristic of large pores with powerful adsorptive properties. The cisplatin solution freely penetrated the pores of xerogel causing its swelling, whereas desorption occurred without any obstacles that would be caused by the formed three-dimensional silica lattice.
The time of release approximated 3 days, with more than half of the trapped cisplatin being released within the first 3 h. Gel with cisplatin obtained by the predoping method was characteristic of smaller pores due to the application of the acid catalyst. The use of ammonia as the catalyst was abandoned because ammonia molecules are strong ligands and could exchange chloride ions Cl- for NH3 in cisplatin, thus damaging the drug. Notwithstanding, this also caused a smaller degree of medicine release from pores (60–70%).
A smaller percent of drug release also resulted from the fact that the medicine was added to the matrix being formed (at the stage of sol). Thus, the forming lattice of silica effectively immobilized cisplatin molecules, making it difficult for the medicine, due to steric reasons, to pass to the water phase. However, this did not extend the time of medicine release from the xerogel matrix. Over 90% of the drug was released during the first 5 h.
CONCLUSIONS
Based on the presented results of the study we may state that:
The process of cisplatin release from silica gel is repeatable, regular, and proceeds with dynamics characteristic of the regression equation: c = a(ln t) + b.
Of the applied two techniques of obtaining such a medicine form, the postdoping technique appeared to be more useful (the release of the therapeutic agent from gel was accomplished in 94–96%) compared with gels obtained by the predoping method (release percent is 68–71%).
The time of cisplatin release in the postdoping method was 3 days.
The sol-gel method is a useful method of obtaining a carrier for a medicinal substance administered in the form of removable implants positioned in the vicinity of a new-growth tumor.
REFERENCES
- Ahola M. S., Sailynoja E. S., Raitavuo M. H., Vaahtio M. M., Salonen J. I., Yli-Urpo A. U.. 2001. In vitro release of heparin from silica xerogel. Biomaterials. 22: 2163–2170. [PUBMED], [INFOTRIEVE], [CSA]
- Buckley A. M., Greenblatt M.. 1994. The sol-gel preparation of silica gels. J. Chem. Educ.. 71: 599–602
- Klein L. C.. 1993. Sol-gel optical materials. Annu. Rev. Mater. Sci.. 23: 437–452
- Livage J., Sanchez C.. 1994. Sol-gel chemistry. J. Non-Cryst. Solids. 45: 11–19
- Nguyen D. T., Smit M., Dunn B., Zink J. I.. 2002. Stabilization of creatine kinase encapsulated in silicate sol-gel materials and unusual temperature effects on its activity. Chem. Mater.. 14: 4300–4306. [CROSSREF]
- Radin S., Falaize S., Lee M. H., Ducheyne P.. 2002. In vitro bioactivity and degradation behavior of silica xerogels intended as controlled release materials. Biomaterials. 23: 3113–3122. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Schmidt H.. 1988. Chemistry of material preparation by the sol-gel process. J. Non-Cryst. Solids. 100: 51–64. [CROSSREF], [CSA]