Abstract
We prepared new ternary interpenetrating polymeric networks (IPN) systems containing chitosan, poly(N-vinylpyrrolidone) and poly(acrylamide) polymers. IPNs were synthesized by radical polymerization of acrylamide monomers in presence of glutaraldehyde (G) and N,N′-methylenebisacrylamide as crosslinkers and the other polymers. These IPNs were named as C-P-A. Glutaraldehyde were used in different concentration to control the network porous of IPNs. Spectroscopic and thermal analyses of these cylindrical shaped IPNs were made with fourier transform infrared spectroscopy analysis, thermogravimetric analysis, and thermomechanical analysis. Swelling studies of IPNs were carried out at pH 1.1 and pH 7.4 at 37°C. The swelling and diffusion parameters of IPNs in these solutions were calculated. Amoxicillin as a bioactive species was entrapped to the IPNs during synthesis. In vitro release kinetics of IPNs were investigated. The experimental data of swelling and release studies suggest clearly that the swelling and release process obeys second-order kinetics. The release of the entrapped bioactive species from IPNs depends on the degree of crosslinking of the polymer and pH of the medium at body temperature. We observed that amoxicillin release at pH 1.1 was higher than at pH 7.4. As a result, IPNs-based chitosan with different cross-linker concentration could be promising candidates for formulation in oral gastrointestinal delivery systems.
Interpenetrating polymer networks (IPNs) are threedimensional networks formed from homogeneous or heterogeneous polymers crosslinked in the presence of one another. Materials formed from IPNs share properties characteristic of each network (Kosmala, Henthorn, and Peppas Citation2000; Chakrabarty Citation1998). Today many natural polymers such as chitosan, chitin, gelatin, etc. and synthetic polymers such as poly(acrylamide), poly(acrylic acid), poly(N-isopropylacrylamide), and poly(N-vinylpyrrolidone) have been used extensively for preparing IPNs.
Chitosan is a deacetylated derivative of chitin, a naturally occurring polymer. It is widely studied in pharmaceutical and biomedical fields because of biodegradability, biocompatability and interesting structural properties (presence of amino and hydroxyl groups). Chitosan hydrogels can be used as carriers for the release of drugs and bioactive molecules (Khalid et al. Citation2002; Koseva et al. Citation1999; Hejazi and Amiji Citation2003; Oztop, Saraydin, and Cetinus Citation2002). Poly(N-vinyl pyrrolidone) (PVP) is a polymeric compound that is widely used for textiles, cosmetics and toiletries, pharmaceuticals, and medical applications, (Ekici et al. Citation2003; Peppas et al. Citation2000). Polyacrylamide hydrogels and acrylamide-based matrices have been used in in vitro and in vivo systems and biocompatibility studies (Risbud and Bhonde Citation2000; Saraydin et al. Citation1995; Karadag et al. 1995). Moreover, several acrylamides (AAms) have been reported as hydrogels with adjustable swelling kinetics, with applications for insulin release, to improve osteoblast adhesion, and show special properties such as superabsorbent hydrogels (Elvira et al. Citation2002).
Some of the significant applications of IPNs include artificial implants, dialysis membranes, drug-delivery systems and burndressing (Bajpai, Bajpai, and Shukla Citation2001). Hydrogel IPNs are one of the promising and versatile materials with enormous possibilities and potential. In particular, controlled release systems are capable of delivering drug at constant rate over an extended period of time. Homopolymer alone cannot meet such divergent demand in terms of both properties and performance. Therefore, a composite or IPN of two or three different polymers would be a better approach (Changez et al. Citation2003; Peppas et al. Citation2000).
The localized treatment of Helicobacter pylori infections of the stomach could be scientifically improved if a site-specific antibiotic drug delivery system could be developed. In vivo H. pylori is closely associated with the adherent mucus layer, living both within and beneath it and adhering to the gastric epithelial cells. The failure of conventional treatments could be due to poor permeability of the antibiotics across the mucus layer or from subtherapeutic antibiotic concentrations at the site of infection after administration from conventional tablets or capsules. pH-sensitive swelling hydrogels seem to be useful for localized antibiotic delivery in the acidic environment of the gastric fluid. One of the most important advantages of these hydrogels is that formulations remain on the targeted site more time than conventional ones (Torre et al. Citation2003).
Here we developed new chitosan-poly(N-vinylpyrrolidone)-poly(acrylamide) (C-P-A) IPNs with different glutaraldehyde concentration to take advantage of the desirous properties of all polymers. Because chitosan is not an easy polymer to use, we have chosen to work with other hydrophilic polymers with chitosan.
Then, these cylindrical shaped IPNs were characterized by Fourier transform infrared spectroscopy (FT-IR), thermogravimetric Analysis (TG) and thermomechanical Analysis (TMA). Swelling studies were carried out in simulated gastric fluid (SGF) of pH 1.1 and simulated intestinal fluid (SIF) of pH 7.4 to achieve site-spesific drug delivery. Amoxicillin were selected as a model drug and entrapped into the polymeric networks during polymerization. The in vitro release studies also were established in SGF and SIF at body temperature (37°C).
MATERIALS AND METHODS
Materials
Chitosan (Mr ∼ 600.000) was purchased from Fluka (Steinheim, Switzerland). Poly(N-vinylpyrrolidone) (M.W. 40.000) was obtained from Calbiochem (Darmstadt, Germany). Acrylamide was obtained from Merck (Darmstadt, Germany). N,N′-methylenebisacrylamide, glutaraldehyde (25%), and ammonium persulfate were received from Merck (Schuchardt, Germany). Amoxicillin trihydrate was obtained from Fluka (Steinheim, Switzerland). Hydrochloric acid, potassium chloride, disodium hydrogen phosphate, and potassium dihydrogen phosphate were purchased from Merck. All chemicals were analytical grade and used as received. Double distilled water was used throughout the investigations.
Preparation of C-P-A IPNs
IPNs were prepared by free radical polymerization of acrylamide monomers in presence of chitosan and poly(N-vinyl-pyrrolidone). Poly(N-vinylpyrrolidone), acrylamide monomers, N,N′-methylenebisacrylamide, and glutaraldehyde were added to chitosan solution (10 mL, 1% in 0.8% acetic acid), and mixed with stirrer. Chitosan, poly(N-vinylpyrrolidone), and acrylamide were used in 4.0:4.0:90 weight ratio. The ratios (w/w) between glutaraldehyde and chitosan in IPNs were 0.5%, 1%, and 2%. The weight ratio between N,N′-methylenebisacrylamide and acrylamide was 2%. Then, ammonium persulfate solution (25 μ L, 5%) was added to this mixture. The mixture was placed in PVC straws of 3 mm diameter. A gel formed 1 hr of reaction at 25°C. After 24 hr, the IPNs obtained in long cylindrical shapes were cut into pieces 4–5 mm length, washed with distilled water, dried in air and vacuum, and stored. The samples synthesized were named as C-P-A/0.5, C-P-A/1, and C-P-A/2 IPNs.
Fourier Transform Infrared Spectroscopy Analysis
FT-IR spectra of C, C-P, and C-P-A IPNs were recorded on a Mattson model FT-IR spectrophotometer for investigation of intermolecular interactions. Samples were thoroughly ground with exhaustively dried KBr and discs were prepared by compression under vacuum. Spectra were taken with a resolution of 1 cm−1.
Thermogravimetric Analysis
The thermal stability measurements were conducted by Shimadzu 50 thermogravimetric analyzer. TA was performed with 10 mg samples under nitrojen atmosphere with a nominal gas flow rate of 25 mL. Experiments were made at a heating rate of 10°C min−1 until 600°C.
Thermomechanical Analysis
To determine the maximum penetration, the TMA of IPNs was made with a Shimadzu 501 thermomechanical analyzer at the temperature range of 20–150°C under 1 g cm−2 load.
Swelling Studies
Dynamic swelling studies of IPNs were made as follows: C-P-A IPNs were swollen in solutions with pH 1.1 (KCl-HCl) and pH 7.4 (Na2HPO4-KH2PO4) at 37°C to determine the parameters of swelling and diffusion. Swollen gels removed from the water bath at regular intervals were dried superficially with filter paper, weighed and placed in the same bath. The radii of cylindrical swollen gels were measured by a micrometer.
Release Studies
Drug loaded samples (50 mg amoxicillin g−1 IPN) also were prepared using a similiar method for release experiments. The in vitro release of the entrapped drug, amoxicillin, were carried out by placing the IPN samples loaded with the drug into 10 mL of solution with pH 1.1 at 37°C in water bath. At periodic intervals 3 mL of solution containing drug were withdrawn and tested at λmax 271 nm using Shimadzu 160A model UV-VIS spectrophotometer. The release media were changed periodically with fresh KCl-HCl solutions (10 mL). The release studies were continued until the absorbance of the final solution was zero. The amount of released amoxicillin was quantified using appropriate calibration curve. The same release experiments also were carried out for solution with pH 7.4 at 37°C.
RESULTS AND DISCUSSION
Characterization of C-P-A IPNs
FT-IR Analysis
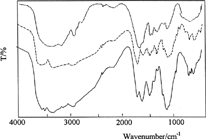
The structural characterization was performed by recording FT-IR spectra of the samples. shows the IR spectra of chitosan (C) crosslinked with glutaraldehyde, chitosan-poly(N-vinylpyrrolidone) (C-P), and C-P-A/2 samples. Chitosan spectrum exhibits 1676 cm−1 (amide I) and 1574 cm−1 bands (-NH2 bending). The absorption bands at 1165 cm−1 (antisymmetric stretching of the C-O-C bridge), 1089 and 1038 cm−1 (skeletal vibrations involving the C-O stretching) are characteristic of its saccharide structure (Peniche et al. Citation1999). The band at 1651 cm−1 in the chitosan spectrum, which can be attributed to the formation of C═N, is due to imine reaction between amino groups of chitosan and aldehydic groups in glutaraldehyde.
We observed that the intensity of the absorption band in the range of 2800–3500 cm−1 represents the –OH groups of chitosan changed in C-P spectrum. This change indicates hydrogen bonding interaction between C═O groups of poly(N-vinylpyrrolidone) and –OH groups of chitosan with saccharide structure. The absorption bands at 1421, 1446, and 1472 cm−1 are assigned to the characteristic vibration of pyridine ring (Wu et al. Citation2003) and the absorption band at 1668 cm−1 is called amid-I (Lau and Mi Citation2002). The bands in spectrum C-P-A/2 IPN are the characteristic bands related to poly(acrylamide) crosslinked with N,N′-methylenebisacrylamide. These data clearly indicate the formation of an interpolymer-type system.
Thermogravimetric Analysis
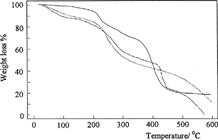
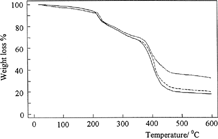
shows the thermograms for the dried samples of C, C-P, and C-P-A/0.5 in nitrogen atmosphere heated at 10°C min−1 from 0°C to 600°C. The thermogravimetric curve of C in shows three degradation steps. The first step, until 157°C, could be attributed to the loss of bound water, the second step, ranging from 218–300°C, corresponded to chitosan degradation. The third step, from 300–600°C, represented the total degradation of chitosan. Similar results were showed by Khalid et al. (Citation2002) with chitosan films.
As shown in , thermal stability of C-P-A/0.5 IPN until 410°C increases in comparison with C and C-P polymers. In the case of IPN, since the polymer chains are more closely tangled together, thermal stability of IPN is higher than those of other polymers. This indicates the formation of an IPN including C, P, and pAAm polymers.
Also, shows were the effect of the crosslinking concentration on thermal stability of IPN. Thermal stability of IPNs has increased with glutaraldehyde concentration in IPNs. This increase can be understood as a result of crosslinking, which significantly lowers the mobility of the chains.
Thermomechanical Analysis
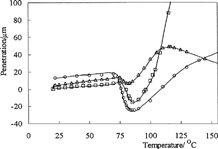
Thermomechanical thermograms of C-P-A IPNs are shown in . The values of maximum penetration of C-P-A/0.5, C-P-A/1, and C-P-A/2 IPNs were found as 25, 15, and 7 μ m, respectively, from the thermomechanical thermograms in . In other words, the values of maximum penetration is decreased with the addition of crosslinker to the IPN. Thus, it the thermomechanical stability of IPNs has increased with glutaraldehyde concentration.
Swelling Studies
To investigate the time-dependent swelling behavior of IPNs in solutions with different pH, we performed dynamic swelling studies. The swelling S% is calculated from the following relation:
(1)
where Mo is the mass of dry gel at time 0, Mt is the mass of swollen gel at time t.
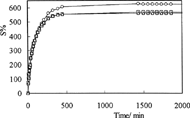
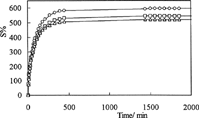
Swelling curves of C-P-A IPNs in solutions with pH 1.1 and pH 7.4 at 37°C are shown in and , respectively. As can be seen in these figures, the values of equilibrium swelling of IPNs have decreased with increasing glutaraldehyde concentrations in IPNs. The swelling degree of a hydrogel depends on its network structure, which is controlled by the concentration of the crosslinker.
For extensive swelling of polymers, the following relation can be written as (Katime, Valderruten, and Quintana Citation2001)
(2)
where S is the degree of swelling at time t, B = 1/Smax is the inverse of the maximum swelling, A = 1/(dS/dt)0 = 1/Smax2ks is the reciprocal of the initial swelling rate (r0) the gel. The relation represents second-order kinetics.
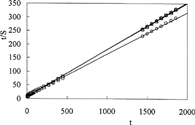
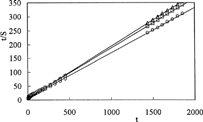
and show the linear regression of the swelling curves obtained by means of Equation [2]. The values of the initial swelling rate, r0 [g solution (g IPN)−1s−1] and maximum swelling, Smax [g solution (g IPN)−1] are calculated from the slope and intersection of the lines and swelling rate constant, ks [g IPN (g solution)−1s−1] are presented in .
Swelling and diffusion parameters of IPNs at pH 1.1
The following equation is used to determine the nature of diffusion of buffer solutions into IPNs:
(3)
Where F is the fractional uptake at time t, k is a constant incorporating characteristic of the macromolecular network system and the penetrant, and n is the diffusional exponent, which is indicative of the transport mechanism. Equation [3] is valid for the first 60% of the fractional uptake. Fickian diffusion and Case II transport are defined by n valuing to 0.5 and 1, respectively. Anomalous transport behaviour (non-Fickian diffusion) is intermediate between Fickian and Case II. That is reflected on n between 0.5 and 1 (Katime et al. Citation2001; Bajpai and Giri Citation2002).
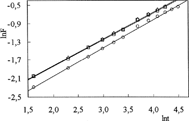
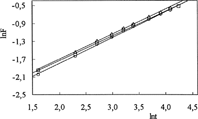
For the IPN hydrogels, lnF versus lnt graphs are plotted and shown in and . n exponents and k parameters are calculated from the slopes and intercepts of the lines, respectively, and are listed in .
The diffusion coefficients D of the cylindrical hydrogels were found by:
(4)
where D is in cm2 s−1, t in seconds, and r is the radius of cylindrical IPN sample. The diffusion coefficients were calculated from Equation [4], and are listed in and .
Swelling and diffusion parameters of IPNs at pH 7.4
and summarize the results of studying the swelling of obtained IPNs. As can be seen, with increasing crosslink concentration in IPN, the degree of IPN swelling decreases. Also, with increasing amount of crosslinking, an increase in network parameters, k, was observed. We observed that experimental Seq% values were close to calculated Smax% values. Results indicated that IPNs exhibited greater swelling at pH 1.1 when compared with swelling at pH 7.4. This can be explained due to the ionic interaction of the amino group of chitosan in stomach pH conditions. and show the number determining type of diffusion (n) is over 0.50 for C-P-A IPNs at both pHs. Hence the diffusion of solutions into the hydrogels is generally taken as a non-Fickian character. When diffusion type is anomalous behavior, the relaxation and diffusion time are of the same order of magnitude. As the solvent diffuses into the hydrogel, rearrangement of chains does not occur immediately.
Release of Drug from the IPNs
Amoxicillin was selected as our model drug; HCl-KCl solution and Na2HPO4-KH2PO4 (pH = 7.4) solution were used as modulated media. The percent cumulative release of amoxicillin from IPNs to solutions was calculated by:
(5)
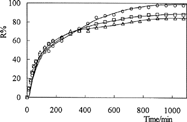
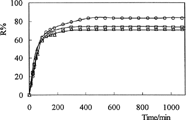
Where Mt and Mo are the amount of drug released at time, t and in the dry IPN, respectively. and depict the percent cumulative release of amoxicillin from IPNs at pH 1.1 and pH 7.4, respectively, at 37°C. The values of R% are presented in .
Release kinetic parameters of IPNs in solutions with different pH
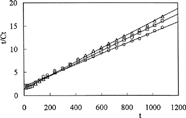
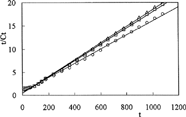
To investigate the parameters of release kinetics, Equation [6] was used and the plots of t/Ct versus t are presented in and .
(6)
Here, Ct is the amount of drug released at time t, β = 1/Cmax is the inverse of the maximum amount of released drug, α = 1/Cmax2krel = 1/ro is the inverse of the initial release rate, and krel is the constant of the kinetic of release. The kinetic parameters calculated from the lines in are presented in .
Drug release is pH-dependent. The percent cumulative release of amoxicillin from IPNs were higher in acidic medium than in basic medium. The diffusion of the drug molecules out of the chitosan-based IPNs was enhanced because of the swelling at low pH. We observed because release results were parellel to swelling results. This can be explained because drug release from IPNs to solutions is a swelling-controlled delivery system. The release time for amoxicillin was found 11 hr at pH 1.1 and 7 hr at pH 7.4. However, the release of drug has decreased when the quantity of glutaraldehyde in IPN increases. IPN hydrogels released 98%, 88%, and 84% of the entrapped drug at pH 1.1, compared with the release of 83%, 73%, and 71% of the drug at pH 7.4.
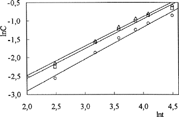
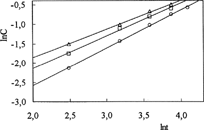
The relative importance of macromolecular relaxation on the mechanism of drug release can be easily assessed by fitting experimental release data to Equation [7] and determining the exponent, n:
(7)
It is of paramount importance that Equation [7] can be applied only to the first 60% of the total amount of drug released. Here, Ct and Ceq are the amounts of drug released at time, t, and at equilibrium, respectively; k is a proportionality constant, and nrel is the diffusional exponent (Soppirnath and Aminabhavi Citation2002; Geresh et al. Citation2004; Ahn et al. Citation2002). From the plot of lnC versus lnt ( and ), drug diffusion parameters, nrel and k, were calculated and presented in .
Release diffusion parameters of IPNs in solutions with different pH
Drug diffusion through swollen elastomeric polymers in thermodynamic equilibrium with the solvent is Fickian if nrel < 0.5, while diffusion in elastomeric polymers during continuous swelling can be Fickian or anomalous if nrel > 0.5. As can be seen from , IPNs presented a non-Fickian mechanism because nrel0 > 0.5, indicating that the process is partially controlled by the viscoelastic relaxation of the matrix during water penetration. Also, by raising the amount of crosslinker, the kinetic parameter, k, has generally increased.
CONCLUSION
We have been developing an IPN system by using three different polymer and crosslinker concentration to control network pores of IPNs. IPNs were prepared by polymerization of acrylamide monomers in presence of chitosan and poly(N-vinylpyrrolidone) as polymers and glutaraldehyde and N,N′-methylenebisacrylamide as crosslinkers. C-P-A IPNs obtained with cylindrical shape and containing different glutaraldehyde concentration display remarkable good mechanical strength during all experiments particularly swelling and release studies. We found that chitosan crosslinked with glutaraldehyde, poly(acrylamide) crosslinked with N,N′-methylenebisacrylamide and poly(N-vinylpyrrolidone) polymers formed IPN structure from the FT-IR results. Thermogravimetric and thermomechanical analysis results show that the properties of thermal stability and thermomechanical strength of IPNs have increased with the amount of glutaraldehyde in IPNs.
The swelling kinetics of IPNs have been carried out in SGF (pH 1.1) and SIF (pH 7.4) solutions at body temperature (37°C), together with amoxicillin release kinetics in the same solutions and temperature. We observed that the swelling and release kinetics follow second-order kinetics. The values of swelling and release were found higher in SGF solution than SIF solution due to the ionic interaction of the amino group of chitosan in stomach pH conditions. Also, the release time of amoxicillin from C-P-AAm IPNs to solutions with different pH was about 7–11 hr indicating that gastrointestinal transit time of oral dosage forms in human.
Consequently, this type of IPN device may be ideal to deliver drugs to the primary target in the stomach or in the upper small intestine.
REFERENCES
- Ahn J. S., Choi H. K., Chun M. K., Ryu J. M., Jung J. H., Kim Y. U., Cho C. S.. 2002. Release of triamcinolone acetonide from mucoadhesive polymer composed of chitosan and poly(acrylic acid) in vitro. Biomaterials. 23: 1411–1416. [PUBMED], [INFOTRIEVE], [CSA]
- Bajpai A. K., Bajpai J., Shukla S.. 2001. Water sorption through a semi-interpenetrating polymer network (IPN) with hydrophilic and hydrophobic chains. React. Funct. Polym.. 50: 9–21. [CROSSREF]
- Bajpai A. K., Giri A.. 2002. Swelling dynamics of a macromolecular hydrophilic network and evaluation of its potential for controlled release of agrochemicals. React. Funct. Polym.. 53: 125–141. [CROSSREF]
- Chakrabarty D.. 1998. Interpenetrating polymer networks: engineering properties and morphology. Polym. Gels Networks. 6: 191–204. [CROSSREF]
- Changez M., Burugapalli K., Koul V., Choudhary V.. 2003. The effect of composition poly (acryl acid)-gelatin hydrogel on gentamicin sulphate release in vitro. Biomaterials. 24: 527–536. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ekici S., Isikver Y., Sahiner N., Saraydin D.. 2003. Adsorption of some textile dyes onto crosslinked poly(N-vinylpyrrolidone). Adsorpt. Sci. Technol.. 21: 651–659. [CROSSREF]
- Elvira C., Mano J. F., Roman S. J., Reis R. L.. 2002. Starch-based biodegredable hydrogels with potential biomedical applications as drug delivery systems. Biomaterials. 23: 1955–1966. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Geresh S., Gdalevsky G. Y., Gilboa I., Voorspoels J., Remon J. P., Kost J.. 2004. Bioadhesive grafted starch copolymers as platforms for peroral drug delivery: a study of theophylline release. J. Control. Rel.. 94: 391–399. [CROSSREF], [CSA]
- Hejazi R., Amiji M. J.. 2003. Chitosan-based gastrointestinal delivery systems. J. Control. Rel.. 89: 151–165. [CROSSREF], [CSA]
- Karadag E., Saraydin D., Cetinkaya, S., Guven. O.. 1996. In vitro swelling studies and preliminary biocompatibility evaluation of acrylamide-based hydrogels. Biomaterials. 17: 67–70. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Katime I., Valderruten N., Quintana J. R.. 2001. Controlled release of aminophylline from poly(N-isopropylacrylamide-co-itaconic acid) hydrogels. Polym. Int.. 50: 869–874. [CROSSREF]
- Khalid M. N., Agnely F., Yagoubi N., Grossiord J. L., Couarraze G.. 2002. Water state characterization, swelling behavior, thermal and mechanical properties of chitosan based networks. Eur. J. Pharm. Sci.. 15: 425–432. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Koseva N., Manolova N., Markova N., Radoucheva T., Rashkov I.. 1999. Chitosan gel beads as drugs carriers. Polym. Bull.. 43: 101–107. [CROSSREF]
- Kosmala J. D., Henthorn D. B., Peppas L. B.. 2000. Preparation of interpenetrating networks of gelatin and dextran as degradable biomaterials. Biomaterials. 21: 2019–2023. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lau C., Mi Y.. 2002. A study of blending and complexation of poly(acrylic acid)/poly(vinyl pyrrolidone). Polymer. 43: 823–829. [CROSSREF]
- Oztop H. N., Saraydin D., Cetinus S.. 2002. pH-sensitive chitosan films for baker's yeast immobilization. Appl. Biochem. Biotech.. 101: 239–249. [CROSSREF], [CSA]
- Peniche C., Argüelles-Monal W., Davidenko N., Sastre R., Gallardo A., Roman J. S.. 1999. Self-curing membranes of chitosan/PAA IPNs obtained by radical polymerization: preparation, characterization and interpolymer complexation. Biomaterials. 20: 1869–1878. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Peppas N. A., Huang Y., Torres-Lugo M., Ward J. H., Zhang J.. 2000a. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng.. 2: 9–29. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Peppas N. A., Bures P., Leobandung W., Ichikawa H.. 2000b. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm.. 50: 27–46. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Risbud M. V., Bhonde R. R.. 2000. Polyacrylamide-chitosan hydrogels: in vitro biocompatibility and sustained antibiotic release studies. Drug Deliv.. 7: 69–75. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Saraydin D., Karadag E., Cetinkaya S., Guven O.. 1995. Preparation of acrylamide maleic acid hydrogels and their biocompatibility with some biochemical parameters of human serum. Radiat. Phys. Chem.. 46: 1049–1052. [CROSSREF]
- Soppirnath K. S., Aminabhavi T. M.. 2002. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur. J. Pharm. Biopharm.. 53: 87–98. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Torre P. M., Enobakhare Y., Torrado G., Torrado S.. 2003. Release of amoxicillin from polyionic complexes of chitosan and poly(acrylic acid), study of polymer/polymer and polymer/drug interactions within the network structure. Biomaterials. 24: 1499–1506. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Wu K. H., Wang Y. R., Hwu W. H.. 2003. FTIR and TGA studies of poly(4-vinylpyridine-co-divinylbenzene)-Cu(II) complex. Polym. Degrad. Stabil.. 79: 195–200. [CROSSREF]