Abstract
The purpose of this study was to develop and assess the in vitro characteristics of carbamazepine-loaded microspheres. A solvent evaporation method was used to incorporate carbamazepine (CBZ) into poly (D,L-lactide-co-glycolide) (PLGA) with different molecular weights. The optimum conditions for CBZ-PLGA microspheres preparation were considered and the in vitro release of CBZ of PLGA microspheres were followed up to 24 hr in USP dissolution medium. The effect of using different ratios of PLGA microspheres, prepared with different molecular weights, for optimizing CBZ release also was investigated. CBZ encapsulation efficiency was 68 to 82% for all prepared formulations. Thermograms of CBZ-PLGA microspheres suggest that CBZ was totally entrapped with the PLGA polymer. The presence of Pluronic F-68 has improved the encapsulation of CBZ, resulted in better and smoother microspheres surfaces and enhanced its release pattern. CBZ release profiles were biphasic patterns; after an initial burst, a constant CBZ release rate was observed up to 24 hr. The release from these PLGA-based spherical matrices was consistent with the diffusion mechanism. CBZ dissolution T50% was significantly affected (> 3-fold) by increasing the lactide percent from 33.3 to 66.6% from different microspheres mixtures. The present study provides evidence that the encapsulation of CBZ to PLGA microspheres, either as a single polymer or mixture of two, was a successful attempt to control the release of CBZ.
Epilepsy is one of the more common neurologic disorder affecting at least 0.5 to 1% of any population (Vickrey Citation1995; Annegers Citation1997). Antiepileptic drugs (AEDs) remain the cornerstone of therapy. The primary treatment goal is to achieve complete control of seizures without adverse events. However, for many patients becoming seizure-free remains a challenge. Toxicity and poor adherence to AED therapy are two common reasons why achieving seizure freedom is problematic (Pedley, Scheuer, and Walczak Citation1995; Leppik Citation2002).
Carbamazepine (CBZ), a tricyclic iminostilbene derivative, is among the most important antiepileptic drugs. It is the drug of choice for simple and complex partial and secondarily generalized seizures (Karceski, Morrell, and Carpenter Citation2001) as well as being a mood stabilizer in manic-depressive patients (DeVane Citation1990).
CBZ belongs to class II of the biopharmaceutical classification system. Compounds in this category have high intestinal permeability and low water solubility. Subsequently, the bioavailability of such compounds is limited by their solubility in water. There have been several reports concerning the polymorphism of CBZ and its influence on solubility and bioavailability. At least four different anhydrates and one dihydrate form have been identified for CBZ. Differences in bioavailability have been observed among various commercial formulations of CBZ as well as among the different polymorphic forms (Meyer et al. Citation1992).
During repeated administration of CBZ, its elimination half-life (t1/2) is significantly decreased due to autoinduction of its own metabolism. CBZ t1/2 is ∼24 hr, after single dosing, where as on chronic dosing it is lowered to ∼12 hr under monotherapy or ∼8 hr in those patients who take other enzyme-inducing drugs (Ramsay et al. Citation1990).
The development of sustained-release or controlled-release formulations of CBZ is therefore of therapeutic relevance and has caught the attention of the pharmaceutical industry. These extended-release formulations can improve medication compliance, modify AED pharmacokinetics, and provide clinicians with greater ability to individualize therapy. Various methods, including the formulation of CBZ-modified release dosage forms (granules and tablets) as well as CBZ-controlled release matrix tablets were studied (Giunchedi et al. Citation1993; Ikinci et al. 1999; Rafiee-Tehrani et al. Citation2000; Barakat Elbagory, and Almurshedi Citation2003). Bioavailability studies in patients with epilepsy have shown that the commercially available CBZ extended-release formulations are bioequivalent to the immediate-release formulations (Garnett, et al. Citation1998; Thakker et al. Citation1992). Miller, Bergey, and Krauss (Citation2002) have shown that the central nervous system (CNS) side effects associated with immediate-release formulation of CBZ were reduced when patients were switched to an extended-release formulation.
In recent years, the application of biodegradable polymers to the preparation of pharmaceuticals has gained importance, fundamentally from their capacity to modulate the release of drugs or even to modify their distribution within the body (Brannon-Peppas Citation1995). Biodegradable polyester polymers such as poly (lactide-co-glycolide) (PLGA), a copolymer of poly lactic acid (PLA) and poly glycolic acid (PGA), is the most widely used biodegradable synthetic polymer. These polymers are utilized for long-term delivery of different classes of drugs in the form of either microspheres or nanoparticles (Allémann, Leroux, and Gurny Citation1998; Kawashima et al. Citation1998). Polyester polymers, approved by the Food and Drug Administration, have raised great interest due to their physicochemical and biological properties (Vert et al. Citation1998). In addition to their biocompatibility and bioresorbability properties, the possibility of modulating drug release profiles by selecting the appropriate polymer is particularly interesting for the development of sustained-release drug products. Drugs with diverse physicochemical properties and various therapeutic effects such as antibiotics, anti-inflammatory drugs, anticancer drugs, steroid, peptides, and proteins have been incorporated in the lactide/glycolide copolymer systems (Sanders et al. Citation1986; Ike et al. Citation1992; Mauduit, Bukh, and Vert Citation1993; Chandrashekar and Udupa Citation1996; Radwan and Abu El-Enein Citation2004).
To our knowledge, there not been has a report on the incorporation of CBZ with PLGA as drug delivery system. Therefore, the present study was undertaken to develop and optimize the release characteristics of CBZ loaded to various molecular weights of PLGA. The particle size, microspheres yield, drug loading, and surface morphology also were investigated.
MATERIALS AND METHODS
Carbamazepine was kindly supplied by Novartis Pharma (Cairo, Egypt). Poly (D, L-lactide-co-glycolide) 50:50, i.v. of 0.8, average molecular weight of 98000 (Resomer RG 506); poly (D, L-lactide-co-glycolide) 85:15, i.v. of 1.4, average molecular weight of 220000 (Resomer RG858); and poly (D, L-lactide) average molecular weight of 98000 (Resomer R208) were purchased from Boehringer Ingelheim (Ingelheim, Germany). Span 80 was purchased from Fluka Chemie (Buchs, Switzerland) and Arlacel 86 was obtained from Atlas Chemicals (USA). Pluronic F-68 Prilled was obtained from Ruger Chemical Co. (Irvington NJ, USA). All other reagents and chemicals were analytical grade and were used as received.
Microsphere Preparation
CBZ-PLGA microsphers were prepared by emulsion solvent evaporation method (Arshady Citation1991). The polymer solution (9 ml) was prepared by dissolving a fixed amount, 1.2 g of the polymer, in acetonitrile without or with 0.5% Pluronic F- 68. The polymer concentration in acetonitrile was kept constant (13.3%) in all formulations to avoid any change in polymer viscosity that could affect the droplet size of the inner phase and hence the particle size of the final microspheres. The inner phase was prepared by dissolving different amounts of drug in the polymer solution, based on drug-to polymer ratio of 1:2, 1:3, and 1:4. This phase was added using a 10-ml syringe to heavy liquid paraffin (125 ml) containing 0.5% Span 80 heated to 50–55°C and stirred at a constant stirring rate of 800 rpm, using a mechanical stirrer (Fisher Scientific, USA, connected to Fisher Dyna-Mix for speed adjustment). Stirring was continued for 3 hr to ensure complete evaporation of acetonitrile. The microspheres were collected by vacuum filtration and washed twice with n-hexane containing 1% Arlacel 86 to remove mineral oil, and twice with n-hexane to remove any traces of surfactant. Microspheres were filtered, dried at room temperature, and stored refrigerated in desiccators until used. Each formulation was prepared in triplicate.
Microsphere Yiel
Microspheres were sieved to remove any formed polymeric sheets and microsphere aggregates. The yield was calculated as a percentage of the original amount of polymer and drug. The measurement was performed in triplicate sets.
Particle Size and Morphological Characteristics
An optical microscope (Olympus–Cover-018) with a camera attachment (Minolta) was used to observe the shape of the prepared microspheres for each drug: PLGA ratio. The shape and surface characteristics of selected CBZ-PLGA microspheres were studied by scanning electron microscopy (Joel Scanning Microscopy, Tokyo, Japan).
Drug Content
Microspheres with particle size 315–500 μm were used in the rest of the study. CBZ content in the CBZ-PLGA microspheres was assessed by dissolving known amount of CBZ-PLGA microspheres in acetonitrile. The mixture was kept in ultrasonic bath for 15 min (at 5 C) and shacked for 1 hr at 200 cycles / min. The resultant solution was filtered through 0.22 μm Millipore filter and was measured by spectrophotometer at 285 nm. A blank was used containing the same weight of polymer. There was no interference from the polymer in the drug measurements. The drug content was calculated as the mean percentage of three replicates.
Infrared Spectroscopy (IR)
CBZ-loaded microspheres were evaluated by comparing the IR spectra of the solid microspheres and of pure CBZ and pure polymers. Blends corresponding to 1.5 mg of samples and 150 mg of KBr were produced compressed and recorded in the region of 4000–400 cm-1
Thermal Analysis
Thermal analysis of CBZ, PLGA, and prepared microspheres was done with a differential scanning colorimeter (DSC912, Dupont Instruments, UK) to evaluate the change in glass transition temperature between the raw materials and the prepared microspheres. Approximately 5 mg of samples were placed in hermetically sealed aluminum pans and scanned at a rate of 10°C/min over a temperature range of 25 to 200°C in closed aluminium pans under an argon purge.
In Vitro Dissolution Testing
CBZ in vitro release from CBZ-PLGA was followed in 900 ml water containing 1% sodium lauryl sulphate using USP apparatus, paddle system. Samples containing 200 mg of CBZ were added to dissolution medium set at 37 ± 0.5°C and 75 oscillations per min. At each sample time interval, an exact volume of sample (5 ml) was withdrawn (through a 0.22 μm Millipore filter) from each vessel and immediately replaced with an identical volume of fresh medium, kept under the same condition, to maintain a sink condition. The drug release was followed up to 24 hr. The CBZ released was determined by ultraviolet spectroscopy at 285 nm (placebo PLGA microspheres were treated in the same manner). The experiment was performed in triplicate sets.
Data Analysis
All in vitro data were reported as the mean ± SD of at least three parallel studies. The release kinetic model and mechanism of the microspheres that showed slow release in the dissolution media were investigated. Three release kinetic equations models including zero-order (Eq. 1), first-order (Eq. 2), and Higuchi model (Eq. 3) were applied to the in vitro release data to determine the equation that best fit (James et al. Citation1994).
where Q is the release percentage at time (t) and k0, k1, and k are the zero-order, first-order, and Higuchi rate constants, respectively. The amount of CBZ released was calculated from the linear regression equation of the drug dissolution data. The Peppas equation (Eq. 4) is commonly used to assess the mechanism of drug release from extended-release matrix (Peppas Citation1985; Ritger and Peppas Citation1987). It is simply more generalized than the Higuchi's relationship. Where n represents the diffusional release exponent, K is a parameter characteristic of the polymer network and active agent, and Mt/M_∞ is the fractional drug released:
RESULTS AND DISCUSSION
CBZ-PLGA microsphers prepared by emulsion solvent evaporation technique showed a promising microspheres yield (75.1–89.9%) for all tested drug-to-polymer ratio of CBZ. This finding is agrees with previously published data using this polymer (Elkheshen and Radwan Citation2000).
Different ratios of initial drug loading to polymer (1:2, 1:3 and 1:4) were selected to study the encapsulation efficiency of PLGA to CBZ. As the percentage of drug loading increases, from 20% to 33%, there was insignificant increase in the encapsulation efficiency (P > 0.05) as illustrated in .
TABLE 1 The effect of formulations on Cadrbamzepine microspheres characteristics
The CBZ content in PLGA-CBZ microspheres was almost similar in all formulations (68.2%–82.0%). The same trend was observed for the mean diameter of PLGA-CBZ microspheres (298–453 μ m). Interestingly, microspheres prepared by low molecular weight polymers have mean diameters of 298 μm, whereas the ones prepared by higher molecular weight polymers have mean diameters (453 μ m) and loading efficiency that were not significantly changed. Jallin and Nixon (Citation1990) reported that lowering the microspheres diameters were observed with respect to lower polymer molecular weight.
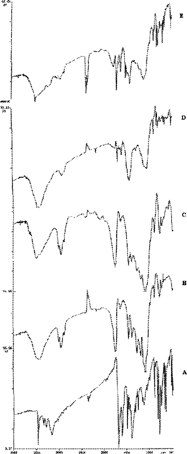
IR spectra of CBZ, PLGA, and CBZ loaded to PLGA are shown in . Bands of CBZ are observed at 3474 cm-1 ( NH valence vibration), 1686 cm-1 (
NH valence vibration), 1686 cm-1 ( CO
CO R) vibration, 1603 and 1593 cm-1 (range of
R) vibration, 1603 and 1593 cm-1 (range of  C
C![]() C
C![]() and
and  C
C![]() O vibration and
O vibration and  NH deformation), and 1395 cm-1 which are quite the same described for CBZ polymorph II (Rutichelli et al. Citation2000). The presence of
NH deformation), and 1395 cm-1 which are quite the same described for CBZ polymorph II (Rutichelli et al. Citation2000). The presence of  NH valence vibration at an intermediate wave number (3474 cm-1) was the major indicative sign that CBZ could be neither polymorph III (3464 cm-1) nor polymorph I (3484 cm-1). A PLGA spectrum presents a large band and a peak in the region of 2955–3750 cm-1, a distinct peak at 1760 cm-1, and a band in the region of 2348–2360 cm-1. A spectrum of CBZ-loaded microspheres shows that PLGA broad bands would not affect CBZ main characteristic peaks. Although the peak at 3474 cm-1 was partially hidden by the broad band of PLGA, the peaks at 1678 cm-1 and 1599 cm-1 are less intense in CBZ microspheres. This was expected since CBZ content is ∼20–33% in the final microspheres. Additionally, the band corresponding to PLGA at 1750 cm-1 appears in the IR spectrum of the microspheres.
NH valence vibration at an intermediate wave number (3474 cm-1) was the major indicative sign that CBZ could be neither polymorph III (3464 cm-1) nor polymorph I (3484 cm-1). A PLGA spectrum presents a large band and a peak in the region of 2955–3750 cm-1, a distinct peak at 1760 cm-1, and a band in the region of 2348–2360 cm-1. A spectrum of CBZ-loaded microspheres shows that PLGA broad bands would not affect CBZ main characteristic peaks. Although the peak at 3474 cm-1 was partially hidden by the broad band of PLGA, the peaks at 1678 cm-1 and 1599 cm-1 are less intense in CBZ microspheres. This was expected since CBZ content is ∼20–33% in the final microspheres. Additionally, the band corresponding to PLGA at 1750 cm-1 appears in the IR spectrum of the microspheres.
FIG. 1 INfrared spectra of CBZ, PLGA, and CBZ loaded to PLGA, where: A = Pure CBZ, B = RG506, C = RG 858, D = CBZ:RG 506 (1:3) microspheres and E = CBZ:RG 506 (1:3) microspheres.
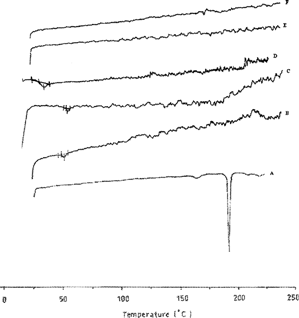
Differential scanning calorimetry (DSC) is a thermal analytical technique there provides information about the physical properties of the products, as the crystalline or amorphous nature of the tested samples. Quantitative information about exothermic, endothermic and heat capacity changes as a function of temperature or time is provided (Clas, Dalton, and Hancock 1991). DSC also can demonstrate a possible interaction between different compounds of a mixture (Fathy, Hassan, and Mohamed Citation2002). The respective thermograms of CBZ, PLGA microspheres are shown in . It was demonstrated that PLGA 858, 506, and PLA 208 were amorphous and their glass transition temperatures (Tg) are 43, 52, and 58°C, respectively. The DSC curves also showed large relaxation endotherm. The DSC trace of the pure CBZ shows two endothermic peaks; one at 158.8°C, corresponding to transition from polymorph II to I and the second at 189.9°C, corresponding to melting of polymorph I. This attribute of polymorph II is in agreement with Rustichelli et al. Citation2000 and Naima et al. Citation2001.
FIG. 2 Differential scanning calorimetry of CBZ, PLGA microspheres where: A = Pure CBZ, B = RG506, C = R208, D = RG858, E = CBZ:(RG858 + 208) 1:3, and F = CBZ:(RG858 + 506) 1:3.
There is consensus in the literature that CBZ crystallizes to three forms, at least: a trigonal (form II), a monoclinic (form III), and a high-temperature phase (form I) (Krahn and Mielck Citation1989).
Thermograms of CBZ-PLGA microspheres displayed corresponding peaks to CBZ melting, at 192°C, which is supposed to be caused by an interaction with PLGA, during heating. Absence of CBZ melting peak suggests that CBZ was totally entrapped inside the polymer; the drug was present as an amorphous phase in the microspheres. This is similar to the recent report by Passerini and Craig (Citation2002) of cyclosporine A loaded to poly (d,l-lactide-co-glycolide) microspheres using modulated temperature DSC.
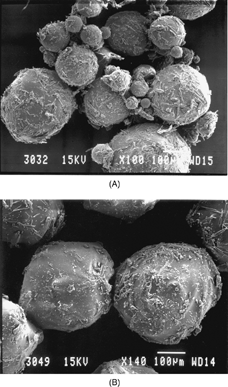
and represent the electron scanning micrographs of CBZ-PLGA microspheres, in the presence or absence of Pluronic (0.5%) in the formulation process, respectively. The presence of Pluronic has improved the encapsulation of CBZ and resulted in better and smoother microsphere surfaces.
FIG. 3 The electron scanning micrographs of CBZ-PLGA microspheres, in the presence “A” or absence “B” of pluronic (0.5%) in the formulation process, respectively.
The drug release from PLGA microspheres depends upon various factors including the polymer composition, polymer molecular weight, drug loading, and the microstructure of microspheres (Bodmeier Citation1994; Jain et al. Citation1998). CBZ was released in a biphasic manner from the microspheres, characterized by burst effect followed by a slow release. The burst effect corresponds to the release of the drug located on or near the surface of the microspheres or release of poorly entrapped drug. The slow release period may be due to the medium being diffused into the polymer matrix, whereby degradation occurs and the drug diffuses out of the microspheres. The formulation with higher drug loading released the drug faster.
The microspheres prepared with polymer with higher glycolide content degrades faster than microspheres prepared with lower glycolide content; i.e. PLGA 50:50 degraded faster than PLGA 85:15 and D.L, Lactide (Jeffery Davis, and O'Hagan Citation1991; Vert, Li, and Garreau Citation1991). This could explain CBZ faster release from PLGA 50:50 compared with PLGA 85:15 and D, L, Lactide, at 1:3 ratios as shown in . PLGA (50:50) molecular weight (M Wt) 98,000 degraded faster compared with PLGA (85:15) M Wt 220,000. It is an indication that degradation was faster with a polymer of higher glycolide content and lower M Wt. Therefore, polymer M Wt has an effect on the extent of drug release (Watts, Davies, and Melia Citation1990). The glycolic units are more hydrophilic than lactic units and promote water uptake into the polymer which encourages hydrolytic cleavage (Dunn et al. Citation1988).
FIG. 4 The in vitro release of CBZ loaded to microspheres prepared with polymers with different Lacide and glycolide content.
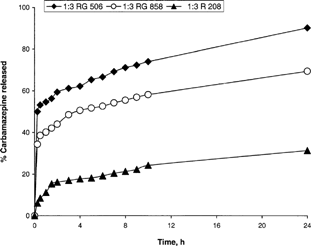
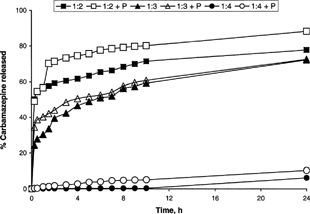
A steady drug release was observed for the studied ratios. There was significant difference between the drug released of 1:2 and 1:3 drug-to-polymer ratios. Surprisingly, the percent drug release of the 1:4 ratios was significantly lower compared with the other two. Fitting the drug release of the three tested ratios to different release kinetics showed approximately Higuchi release up to 24 hr (Eq. 3 above). demonstrated that 92% and 67% CBZ was released from RG858 and RG506, respectively, after 24 hr, while less than 25% CBZ was released from R 208 at the same time. To optimize CBZ release from PLGA microspheres, CBZ-loaded microspheres were prepared using mixture of RG858 and R208 and RG506 and R208 in different ratios of R208 ranging from 33.3 to 66.6% of the total polymer mixture.
The effect of 0.5% Pluronic F-68 (P) on the in vitro release of CBZ from CBZ-PLGA was followed for polymer RG858 prepared in different ratios. demonstrates that the addition of Pluronic F-68 to the drug-polymer solution enhanced CBZ release. Interestingly, this was in agreement with the morphological characteristic observed under electron scanning microscope (). At drug: polymer ratio of 1:3, the release enhancement was not significant (p > 0.05).
FIG. 5 The effect of 0.5% Pluronic F-68 (P) on the in vitro release of CBZ from CBZ-PLGA (RG 858) prepared in different ratios.
and show the in vitro release profile of CBZ from mixtures of RG858 and R208 or RG506 and R208-based microspheres, respectively. One can observe that increased-lactide percent from 33.3% to 66.6% can significantly influence CBZ release from different microspheres mixtures. T50% was increased more than 3 fold (7.2 to 22.2 hr or 1.5 to 5 hr) upon increasing R208 from 33.3 to 66.6% in the microspheres prepared with RG858 and R208 or RG506 and R208, respectively ().
FIG. 6 The in vitro release profile of CBZ from mixtures of RG858 + R208 PLGA-PLA-based microspheres in the presence of 0.5% Pluronic F-68.
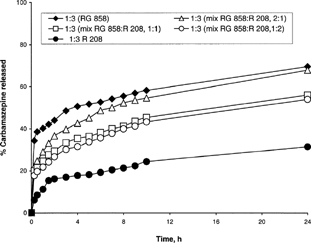
FIG. 7 The in vitro release profile of CBZ from mixtures of RG506 + R208 PLGA-PLA = based microspheres in the presence of 0.5% Pluronic F-68.
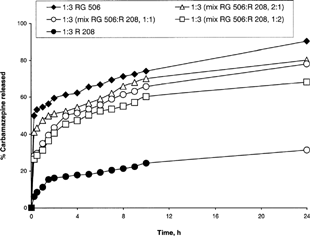
TABLE 2 Mean release kinetic parameters of carbamazepine from different microsphere formulations
Two possible mechanisms could explain the release of CBZ of PLGA microspheres: the dissolution/diffusion from the spherical matrices, or the matrix erosion resulting from degradation/dissolution of PLGA polymers. Since the bulk degradation of PLGA is trivial during the initial period, the drug release time, 24 hr in this study, is considerably shorter than the degradation lifetime of PLGA (Shih Citation1995; Hsu et al. Citation1996). The polymer backbone may retain its integrity without significant degradation/dissolution. The release rate constants according to different mechanisms were calculated and are shown in . In all cases, the most suitable mathematical model for describing the present data was the Higuchi equation. This is an indication that the diffusion was the main factor controlling the drug release rate, and the release mechanism was not significantly influenced by formulation variations. The n value, indicative of the release mechanism, has a value range of 0.339–0.505. As a result, drug release from the microspheres may be attributed to the Fickian diffusion mechanism. According to the model developed by Peppas, for a drug incorporated in a spherical matrix, by fitting the observed data shown in and to the spherical matrix model (for up to 60% of the total drug released), an average correlation coefficient of 0.995 was obtained. This simple analysis of CBZ release data suggests that the release from these PLGA-based spherical matrices was consistent with the diffusion mechanism (Yen et al. Citation2001).
The present study provides evidence that the encapsulation of CBZ to PLGA microspheres, either as a single polymer or mixture of two, was a successful attempt to control the release of CBZ. Two CBZ microspheres formulations, prepared with mixtures of CBZ and two polymers (1:3), were selected based on their dissolution T50%. These mixtures are RG858: R208, 2:1 (T50%= 7.2 hr) and RG506:R208, 1:2 ((T50%= 5 hr). These microspheres mixtures will be subject to future experiments to study their in vivo performance in animals, which would be beneficial in examining their practicality as drug delivery system.
REFERENCES
- Allémann E., Leroux J C., Gurny R. Biodegradable nanoparticles of poly(lactic acid) and poly(lactic-co-glycolic acid) for parenteral administration. Pharmaceutical Dosage Forms: Disperse Systems, H. A. Lieberman, M. M. Rieger, G. S. Banker. Marcel Dekker, New York 1998
- Annegers J. F. Epidemiology of epilepsy. The treatment of Epilepsy: Principles and Practice, 2nd ed, E. Wyllie. Williams & Wilkins, Baltimore 1997; 165–172
- Arshady R. Preparation of biodegradable microspheres and microcapsules: Polylactides and related polyesters. J. Control. Rel. 1991; 17: 1–22, [CSA]
- Barakat N. S., Elbagory I. M., Almurshedi A. S. (2003) Evaluation of prolonged release carbamazepine granules prepared from lipophilic matrices. 6th Saudi Pharmaceutical International Conference. October, 6–92003
- Brannon-P L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int. J. Pharmaceut. 1995; 116 1–9, [CROSSREF], [CSA]
- Bodmeier R. Polimeric microparticles prepared by solvent evaporation and spray-drying techniques. Third European Symposium on Controlled Drug Delivery, The Netherlands, 1994; 45–48
- Chandrashekar G., Udupa N. Biodegradable injectable implant systems for long term drug delivery using poly(Lactide-co-glycolic) and copolymers. J. Pharm. Pharmacol. 1996; 48: 669–674, [PUBMED], [INFOTRIEVE], [CSA]
- Clas S. D., Dalton C. R., Hancock B. C. Differential scanning calorimetry: applications in drug development. Pharmaceut. Sci. Tech. Today 1999; 2: 311–320, [CROSSREF], [CSA]
- DeVane L. Fundamentals of monitoring psychoactive drug therapy in drug therapy for mood disorders (E) intraclass comparisons of antidepressants and mood stabilizers. Williams & Wilkins, Baltimore 1990; 128–130
- Dunn R. L., English J. P., Strobel J. D., Cowsar D. R., Tice T. R. Preparation and evaluation of Lactide/glycolide co-polymers for drug delivery. Polymers in Medicine III, C. Migliaresi. Elsevier, Amesterdam 1988; 98–161
- Elkheshen S. A., Radwan M. A. Sustained release microspheres of metoclopramide using poly (D,L-lactide-co-glycolide) copolymers. J. Microencap. 2000; 17: 425–435, [CROSSREF], [CSA]
- Fathy M., Hassan M. A., Mohamed F. A. Differential scanning calorimetry to investigate the compatibility of ciprofloxacin hydrochloride with excipients. Pharmazie 2002; 57: 825–828, [PUBMED], [INFOTRIEVE], [CSA]
- Garnett W. R., Levy B., McLean A. M., et al. Pharmacokinetic evaluation of twice-daily extended-release carbamazepine (CBZ) and four-times-daily immediate-release CBZ in patients with epilepsy. Epilepsia 1998; 39: 274–279, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Giunchedi P., Maggi U., Conte U., La Manna A. Linear extended release of water insoluble drug, carbamazepine, from erodible matrices. Int. J. Pharmaceut 1993; 94: 15–22, [CROSSREF], [CSA]
- Hsu Y. Y., Gresser J. D., Stewart R. R., Trantolo D. J., Lyons C. M., Simons G. A., Gangadharam P. R. J., Wise D. L. Mechanism of izoniazid release from poly (d,l-lactide-co-glycolide) matrices prepared by dry mixing and low density polymeric foam methods. J. Pharmaceut. Sci. 1996; 85: 706–713, [CROSSREF], [CSA]
- Ike O., Schimizu Y., Wada R., Hyon S. H., Ikada Y. Controlled cisplatin delivery system using poly(d, L-Lactic acid). Biomaterials 1992; 13: 230–234, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ikinici G., Capan Y., Senel S., Dalkara T., Hincal A. A. Formulation and in-vitro/in-vivo investigation of carbamazepine controlled-release matrix tablets. Pharmazie 1999; 54: 139–141, [CSA]
- Jain R., Shah N. H., Malick A. W., Rhodes C. T. Controlled drug delivery by biodegradable poly(ester) devices: different preparative approaches. Drug Del. Ind. Pharm. 1998; 24: 703–727, [CSA]
- Jallin R., Nixon J. R. Microencapsulation using poly(DL-Lactic acid) II: effect of polymer molecular weight on the microcapsule properties. J. Microencap. 1990; 7: 245–254, [CSA]
- James E., Singh G., Larry L., Vinod P. Method to compare dissolution profiles and a rationale for wide dissolution specification for metoprolol tartrate tablets. J. Pharmaceut. Sci. 1994; 86: 690–700, [CSA]
- Jeffery H., Davis S. S., O'Hagan D. T. The preparation and characterization of poly (lactic-co-glycolide) microparticles: I. Oil-in-water emulsion solvent evaporation. Int. J. Pharmaceut. 1991; 77: 169–176, [CROSSREF], [CSA]
- Karceski S., Morrell M., Carpenter D. The expert consensus guideline series: treatment of epilepsy. Epilepsy Behav, 2001; 2: A1–A50, [CROSSREF], [CSA]
- Kawashima Y., Yamamoto H., Takeuchi H., Hino T., Hiwa T. Properties of a peptide containing DL-lactide/glycolide copolymer nanospheres prepared by novel emulsion solvent diffusion methods. Euro. J. Pharmaceut. Biopharmaceut. 1998; 45: 41–48, [CROSSREF], [CSA]
- Krahn F. U., Mielck J. B. Effect of type and extent of crystalline order on chemical and physical stabilityof carbamazepine. Int. J. Pharmaceut. 1989; 53: 25–34, [CROSSREF], [CSA]
- Lambert W. J., Peck K. D. Development of an in situ forming biodegradable poly-lactide-co-glycolide system for the controlled release of protein. J. Control. Rel. 1995; 33: 189–195, [CROSSREF], [CSA]
- Leppik I. E. Contemporary Diagnosis and Management of the Patient with Epilepsy 6th ed. Handbooks in Healthcare, Newtown, PA 2002; 119–127
- Mauduit J., Bukh N., Vert M. Gentamicin/poly(lactic acid) blends aimed at sustained release local antibiotic therapy administered per-operatively: III. The case of gentamicin sulfate films prepared from high and low molecular weight poly (d, l-lactic acids). J. Control. Rel. 1993; 25: 43–49, [CROSSREF], [CSA]
- Meyer M. C., Straughn A. B., Jarivi E. J., Woods G. C., Pelsor F. R., Shah V. P. The bioinequivalance of carbamazepine tablets with a history of clinical failures. Pharmaceut. Res. 1992; 9: 1612–1616, [CROSSREF], [CSA]
- Miller A., Bergey G., Krauss G. Conversion from immediate- to extended-release carbamazepine markedly reduces CNS-related side effects in patients with partial-onset epilepsy. Epilepsia 2002; 43(Suppl 7)196, [CSA]
- Naima Z., Toscani S., Gines-Dorado J.-M., Chemtob C., Cèolin R., Duguè J. Interactions between carbamazepine and polyethylene glycol (PEG) 6000: characterizations of the physical solid dispersed and eutectic mixtures. Eur. J. Pharmaceut. Sci. 2001; 12: 395–404, [CROSSREF], [CSA]
- Passerini N., Craig D. Q. M. Characterization of ciclosporin A loaded poly (D,L-Lactide-Co-glycolide) microspheres using modulated temperature differential scanning calorimetry. J. Pharm. Pharmacol. 2002; 54: 913–919, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Pedley T. A., Scheuer M. L., Walczak T. S. Epilepsy. Merritt's Textbook of Neurology, 9th ed., L. P. Rowland. Williams & Wilkins, Baltimore 1995; 845–68
- Peppas N. A. Analysis of Fickian and non-Fickian drug release from polymers. Pharmaceutica Acta Helvetia 1985; 60: 110–111, [CSA]
- Radwan M. A., Abu El-Enein H. In vitro release and stereoselective disposition of flurbiprofen loaded to poly (D,L-lactide-co-glycolide) nanoparticles in Rats. Chirality 2004; 16: 119–125, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rafiee-Tehrani M., Zehtabchii B., Aghaii-Moghaddam F., Mehdizadehi A. Effect of different polymers on the release characteristics of carbamazepine from stable controlled-release tablets prepared by the air suspension and wet granulation techniques. Acta Pharmaceutica 2000; 50: 291–301, [CSA]
- Ramsay R. E., McManus D. Q., Guterman A., Briggle T. H., Vazquez D., Perchalski R. Carbamazepine metabolism in humans: effect of concurrent anticonvulsant therapy. Therapeut. Drug Monit. 1990; 12: 235–241, [CSA]
- Ritger P. L., Peppas N. A. A simple equation for description of solute release II. Fickian and anomalous release from swellable device. J. Control. Rel. 1987; 5: 37–42, [CROSSREF], [CSA]
- Rustichelli C., Gamberini G., Ferioli V., Gamberini M. C., Ficarra R., Tommasini S. Solid-state study of polymorphic drugs: carbamazepine. J. Pharmaceut. Biomed. Anal. 2000; 23: 41–54, [CROSSREF], [CSA]
- Sanders L. M., Kell B. A., McRae G. I., Whitehead G. W. Prolonged controlled-release of nafarelin, a luteinizing hormone-releaseing hormone analogue, from a biodegradable polymeric implants: influence of composition and molecular weight of polymer. J. Pharmaceut. Sci. 1986; 75: 356–360, [CSA]
- Shih C. Chain-end scission in acid catalyzed hydrolysis of poly (d,l-lactide) in solution. J. Control. Rel. 1995; 34: 9–15, [CROSSREF], [CSA]
- Thakker K. M., Mangat S., Garnett W. R., Levy R. H., Kochak G. M. Comparative bioavailability and steady state fluctuations of Tegretol commercial and arbamazepine OROS tablets in adult and pediatric epileptic patients. Biopharmaceut. Drug Disp. 1992; 13: 559–69, [CSA]
- Vert M. Structure et comportement des polymères. Exemples des polymers biorésorbables. S.T.P. Pharma Science 1987; 3: 216–222, [CSA]
- Vert M., Li S., Garreau H. More about the degradation of LA/GA derived matrices in aqueous media. J. Control. Rel. 1991; 19: 15–26, [CROSSREF], [CSA]
- Vert M., Schwach G., Engel R., Coudane J. Something new in the field of PLA/GA bioresorbable polymers. J. Control. Rel. 1998; 53: 85–92, [CROSSREF], [CSA]
- Vickrey B. G. Special issue: advancesin the measurement of health-related quality of life in epilepsy. Qual. Life Res. 1995; 4: 83–85, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Watts P. J., Davies M. C., Melia C. D. Microencapsulation using emulsification/solvent evaporation: an overview of techniques and applications. Crit. Rev. Therap Drug Carrier Sys. 1990; 7: 235–259, [CSA]
- Yen S. Y., Sung K. C., Wang J. J., Hu O. Y. Controlled release of nalbuphine propionate from biodegradable microspheres: in vitro and in vivo studies. Int. J. Pharmaceut. 2001; 220: 91–99, [CROSSREF], [CSA]