Abstract
Environmentally responsive gel formulation for ocular controlled delivery of carteolol hydrochloride (HCl) was developed in an attempt to improve ocular bioavailability and hence decrease its systemic absorption and side effects. The viscosity and the ability of the prepared formulations to deliver carteolol HCl in vitro and in vivo were monitored and compared with an aqueous commercial solution. The effect of polymer concentration and drug concentration on the in vitro release of carteolol HCl was examined. Gelrite formulations showed pseudoplastic behavior with thixotropic characteristics and the viscosity of the prepared systems increased as the concentration of the polymer increased. At fixed drug concentrations, as the Gelrite concentration increased, the drug release decreased. At fixed polymer concentrations, as the drug concentration increased the release of drug increased. Gelrite formulation (0.4% w/w) containing 1% drug showed significantly improved bioavailability compared with the commercial aqueous solution (Arteoptic® 1%). The developed in situ gel formulation showed potential for use as delivery systems with superior ocular bioavailability of carteolol HCl.
The most common way to deliver drugs to the eye is to instill an aqueous solution of the drug into the eye. The bioavailability of a drug introduced in this way is often very low, typically 1% or less (Järvinen et al. Citation2000). The low bioavailability is attributed to extensive precorneal drug loss by nasolachrymal drainage, in conjunction with low drug permeability of the cornea (Järvinen et al. Citation2000). The residence time can be prolonged by adding a polymer to the drug solution and hence increasing the viscosity of the vehicle (Lee and Robinson Citation1986). Other ways of increasing the contact time involve the use of ointment (Lee and Robinson Citation1986) or inserts (Gurtler and Gurny Citation1995). Hydrogels are better tolerated by patients than ointments; they do not obstruct vision and when formulated as in situ gels preparations offer the advantage of convenient administration. Environmentally responsive gels (in situ gels) are instilled as low viscosity solutions into the conjunctival sac of the eye. Upon contact with the eye, the polymer changes conformation producing a gel that combines the advantages of a solution, patient convenient, and favorable residence time.
Gelrite (deacetylated gum), one of the most interesting environmentally responsive gelling polymers, is a polysaccharide composed of tetrasaccharide repeating units (Rozier et al. Citation1989). It is gelled in the presence of cations in the tear fluid. Once gelled, the formulations resist the natural elimination process of drainage from the precorneal area; their residence time at the site of drug absorption is prolonged (Rozier et al. Citation1989; Carlfors et al. Citation1998) and subsequently the amount of drug absorbed is increased.
Carteolol is a nonselective β -adrenergic receptor blocking agent and commonly used in the treatment of glaucoma (Chrisp and Sokin Citation1992). However, it has not been marketed as an ocular in situ gel yet. The aim of our work is to develop and evaluate environmentally responsive gel (in situ gel) formulations to improve ocular bioavailability and hence decrease the systemic absorption and side effects of carteolol hydrochloride (HCl).
MATERIALS AND METHODS
Chemicals include carteolol HCl (CIBA Vision Ltd., Switzerland), Gelrite®, or gellan gum (Kelcogel® F) (CP Kelco, Liverpool, UK), commercial eyedrops (Arteoptic® 1%, CIBA Vision), theophylline anhudrous (Boehringer Ingelheim, Germany), and methanol HPLC (BDH Laboratory). All other chemicals and organic solvents were commercially available products of analytical grade.
Preparation of Gelrite–Based Formulations
Appropriate quantities of Gelrite were dispersed in ultrapure deionized water containing 0.005% w/w Tween 80 and sorbitol (isotonicity agent) to obtain 0.2, 0.4, and 1% w/w polymer concentration. The dispersions were heated to 90°C for 20 min while stirring. The solutions were allowed to cool at room temperature, then various amounts of carteolol HCl were added to obtain drug concentrations of 0.5, 1, 2%. The pH was adjusted to 7.4 ± 0.1 using drops of 0.5 M NaoH, and the dispersion was equilibrated at 4°C overnight.
Rheological Studies
For rheological measurements, Gelrite gels were prepared using simulated tear fluid (STF) as solvent. All examined gel samples contained no drug and were equilibrated at 34° C ± 0.1°C prior to each measurement. Viscosity was measured by Brookfield viscometer RVDV-II using spindle no. 6. The speed was increased from 0 to 6 rpm with 15 sec between each two successive speeds, and then in descending order speed setting from 6 to 0.3 rpm. The gel formulations were sheared at a relatively low speed (0.3–6 rpm) to avoid destruction of the structure of the gel. The viscosity was read directly from the viscometer display. All measurements were made in triplicate.
In Vitro Release Studies
The in vitro drug release from Gelrite formulations and the commercial brand (Arteoptic® 1%) was carried out using a small cylindrical glass container with surface area of 1.766 cm2. Cellophane membrane, molecular weight cut-off: 6000–8000, previously soaked in simulated tear fluid, was placed over one end of the cylindrical container and clamped into position. An aliquot of 0.5 ml of the examined formulations was placed over the cellophane membrane and the end of cylindrical container was immersed in 100 ml of simulated tear fluid kept at 34°C and stirred at 20 rpm. Samples, 2 ml each, were drawn at the following time intervals: 5, 10, 15, 30, 45, 60, 90, 120, 180, 240, and 300 min. Each sample was replaced by fresh STF. The concentration of carteolol HCl was analyzed spectrophotometrically at λ max 252 nm (Sasaki et al. Citation1997). Each experiment was performed in triplicate.
In Vivo Evaluation
For each examined formulation, a group of 36 rabbits was assigned and subdivided into 4 subgroups each of 9 animals. All animals in the study conformed to the guidelines of animal experimentation in King Saud University. First, 25 μl of 0.4% Gelrite-1% carteolol HCl formulation or Arteoptic® 1% were placed in the lower conjunctival sac. The animal was sacrificed at the following time intervals: 0, 15, 30, 60, 90, 120, 180, 240, 300 min and the aqueous humor samples were withdrawn. The samples were withdrawn through the corneal-scleral junction and slightly upward into the anterior chambers (Sasaki et al. Citation1997). The aqueous humor samples were immediately centrifuged for 20 min at 10°C and 20,000 rpm. Aqueous humor (175 μ l) was mixed with 25 μ l theophilline solution (20 μ g/ml) as internal standard and 150 μ l methanol. The mixture was vortexed for 30 sec then centrifuged for 20 min at 10°C and 20,000 rpm. An aliquot of the supernatant was injected onto the HPLC. Each experiment was repeated 4 times. Calibration curve was constructed in a concentration range of 1–7 μg/ml of carteolol HCl.
HPLC Assay
The HPLC system (Jasco, UV-1575 detector, Pu-1580 pump, Jasco Corporation, Tokyo, Japan; Shimadzu C-R6A Chromatopac integrator, Tokyo, Japan) was used in the reversed-phase mode (Sasaki et al. Citation1995). Analysis was performed on a Novapack C18 packed column (150 mm length × 3.9 mm i.d.). The mobile phase was a mixture of methanol and 50 mM NaH2 PO4 (25: 75 v/v). The flow rate was 1 ml min-1. Ultraviolet detector was used at 252 nm. The retention time of carteolol HCl and theophylline was 5.7 and 4 min, respectively.
Calculation of Pharmacokinetic Parameters
The area under the aqueous humor concentration-time curve (AUC0 –300min μg.min/ml) was calculated based on the trapezoidal rule using Stripe computer program (College of Pharmacy, University of Illinois, Chicago, IL, USA 1984). The Cmax and Tmax were obtained directly from the aqueous humor concentration-time profiles. The relative bioavailability (Frel) of tested formulation was finally calculated according to the following formula:
Local Irritation Study
Local irritation was determined in albino rabbits. An aliquot of 25 μl of 0.4% Gelrite-1% carteolol HCl was applied in one eye and compared with that of the other eye of the same animal in which phosphate buffer (pH 7.4) was instilled as control drops (Sasaki et al. Citation1995). The potential ocular irritation of the tested formulations was evaluated based on the blinking count for 3 min after instillation of 25 μ l of the in situ gel formulation and by observing any redness or increased lachrymation before treatment, and 0.5, 1, 2, and 3 hr after instillation. Each experiment was performed in triplicate.
Statistical Analysis
Analysis of variance (ANOVA) was used to test the differences between the calculated parameters using SPSS Statistical Package (Version 10, SPSS Inc., USA 1999). Statistical differences yielding p≤ 0.05 were considered significant. Duncan multiple comparison was applied when necessary to identify which of the individual formulations was significantly different.
RESULTS AND DISCUSSION
Rheological Study
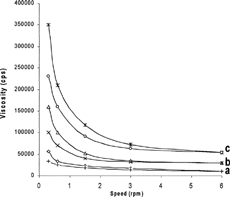
The flow curves of Gelrite gels (viscosity against speed) () indicated that at the examined polymer concentrations (0.2–1.0%), pseudoplastic systems were obtained. Anticlockwise hysteresis loops (thixotropy), a pseudoplastic nature characteristic, were observed in the rheograms of Gelrite gels. Thus, the prepared gels tend to thin after being exposed to a shearing force, followed by a tendency to thicken when the stress is removed. It is also obvious that as the concentration of polymer increased, the viscosity also increased. As the concentration of Gelrite increased, the polymer chains approach closer and the number of interactions between polymer chain increases which lead to more dense three-dimensional network structure. It also was proposed that, in polymer solutions, the various interactions between polymer chains regarded as localized junctions are responsible for an increase in viscosity and exhibit non-Newtonian flow (Lodge Citation1956).
Release Study
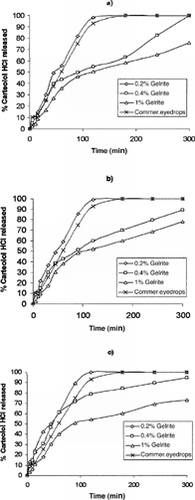
Statistical analysis (ANOVA) of the percent carteolol HCl released showed that the release of carteolol from 0.4% and 1% Gelrite gels () was significantly lower than that of commercial solution (p ≤ 0.001). Understanding the nature of the gelation process exhibited by Gelrite can help to explain why Gelrite showed higher drug release in vitro. X-ray diffraction study conducted on gellan indicated that the molecular structure is an extended, intertwined, 3-fold left-handed double helix where the polysaccharide is stabilized by interchain hydrogen bonds (Hernandez et al. Citation2003). It is worth mentioning that the internal structure of the formed gel as well as the mode of drug entrapment within the gel matrix and the viscosity of the gel would have appreciable effect on the drug release from the prepared gel formulations.
FIG. 2 Release profiles of drug from various Gelrite formulations containing (a) 0.5%, (b) 1%, and (c) 2% carteolol HCl in comparison with 1% carteolol HCl commercial eyedrops.
The in vitro release data were kinetically analyzed according to zero-order, first-order, and diffusion-controlled release mechanism (Higuchi model) (Higuchi Citation1962). The relative high correlation coefficient values obtained from the analysis of amount of drug released versus square root of time indicated the fitting of the data to the Higuchi kinetic model, as shown in .
TABLE 1 Correlation coefficients for kinetic analysis of the release data and diffusion coefficients of carteolol HCl calculated for various formulations
We think only one study has been reported that investigated drug release kinetics from Gelrite gels (Sanzgiri et al. Citation1993). It documented that the release of methylprednisolone from the gellan eyedrops followed a square root of time relationship. The release of carteolol HCl decreased significantly (p ≤ 0.01) as the concentration of in situ gel polymer increased. The release from various Gelrite formulations can be ranked as follows at all levels of drug concentrations: 0.2% > 0.4% >1%. These results indicated that the structure of the gel became more closely packed and functioned as an increasingly resistant barrier to drug release as the concentration of polymer increased. This also was due to increased viscosity of Gelrite gels, as the polymer concentration increased as indicated by the results of rheological study.
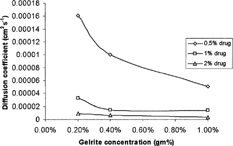
The calculated diffusion coefficient () decreased nonlinearly as the concentrations of polymer increased, as shown in . Regression analysis showed that the data were best fitted by quadratic equations. The lower diffusion coefficients with the higher polymer concentration was in agreement with Lauffer's diffusion theory in gels (Lauffer Citation1961) which stated that the diffusion coefficient of solute is inversely proportional to the volume fraction occupied by the gel forming agent.
FIG. 3 Effect of concentration of Gelrite on the diffusion coefficient of carteolol HCl from various prepared formulations.
At fixed polymer concentration, as the drug concentration increased there was significant change (p ≤ 0.01) in percent drug released as indicated by the statistical test (ANOVA). Duncan test revealed that the effect of drug concentration on the percent released of carteolol HCl from Gelrite (at all levels of polymer concentrations) can be ranked as follows: 2% > 1% > 0.5%; i.e., as the drug concentration increased, the percent drug released also increased.
It is surprising that the calculated diffusion coefficient decreased as the drug concentration increased at all tested polymer concentrations (). Theoretically, the diffusion coefficient should be independent of initial concentration (Co) as long as Co is below the solubility of drug in the vehicle (Chen-Chow and Frank Citation1981). However, it has been reported that other factors were influencing drug release in addition to the concentration of the drug in the gel. Bottari et al. (Citation1979), reported that the dependency of apparent diffusion coefficient on initial drug concentration was not an uncommon phenomenon, and results from various physico chemical changes, such as that of the partition coefficient of a drug at an elevated initial concentration, also could affect the drug release. The dependence of apparent diffusion coefficients on drug concentration was also reported by Veyries et al. (Citation1999) and Chen-Chow and Frank (Citation1981).
Although the in vitro drug release conditions may be very different from those likely to be encountered in the eye, the results clearly showed that the gel formulations have better ability to control the release of carteolol HCl than the commercial eyedrops.
In Vivo Studies
A selected formulation, namely, 0.4% Gelrite-1% carteolol HCl was chosen to be tested in vivo and compared with commercial eyedrops, Arteoptic® 1%. shows the level of carteolol HCl in aqueous humor after instillation of the selected gel formulation and the commercial product. The aqueous humor drug content was significantly higher at all time points after administration of carteolol HCl formulation than that obtained after instillation of 1% commercial product.
FIG. 4 Concentration of carteolol HCl in aqueous humour after instillation of aqueous commercial solution (Arteoptic®), and 0.4% Gelrite-1% carteolol HCl in situ gels.
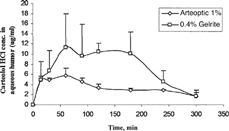
lists AUC0–300min, Cmax, and Tmax of carteolol HCl after instillation of the selected gel formulations as well as commercial eye drops (Arteoptic® 1%). Maximum concentration (Cmax) achieved from commercial product was 6.1 μg/ml compared with 15.4 for 0.4% Gelrite formulation. The faster precorneal clearance for aqueous solution may result in a significant low Cmax obtained after application of commercial eyedrops. Time to reach maximum concentration (Tmax) for the examined formulations was almost the same. AUC 0-300min observed for 0.4% Gelrite formulation was about 2.3 times higher than that calculated for commercial eyedrops (Arteoptic® 1%). This confirmed the superior bioavailability of carteolol HCl administered as in situ gel formulations when compared with the commercial eyedrops (Arteoptic® 1%).
TABLE 2 Pharmacokinetic parameters of carteolol HCl following ocular application of 25 μl of 0.4% Gelrite-1% carteolol HCl formulation and commercial eye drops (Arteoptic® 1%)
Local irritation test was performed to provide estimation of human ocular response to the tested products. Griffith et al. (Citation1980) have reported a good correlation between rabbit eye response and human eye response. We did not observe any redness, swelling, or excessive lachrymation after installation of 0.4% Gelrite -1% carteolol HCl formulation. The normal average of blinking counts in rabbits was documented to be 2–5 times/min (Sasaki et al. Citation1995). The counted blinking rates obtained after instillation of tested formulation was within the normal range (3–5 blink/min).
CONCLUSION
The examined in situ gel formulation, 0.4% Gelrite-1% carteolol HCl, showed significantly improved ocular bioavailability compared with the commercial aqueous solution (Arteoptic®1%). The developed formulation seemed to be a viable alternative to conventional eyedrops by virtue of ease of development of formulation, its good ocular tolerance, and its ability to enhance ocular bioavailability.
REFERENCES
- Bottari F., Carelli V., DiColo G., Saettone M. F., Serafini M. F. A new method for determining the diffusional coefficient of drugs in semi-solid vehicles from release data. Int. J. Pharm. 1979; 2: 63–79, [CSA]
- Carlfors J., Edsman K., Petersson R., Jornuing K. Rheological evaluation of Gelrite® in situ gels for ophthalmic use. Eur. J. Pharm. Sci 1998; 6: 113–119, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Chen-Chow P. C., Frank S. In vitro release of lidocaine from pluronic F-127 gels. Int. J. Pharm. 1981; 8: 89–99, [CSA]
- Chrisp P., Sokin E. M. Ocular carteolol. A review of its pharmacological properties, and therapeutic use in glaucoma and ocular hypertension. Drug Aging 1992; 2: 58–77, [CSA]
- Griffith J. F., Nixon G. A., Bruce R. D., Reer P. J., Bannan E. A. Dose–response studies with chemical irritants in the albino rabbit eye as a basis for selecting optimum testing conditions for predicting hazard to the human eye. Toxicol. Appl. Pharmacol. 1980; 55: 501–513, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Gurtler F., Gurny R. Patent literature review of ophthalmic inserts. Drug Develop. Ind. Pharm. 1995; 21: 1–18, [CSA]
- Hernandez A. I., Durand S., Garnier C., Tecante A., Doublier J. L. Rheology-structure properties of gellan systems: evidence of network formation at low gellan concentrations. Food Hydrochlolloids 2003; 17: 621–628, [CROSSREF], [CSA]
- Higuchi W. I. Analysis of data on the medicament release from ointments. J. Pharm. Sci. 1962; 51: 802–804, [PUBMED], [INFOTRIEVE], [CSA]
- Järvinen T., Pate D. W., Lain K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2000; 295: 203–220, [CSA]
- Lauffer M. A. Theory of diffusion in gels. Biophys. J. 1961; 1: 205–213, [PUBMED], [INFOTRIEVE], [CSA]
- Lee V. H. L., Robinson J. R. Review: topical ocular drug delivery: recent developments and future challenges. J. Ocul. Pharmacol. 1986; 2: 67–108, [PUBMED], [INFOTRIEVE], [CSA]
- Lodge A. S. A network theory of flow birefringence and stress in concentrated polymer solutions. Trans. Faraday Soc. 1956; 52: 120–130, [CROSSREF], [CSA]
- Rozier A., Mazuel C., Grove J., Plazonnet B. Gelrite—a novel, ion-activated, in situ gelling polymer for ophthalmic vehicles—effect on bioavailability of timolol. Int. J. Pharm. 1989; 57: 163–168, [CROSSREF], [CSA]
- Sanzgiri Y. D., Maschi S., Crescenzi V., Callegaro L., Topp E. M., Stella V. J. Gellan-based systems for ophthalmic sustained delivery of methylprednisolone. J. Control. Rel. 1993; 26: 195–201, [CROSSREF], [CSA]
- Sasaki H., Ichikawa M., Kawakami S., Yamamura K., Mukai T., Nishida K., Nakamura J. In situ ocular absorption of ophthalmic beta-blockers through ocular membranes in albino rabbits. J. Pharm. Pharmacol. 1997; 49: 140–144, [PUBMED], [INFOTRIEVE], [CSA]
- Sasaki H., Igarachi Y., Nagano T., Nishida K., Nkamura J. Different effects of absorption promoter on corneal and conjunctival penetration of ophthalmic beta blockers. Pharm. Res. 1995; 12: 1146–1150, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Veyries M. L., Couarraze G., Geiger S., Agnely F., Massias L., Kunzli B., Faurisson F., Rouveix B. Controlled release of vancomycin from poloxamer 407 gels. Int. J. Pharm. 1999; 192: 183–193, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]