Abstract
The objective of our study was to prepare and characterize a stable microemulsion formulation for oral administration of a peptide, e.g., rh-insulin. The microemulsions were prepared using Labrafil M 1944 CS, Phospholipon 90G (lecithin), absolute alcohol, and bidistilled water. Commercially available soybean lecithins (namely, Phospholipon 80, phosphatidylcholine purity 76 ± 3%, and Phospholipon 90G, phosphatidylcholine purity 93 ± 3%) were used in the study. The results showed that the phase diagram obtained using a low purity lecithin was not similar to that obtained with a high purity lecithin. We observed that the microemulsion area was wider at the phase diagram obtained with the higher purity lecithin. We found that the extent of the microemulsion region depended upon both the purity of the lecithin and the surfactant/co-surfactant (s/co-s) mixing ratios (Km). The rheological studies showed that microemulsions followed a Newtonian behavior. Such physical characteristics as viscosity, turbidity, density, conductivity, refractive index, droplet size, physical appearance, and phase separation of the microemulsion were measured at different temperatures (4°C, 25°C, and 40°C) during 6 months. The results indicated that the physical characteristics of the developed microemulsions did not change under different storage temperatures (p > 0.05).
Microemulsions are isotropic systems constituted by a transparent mixture of aqueous phase, oil phase, surfactant, and co-surfactant. The small droplet sizes of microemulsions are a consequence of the ultra-low interfacial tension between the oil and water phases, arising from the presence of co-surfactant molecules, which intercalate between the surfactant molecules at the oil/water interface (Tenjarla Citation1999; Bagve et al. Citation2001; Lawrence and Rees Citation2000). The interest to potential users of microemulsions as a novel oral drug delivery system is motivated by different reasons: technologically, microemulsions are interesting because of their spontaneous formation at room temperature, their considerable solubilizing properties for active principles, their possible sterilization by filtration, and their high physical stability (Bhargava, Narurkar, and Lieb Citation1987; Sarciaux, Acar, and Sado Citation1995).
Lecithin is a very good surfactant candidate for microemulsion formulation because of its very low toxicity. It is also a biological surfactant and a major component of membrane lipids. Short-chain alcohols are suitable co-surfactants for use with lecithin because, in addition to their incorporation into the interfacial layer; they also have make the aqueous phase less hydrophilic, which shifts the HLB of the lecithin, enabling spontaneous microemulsification to occur (Shinoda et al. Citation1991; Ruth et al. Citation1995; Constantinides Citation1995). Ruth et al. (Citation1995) reported the possibility of using ethanol as a co-surfactant for the formation of a microemulsion composed of lecithin, isopropyl myristate and water. There are a number of studies in the literature concerning the preparation and characterization of lecithin-based microemulsions (Paolino et al. Citation2002; Trotta, Pattarino, and Grosa Citation1998; Park et al. Citation1999).
The oral absorption of peptide and protein drugs after oral administration is generally poor. This low absorption has been attributed to hydrolysis of these drugs by the proteolytic enzymes in the gastrointestinal tract and their poor permeability characteristics. The use of microemulsions as carriers for orally used peptide drugs has been suggested (Sarciaux et al. Citation1995; Ritschel Citation1991).
In light of the above, the two objectives of this study were to develop and characterize a stable microemulsion formulation for oral administration of insulin; to examine the influence of lecithin purity on phase behavior. In the second part of the study, the microemulsion containing rh-insulin was manufactured and the hypoglycemic effect was investigated in diabetic and nondiabetic rats (Çilek et al. Citation2001).
MATERIALS AND METHODS
Labrafil M 1944 CS® (unsaturated polyglycolysed glycerides) was kindly provided by Gattefosse (Saint-Priest, France); lecithins (Phospholipon® 80 and Phospholipon® 90 G) were supplied by Rhone-Poulenc; Nattermann Phospholipid GMBH (Cologne, Germany), and absolute alcohol was purchased from Riedel de Haen (Seelze, Germany).
Preparation of Microemulsions
The microemulsions were prepared using Labrafil M 1944 CS, lecithin, absolute alcohol, and bidistilled water as the oil phase, surfactant, co-surfactant, and aqueous phase, respectively. Pseudoternary phase diagram was constructed to determine the composition of polar, nonpolar, and surfactant phases that would yield a microemulsion. They were constructed by titration of bidistilled water into a mixture of oil, surfactant, and co-surfactant. In preparation of the pseudoternary phase diagrams, the effect of temperature also is important. As a result of the direct relation between the microemulsion region area and the environmental temperature, the experiments were done at 25 ± 0.5°C (constant temperature). The surfactant/cosurfactant (s/co-s) ratio (Km) that yielded the maximum microemulsion area was determined. In this study, the ratios 0.5, 1, 1.5, and 2.0 were used. The mixture of phospholipids and co-surfactant with the determined ratios was mixed with the oil phase, and the obtained mixture was slowly titrated with bidistilled water. The transparent fluid formulation was defined as the microemulsion region.
Physical Characterization of the Microemulsions
The measurements of all the given physical characteristics of the microemulsion were performed in the absence of drug. The physical characteristics were measured at different storage conditions (4°C, 25°C, and 40°C) during a period of 6 months. Explanation regarding the physical characteristics measurements follows.
Type of Microemulsion and Phase Separation Studies
To observe whether or not phase separation occurred, the microemulsion was centrifuged at 1200 g for 5 hrs. To determine if the microemulsion was of o/w or w/o type, the dye test (methylene blue and Sudan III) was performed.
Viscosity Measurements and Rheology Studies
The viscosity of the microemulsion was determined using an Ubbelohde viscometer at 25± 0.1°C. The instrument was calibrated with liquid of known viscosity. The rheological behavior of the microemulsion was determined using a rotating-spindle Brookfield viscometer. It was evaluated by plotting the shear stress versus the shear rate values obtained experimentally.
Turbidity, Density, and Conductivity Measurements
Turbidity measurements were carried out using a Hach model 2100 A turbidimeter. As a blank, a test solution (0–100 NTU Range 16) was used in the measurements. The microemulsion density measurements were done using a picnometer. The microemulsion conductivity measurements were carried out using a YSI Model 35 conductimeter. The measurements were done using a glass dipping cell with platinum electrodes. The cell constant was determined using a standard KCl solution.
Refractive Index and Droplet Size Measurements
Refractive index (RI) measurements were performed using a Shimadzu refractometer. The mean size of the microemulsion droplets was measured using quasielastic light scattering (QELS) Nicomp Model 270 submicron particle sizer.
Statistical Analysis
The one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls multiple comparisons were used for data analysis. Results are expressed as the mean ± confidence interval (C.I.) p<0.05 was considered significant for all comparisons.
RESULTS AND DISCUSSION
Preparation of Microemulsions
The substances to be used in the microemulsion formulation were selected carefully. As lecithin is a nontoxic and biologically compatible substance, the microemulsions that contain lecithin are preferred. In the preparation of the microemulsion formulation, high-purity phosphatidylcholine (93± 3%) containing lecithin (Phospholipon 90G) was used.
In many cases, a requirement for co-surfactant causes difficulty in the formulation of the acceptable microemulsions because the majority of studies have chosen medium chain length alcohols as the cosurfactant of choice. Unfortunately, there are significant toxicity and irritancy issues with these materials that preclude their use in pharmaceutical formulations. The absolute alcohol, used as the co-surfactant, was preferred since it is safe and nontoxic. Absolute alcohol has been used as a co-surfactant in both o/w and w/o microemulsion systems (Lawrence and Rees Citation2000). The co-surfactant is expected to increase the interfacial fluidity by penetrating the surfactant film and, consequently, to create a disordered film resulting from the void spaces among the surfactant molecules (Tenjarla Citation1999). Differences in the ability of alcohols to function as co-surfactants are a consequence of their influence on the nature of the interfacial film around the oil droplets. The presence of alcohol in the interfacial region causes a reduction in the rigidity of the otherwise-condensed lecithin film, allowing the curvature necessary for droplet formation. The distribution of the alcohol between the interface and the aqueous continuous phase depends on its hydrophilicity (Ruth et al. Citation1995).
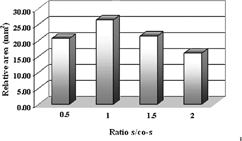
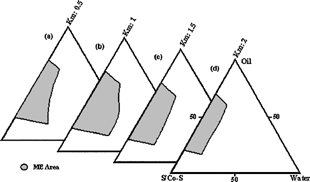
The pseudo-ternary phase diagrams of microemulsions containing lecithin with four different s/co-s ratios (Km) are shown in . The maximum area was found to be 1. shows the relative areas of microemulsion existence field as a function of s/co-s.
FIG. 1 Pseudoternary phase diagrams show existence areas of microemulsion with Labrafil M 1944 CS (oil), lecithin (S), absolute alcohol (Co-S), and water for s/co-s ratios (Km) of (a) 0.5, (b) 1, (c) 1.5, and (d) 2.
Aboofazeli and Lawrence (Citation1993, Citation1994a) conducted a detailed investigation of the formation and characterization of phospholipid microemulsions using lecithin as the surfactant. They determined the influence of several co-surfactants, s/co-s ratios, purity, and different commercial grades of lecithin and of different oils on microemulsion formulation. The major goal of their study was to identify the optimum properties of a co-surfactant that would provide balanced microemulsions stabilized with lecithin. Balanced microemulsions are defined as those systems that provide a large microemulsion area. The authors studied microemulsions formed with isopropyl myristate-water-lecithin and one of the seven short-chain alcohols as co-surfactant. Various ratios of s/co-s were studied with each of the co-surfactants. A stable isotropic w/o microemulsion occurred regardless of the Km value. No significant differences were found in the microemulsion regions when two commercial lecithins of approximately the same purity (e.g., lecithin and soybean lecithin) were used.
In Aboofazeli and Lawrence's study (Citation1994a), a microemulsion composed of lecithin, isopropyl myristate, alcohol, and water was formed and the effect of lecithin purity on formed microemulsion area was investigated. The phase diagrams of microemulsions formed with lecithin including phosphatidylcholine with ratios 76± 3% and 93± 3% were compared. The authors concluded that although there were similarities in the phase diagrams produced by various purities of lecithin, a number of significant differences were observed, but the s/co-s ratio was effective. In our study, as can be seen in , we observed that the microemulsion area formed with lecithin including phosphatidylcholine with a ratio of 93± 3% was wider than the one formed with lecithin including phosphatidylcholine with a ratio of 76± 3%. shows the relative areas of the microemulsion existence field obtained for two lecithin purity levels.
FIG. 3 Pseudoternary phase diagrams show existence areas of microemulsion with Labrafil M 1944 CS (oil), lecithin (S), absolute alcohol (Co-S), and water (Km:1); (a) Phospholipon 90G (b) Phospholipon 80.
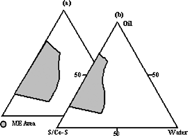
FIG. 4 Relative areas of the microemulsion existence field obtained for two lecithin purity levels (Km:1).

Attwood, Mallon, and Taylor (Citation1992) studied the phase properties of the system isopropyl myristate/lecithin (egg and soybean)/butanol/water. They observed slight differences between these two grades of lecithin, particularly at low concentrations. These differences were attributed to the nature of the fatty acid impurities in the two types of lecithin.
Aboofazeli et al. (Citation1994b, Citation1995) continued their investigation of lecithin microemulsions by testing several other co-surfactants. All the systems except the one in which amines were used as the co-surfactant showed stable isotropic microemulsion regions along the surfactant-oil axes. The influence of 6 polar oils on the formulation of the lecithin-based microemulsion also was investigated. The phase behavior of the system was dependent on the co-surfactant, Km value and polarity of the oil.
Physical Characteristics
Viscosity, turbidity, density, conductivity, RI, and droplet size measurements are important and useful in the characterization testing of microemulsion formulations. The type of the microemulsion was found to be w/o as determined by the dye method. Stable systems were identified as those free of any physical change, such as phase separation (Constantinides and Scalart Citation1997). According to the centrifuge test, no precipitate was observed and the microemulsion remained completely transparent during storage temperatures.
Radomska and Dobrucki (Citation2000) found that no significant changes occurred in the physical appearance of the microemulsion formulation prepared with Epicurone 135 (soybean lecithin), when stored over 6 months at 20°C. In addition, the prepared microemulsion in their study was centrifuged for 30 min at 13000 rpm, and its stability was investigated. They observed that the systems were still clear and had stable dispersion after 6 months. Furthermore, no phase separation could be observed. In our study, there was no change in the appearance of the formulations, at temperatures of 25°C and 40°C, over 6 months, but a mild turbidity occurred in the formulation at temperature 4°C after the second month. However, when brought to room temperature, all the formulations returned to their initial states. In conclusion, at room temperature, the physical appearance of the formulations remained unchanged.
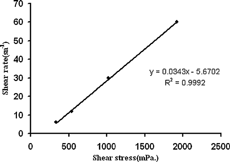
Viscosity studies are necessary determinations for microemulsions to characterize the system physically and to control its physical stability (Ho et al. Citation1996). Several authors have studied the rheological behavior and viscosity of these dosage forms (Baker et al. Citation1984; Ktistis Citation1990). The rheological behavior of dilute systems such as a microemulsion is generally Newtonian. On the other hand, the microemulsion shows Newtonian flow behavior to formation of small spherical particles. The result of our rheological studies showed that the microemulsions followed a Newtonian behavior. Shear stress versus shear rate for the microemulsion was plotted as shown in . Similar results were observed by Brime et al. (Citation2002). Viscosity measurements also were carried out in our study in to obtain some information on the thickness of the surfactant film, and the results are summarized in . No significant difference was encountered for the viscosity of the microemulsion at different temperatures during the 6 months (p>0.05).
TABLE 1 Physical stability of the microemulsion
Turbidity measurements can be used to estimate the extent of clustering/growth (Fletcher and Morris Citation1995). summarizes the insignificant change in turbidity measurements (p>0.05). The density of the microemulsion was between 0.9463 and 0.9679. Change in the densities of the microemulsion formulations occurs as a result of evaporation of the volatile compounds in the formulation over time. No change in the prepared microemulsion density shows that no such damage occurred (p>0.05).
The formation process and gradual changes in microemulsion microstructure can be monitored quantitatively by measuring the electrical conductivity of the system (Lawrence and Rees Citation2000). As shown in , there was no significant change in conductivity at 3 different temperatures (p>0.05). The low electrical conductivity of the microemulsion formulation shows the oil structure of the external phase. In most cases, the aqueous phase contains some electrolyte, while the oil phase does not. Thus, the electrolytic conductivity is the property measured to detect the emulsion type.
Constancy of the RI value is a sign of the constant microemulsion structure. summarizes the RI measurements. No significant difference was found in RI of the microemulsion at different temperatures during the 6 months (p>0.05).
The information on droplet size is particularly important for understanding the behavior of microemulsions during storage. Particle size of the microemulsions upon storage was also determined to assess microemulsion stability in terms of drastic changes in the mean droplet diameter due to droplet coalescence and/or aggregation (Constantinides and Scalart Citation1997). The droplet size of the microemulsion was 5.2 ± 1.1 nm, 5.13 ± 0.66 nm and 5.13± 0.94 nm at 4°C, 25°C and 40°C, respectively, and remained constant throughout the experiment (). Because of the small droplets in the dispersed phase, microemulsions have a very large surface area. For the system to be thermodynamically stable, the positive interfacial energy must be compensated by the negative energy of mixing (Tenjarla Citation1999).
In our previous study (Türkyılmaz et al. Citation1998), the physical stability studies of the microemulsion that consisted of Labrafil M 1944 CS, Arlacel 186/Brij 35, absolute alcohol and distilled water spanned a year, over which time no change was observed in the parameters that described the physical characterization of the microemulsion.
In a second part of this study, the microemulsion was found to be a suitable carrier system for the administration of rh-insulin through the oral route. Addition of aprotinin to the microemulsion increased biological availability of insulin (Çilek et al. Citation2001).
CONCLUSIONS
The results indicated that the physical characteristics of the developed microemulsion did not change under different storage temperatures during 6 months (p>0.05). Furthermore we demonstrated that lecithin-based microemulsions present optimal characteristics to be administered by the oral route. Thus, we have succeeded in preparing a stable microemulsion for potential oral application of peptide.
This study was supported by grants from Gazi University (02/2000-06). The authors thank Gattefosse (France) and Nattermann Phospholipid GMBH (Germany) for generously providing Labrafil M 1994 CS and Phospholipon 90G, respectively. We also thank Prof. Dr Hayat Alkan Önyüksel and Aparna Krishnadas for analysis of the particle size.
REFERENCES
- Aboofazeli R., Lawrence M. J. Investigations into the formation and characterization of phospholipid microemulsions. I. Pseudo-ternary phase diagrams of systems containing water-lecithin-alcohol-isopropyl myristate. Int. J. Pharm 1993; 93: 161–175, [CSA]
- Aboofazeli R., Lawrence M. J. Investigations into the formation and characterization of phospholipid microemulsions. II. Pseudo-ternary phase diagrams of systems containing water-lecithin-isopropyl myristate and alcohol: influence of purity of lecithin. Int. J. Pharm 1994a; 106: 51–61, [CROSSREF], [CSA]
- Aboofazeli R., Lawrence C. B., Wicks S. R., Lawrence M. J. Investigations into the formation and characterization of phospholipid microemulsions. III. Pseudo-ternary phase diagrams of systems containing water-lecithin-isopropyl myristate and either an alkanoic acid, amine, alkanediol, polyethylene glycol alkyl ether or alcohol as cosurfactant. Int. J. Pharm 1994b; 111: 63–72, [CROSSREF], [CSA]
- Aboofazeli R., Patel N., Thomas M., Lawrence M. J. Investigations into the formation and characterization of phospholipid microemulsions. IV. Pseudo-ternary phase diagrams of systems containing water-lecithin-alcohol and oil. Int. J. Pharm 1995; 125: 107–116, [CROSSREF], [CSA]
- Attwood D., Mallon C., Taylor C. J. Phase studies on oil-in-water phospholipid microemulsions. Int. J. Pharm. 1992; 84: R5–R8, [CROSSREF], [CSA]
- Bagve R. P., Kanicky J. R., Palla B. J., Patanjali P. K., Shah D. O. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit. Rew. Ther. Drug Carr. Sys. 2001; 18: 77–140, [CSA]
- Baker R. C., Florence A. T., Ottewill R. H., Tadros T. F. Investigations into the formation and characterization of microemulsions: 2. Light scattering conductivity and viscosity studies of microemulsions. J. Colloid Interface Sci 1984; 100: 332–349, [CSA]
- Bhargava H. N., Narurkar A., Lieb L. M. Using microemulsions for drug delivery. Pharm. Technol. 1987; 11: 46–52, [CSA]
- Brime B., Moreno M. A., Frutos G., Ballesteros P., Frutos P. Amphotericin B in oil-water lecithin-based microemulsions: formulation and toxicity evaluation. J. Pharm. Sci. 2002; 91: 1178–1185, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Constantinides P. P. Lipid microemulsions for improving drug dissolution and oral absorbsion: physical and biopharmaceutical aspects. Pharm. Res. 1995; 12: 1561–1572, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Constantinides P. P., Scalart J. P. Formulation and physical characterization of water-in-oil microemulsions containing long-versus medium-chain glycerides. Int. J. Pharm. 1997; 158: 57–68, [CROSSREF], [CSA]
- Çilek A., Çelebi N., Ocak F., Tay A. Oral absorption of recombinant insulin from microemulsion in rats. EUFEPS World Conference on Drug Absorption and Drug Delivery Benefiting from the New Biology and Informatics, CopenhagenDenmark, June, 18–20, 2001, Proceedings, Abstracts, 81, 82
- Fletcher P. D. I., Morris J. S. Turbidity of oil-in-water microemulsion droplets stabilized by nonionic surfactants. Colloids Surfaces A: Physicochemi Engi Aspects 1995; 98: 147–154, [CROSSREF], [CSA]
- Ho H. O., Hsiao C. C., Sheu M. T. Preparation of microemulsions using polyglycerol fatty acid esters as surfactant for the delivery of protein drugs. J. Pharm. Sci. 1996; 85: 138–143, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ktistis G. Viscosity study on oil-in water microemulsions. Int. J. Pharm. 1990; 61: 213–218, [CROSSREF], [CSA]
- Lawrence M. J., Rees G. D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Del. Rev. 2000; 45: 89–121, [CROSSREF], [CSA]
- Paolino D., Ventura C. A., Nistico S., Puglisi G., Fresta M. Lecithin microemulsions for the topical administration of ketoprofen: percutaneous adsorption through human skin and in vivo human skin tolerability. Int. J. Pharm. 2002; 244: 21–31, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Park K. M., Lee M. K., Hwang K. J., Kim C. K. Phospholipid-based microemulsions of flurbiprofen by the spontaneous emulsification process. Int. J. Pharm. 1999; 183: 145–154, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Radomska A., Dobrucki R. The use of some ingredients for microemulsion preparation containing retinol and its esters. Int. J. Pharm. 2000; 196: 131–134, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ritschel W. A. Microemulsions for improved peptide absorption from the gastrointestinal tract. Meth. Find. Exp. Clin. Pharmacol. 1991; 13: 205–220, [CSA]
- Ruth H. S., Attwood D., Ktistis G., Taylor C. J. Phase studies and particle size analysis of oil-in-water phospholipid microemulsions. Int. J. Pharm. 1995; 116: 253–261, [CROSSREF], [CSA]
- Sarciaux J. M., Acar L., Sado P. A. Using microemulsion formulations for oral drug delivery of therapeutic peptides. Int. J. Pharm. 1995; 120: 127–136, [CROSSREF], [CSA]
- Shinoda K., Araki M., Sadaghiani A., Khan A., Lindman B. Lecithin-based microemulsions: phase behaviour and microstructure. J. Phys. Chem. 1991; 95: 989–993, [CROSSREF], [CSA]
- Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Crit. Rev. Ther. Drug Carr. Sys. 1999; 16: 461–521, [CSA]
- Trotta M., Pattarino F., Grosa G. Formulation of lecithin-based microemulsions containing n-alkanol phosphocholines. Int. J. Pharm. 1998; 174: 253–259, [CROSSREF], [CSA]
- Türkyílmaz A., Çelebi N., Gönül B., Alkan-Önyüksel H. Physical characterization and stability of a microemulsion for potential oral administration of a peptide. Biomedical Science and Technology, A. Híncal, S. Kaş. Plenum Press, New York 1998; 65–72