Abstract
To show the possibility of sustained-release insulin formulation composed of PLGA, the optimum one was administered to BioBreeding rat, a model of spontaneous type I diabetes mellitus (IDDM). Every 2 weeks subcutaneous administration made their blood glucose level depend on the insulin release and food intake. However, all of them kept alive with little change or rather a little gain in body weight. Furthermore, some of pregnant rats with intermittent treatment bore fetuses, although additional insulin therapy seemed necessary. Therefore, the formulation could become a new tool as a provider of basal insulin for IDDM patients.
Insulin-dependent diabetes mellitus patients usually have to boost the basal insulin supply once or twice a day, in addition to doses at mealtimes, because they need a relatively constant basal level of insulin to achieve a near physiological pattern of insulin secretion. Such therapy has been reported not only to improve their general condition, but also to reduce the incidence of diabetic complications (Kawamori Citation1994; Haak Citation1999). If an insulin formulation was available that could release the drug in a constant level for longer periods, patients would be freed from the need to administer multiple doses.
We have investigated the ways to make microcapsules release insulin in a controlled fashion for a long period. To achieve this purpose, we have employed co-poly (D,L-lactic/glycolic) acid (PLGA). This is a biodegradable injectable polymer available for sustained-release preparation of drugs (Ogawa Citation1997; Takada et al. Citation1997; Barichello et al. Citation1999; Lam et al. Citation2000). Although able to release the drug from PLGA microcapsules for longer, the formulation needed to suppress initial rapid release. Our investigations, have demonstrated that the turbidity-reduced oil phase containing PLGA and insulin for preparation of microcapsules resulted in a dramatic little burst (Takenaga et al. Citation2002a). The appropriate amount of glycerin, ethanol, or water was added to improve the turbidity of oil phase. Each compound was confirmed to homogenize the insulin/PLGA mixture and to put it into compact particles (Yamaguchi et al. Citation2002).
As reported by Rizza, Mandarino and Gerich (Citation1981), it is necessary to lower the ratio of peak/through insulin level. Further investigation enabled us to prepare the formulation with the peak/through value of 2.7–2.8, by combination of two additives and zinc compounds (Takenaga et al. Citation2004). The pharmacological study in using streptozotocin-induced hyperglycemic male rats and female normal Cynomolgus monkeys concluded that this formulation provided a flat release of insulin for longer periods (Takenaga et al. Citation2002b).
BioBreeding (BB) rat has been reported to provide a model of spontaneous type I diabetes mellitus (IDDM). They secrete insulin too low which more closely resembles the human disease (Like et al. Citation1982; Chappel and Chappel Citation1982). They show onset of IDDM at 60 to 90 days of age. In addition, they exhibit both hyperglycemia and hyperlipidemia and die within 3 weeks after the onset of diabetes unless insulin therapy is given.
In this study, we planned to administer the optimum insulin formulation to BB rats with intermittent treatment. We aimed to show the possibility that this formulation could be applicable to IDDM patients. Furthermore, we administered the formulation to BB pregnant rats and examined whether it was effective or not.
MATERIALS AND METHODS
PLGA (L/G ratio: 50/50, molecular weights: 5800) was synthesized and kindly provided by Wako Pure Chemical Industries (Osaka, Japan). Human recombinant insulin (26 U/mg), streptozotocin (STZ), and other chemicals were purchased from Wako.
Insulin-Containing PLGA Formulations
Insulin (3 w/w%)-containing PLGA microcapsules were prepared as previously reported by the solvent evaporation method (Takenaga et al. Citation2004). For example, 240 mg of insulin, 39.9 mg of zinc oxide, and 7.84 g of PLGA dissolved in 8 ml of CH2Cl2 were mixed and agitated vigorously to form S/O suspension. At then, the appropriate amount of additive was added for S/O suspension to improve turbidity. This solution was poured into 1.0% (w/v) polyvinyl alcohol (PVA, GOHSENOL:EG-25, average molecular weight:45000, Nippon Gosei Kagaku, Tokyo, Japan) solution under stirring. After stirring to evaporate the organic solvent, we obtained microcapsules. The microcapsules were washed with distilled water by centrifugation and then lyophilized.
Particle size was determined using a particle analyzer (Multisizer IIe, Beckman Coulter, Tokyo, Japan). Mean diameter was 23.45 ± 13.61 μm (mean ± SD, n = 8). The insulin content of the prepared microcapsules was determined after extraction with CH2Cl2 and 0.01 N HCI according to the method of Lowry et al. (Citation1951), using the insulin as a standard. The encapsulation efficiency was > 90%.
Animals
Seven-week-old male Wistar rats (220–240 g, from SLC Experimental Animals, Shizuoka, Japan) were used for STZ-induced diabetic animal model. Three days after intravenously given 60 mg/kg of STZ dissolved in 10 mM citrate-citrate Na buffer (pH 4.5), they were subcutaneously injected with PLGA microcapsules containing insulin.
The diabetes-prone BB/Wor//Tky (BB) rats were originally from BB/Wor (Worcester) rats established by Like et al. (Citation1982). The rats were inbred by mating them around 70 days of age for propagation at the Animal Research Center, Tokyo Medical College. At 50 days of age, blood samples were collected through the tail vein of each rat every other day to monitor their blood glucose levels. Rats were considered diabetic if the value was over 300 mg/dl for 2 consecutive days. Since the rats die within 3 weeks without insulin, daily insulin therapy was continued before dosing.
The animal room had a 12-hr light/dark cycle (lights on from 6:30 to 18:30). Animals were housed at a constant temperature (23 ± 1°C) and humidity (50–60%) with free access to a standard diet and water. The study protocol was approved by Animal Experimentation Committee of St. Marianna University and Tokyo Medical College.
Treatment of Insulin Formulation
Just before administration, PLGA microcapsules containing insulin were dispersed (20 w/v%) with 5% mannitol solution pH 6.5 containing 0.5% carboxymethylcellulose and 0.1% Tween 80. Blood samples were taken from the inferior ophthalmic vein before and after treatment to measure blood glucose and plasma insulin levels. These samples were all taken in the morning (9:00–11:30), except that 6 hr after dosing. In addition, all animals were housed with free access to standard diet and water. Blood glucose level was determined by the glucose oxidase method using a glucose analyzer (Glucoster-M, Sankyo Co., Tokyo, Japan) immediately after sampling blood. Plasma insulin was determined using a radioimmunoassay kit (Shionogi Seiyaku Co., Osaka, Japan).
Statistical Analysis
Results are represented as the mean (± SD). Determination of the significant differences was done using the Mann-Whitney U-test, and p < 0.05 indicated significance.
RESULTS
Plasma Insulin Level
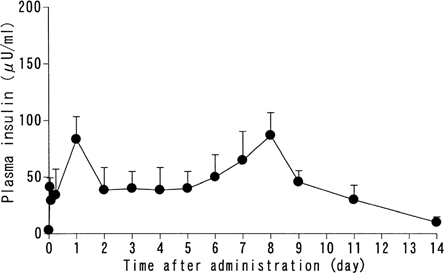
The STZ-treated rats were all hyperglycemic (a morning blood glucose concentration of more than 300 mg/dl) and their plasma insulin concentration ranged from 3.0–5.6 μU/ml. Plasma insulin level in normal untreated animals was between 20.4 and 51.4 μU/ml, and their blood glucose levels were between 98 and 150 mg/dl (morning non fasting level), shows the plasma insulin concentration following a single subcutaneous injection of insulin-containing PLGA microcapsules (125 U/kg). The first peak was seen on day 1 at 83.5 μU/ml. The second release peak produced by the formulation was 87.5 μU/ml (on day 8). The formulation produced a peak/trough ratio of 2.97 when calculated from the data (day 0–day 11).
Blood Glucose Levels and Body Weights
Before starting the experiment, BB rats were daily given 2.0–4.0 U of insulin to keep their blood glucose levels within a normal range and stay alive, because of their low insulin secretion (Zhang et al. Citation2002). It was notable that all animals could live without additional insulin, during the treatment of the formulation.
In the first experiment, the insulin-containing PLGA microcapsules (200 U/kg) were subcutaneously given to BB rats every 2 weeks. Blood glucose levels seemed to be altered corresponding to the insulin release from the formulation and food intake in both animals (). The first administration caused a transient but significant decrease of blood-glucose concentration (female; 57.7 mg/dl on day 7). Thereafter, treated animals gradually increased the level, and again became hyperglycemia by the next dosing.
TABLE 1 Blood glucose levels and body weight change following once a 2 week-treatment (200 U/kg/2 wk) of the insulin in formulation
Female BB rats seemed more sensitive. The second injection reduced female blood glucose levels to 50.0 mg/dl on day 22. On the contrary, male levels were more gradually decreased, and the nadir was 226.6 mg/dl on day 22. There was a significant difference between the two.
Body weight in female animals showed significant augmentation associated with decreased glucose level. Although the weight returned to the basal level prior to the next dosing, the treatment caused a significant gain. Meanwhile, body weight in male animals was relatively stable during the experiment.
Since most of 200 U/kg-treated BB rats became transiently hypoglycemia, the second experiment was planned to treat a lower dose (125 U/kg/2 wk). Both blood glucose levels were relatively low (female; 202.4 mg/dl, male; 192.4 mg/dl) at the beginning of the experiment (). Female rats showed a gradual decrease, and the nadir value was 83.9 mg/dl on day 3. Different from the first experiment, a significant increase was observed by the end of the first administration. The second treatment reduced only to 210.4 mg/dl. Male rats had ∼200 mg/dl of blood glucose level after administration. More than 360 mg/dl glucose was detected by the end of the first dosing as seen in females. The second treatment also showed only a gradual reduction.
TABLE 2 Blood glucose levels and body weight change following once a 2 week-treatment (125 U/kg/2 wk) of the insulin formulation
Reflecting hyperglycemia seen prior to the second administration, female body weight significantly decreased at the end of the first dosing. However, the second treatment did not further reduce their body weight. Male body weight had little change during the experiment.
In the third experiment, the dose was further reduced to 100 U/kg/2 wk. The blood glucose concentration in female rats was significantly higher than that in male rats at the beginning of the experiment (). Significant blood glucose decrease continued in female rats. However, each value did not mean hypoglycemia. Lower dose resulted in only a slight reduction even after the second treatment. This dose seemed insufficient for showing a pharmacological effect, resulting in hyperglycemia.
TABLE 3 Blood glucose levels and body weight change following once a 2 week-treatment (125 U/kg/2 wk) of the insulin formulation
The significant loss was seen in female body weight. There was little change in male rats, although they were hyperglycemia.
Monitoring Blood Glucose Levels and Body Weights
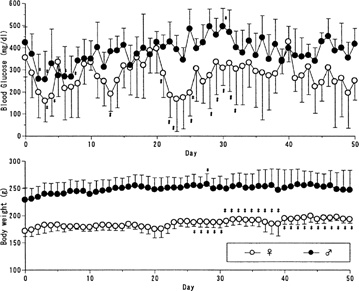
In the course of experiments, these results lead us to think that the formulation would be available for 125 U/kg/10 days-treatment. Blood glucose level was still altered in both animals (). However, every 10 days-treatment with 125 U/kg prevented hypoglycemia. Female rats appeared still more sensitive and their blood glucose was more significantly altered. However, it was noteworthy that female body weight stayed significantly higher since the second treatment, whereas, male body weight changed little for 50 days.
FIG. 2 Blood glucose level and body weight following every 10 days-treatment (125 U/kg) of insulin-containing PLGA microcapsules to BB/wor//Tky diabetic rats BB/Wor//Tky hyperglycemic pregnant rats were subcutaneously given 125 U/kg of PLGA microcapsules containing 3 w/w% insulin every 2 weeks. Blood glucose level (upper) and body weight (lower) were monitored. Mean ± SD. Female n=6; male n=8. *p < 0.05 vs. the first dosing day; #p < 0.05 vs. male group; body weight in male group was significantly higher throughout the experiment.
Intermittent Treatments to Pregnant Rats
BB rats can bear fetuses with daily insulin treatment. If the formulation can keep pregnant rats in a healthy condition just with intermittent treatment and bear fetus, it must be desirable.
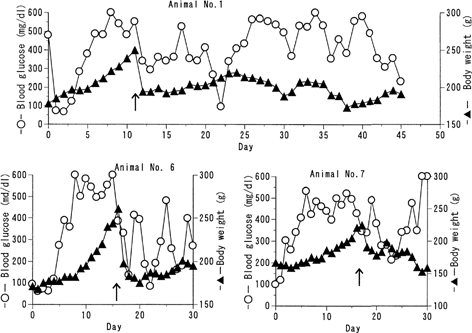
Seven female BB rats were entered in this study (). One was stopped at after one treatment (animal no. 2) because she looked weak and then died. Two of remained sterile during treatment (animals no. 4 and 5). The number of fetus was not different from that in daily insulin-treated ones.
TABLE 4 The trial of intermittent treatment (125 U/kg sc every 10 days) to BB/Wor//TKy pregnant rats
The data of animals having born fetuses are shown in (). Compared with those obtained from the same intermittent treatment to nonpregnant female rats (), all of them had higher glucose concentration and tended to be less sensitive to insulin. Although animal no. 1 had 60.9 μU/ml insulin on day 30, blood glucose concentration was 494 mg/dl. The body weight remained unstable even after the birth. It was regretable that born fetuses could not grow, because all rats lost them owing to lack of milk secretion.
FIG. 3 Trial of intermittent administration of the formulation to BB pregnant rats BB/Wor//Tky hyperglycemic pregnant rats were subcutaneously given 125 U/kg of the insulin formulation every 10 days. Blood glucose level (○) and body weight change (▴) were monitored. Arrow represents the birth of fetuses.
DISCUSSION
BioBreeding (BB) rat has been reported to be a model of IDDM that more closely resembles the human disease (Like et al. Citation1982; Chappel and Chappel Citation1982). It is noteworthy that every 2 weeks subcutaneous treatment of insulin-containing PLGA microcapsules (100, 125, or 200 U/kg) to BB rats could keep them alive without additional treatment. The body weight was relatively stable or sometimes increased during treatments. This finding indicates that it is important to supply insulin in a constant manner as shown in plasma insulin levels following the administration to STZ-induced diabetic rats. This formulation could be quite useful as an insulin provider.
Blood glucose levels seemed to be altered corresponding not only to the release of insulin from the formulation, but also to food intake. This phenomenon also was seen in STZ-induced hyperglycemic rats, although relatively constant insulin had been supplied. This could be ascribed partly to the fact that they could intake food freely. This also indicates that the formulation has no sensory functions.
After all animals treated with the insulin formulation had a transient decrease of blood glucose level, this concentration gradually increased. By the next dosing, hyperglycemia as well as hyperlipidemia was observed probably owing to lack of insulin release from PLGA microcapsules. Blood glucose level in 200 U/kg/2 weeks-treated BB rats reached the nadir on day 7 (female; 57.7 ± 23.5 mg/dl, male; 95.0 ± 51.1 mg/dl). This is almost consistent with the time when the insulin release associated with degradation of the carrier, PLGA, was accelerated ().
FIG. 1 Plasma insulin level following a single subcutaneous administration of insulin-containing PLGA microcapsules PLGA microcapsules containing 3 w/w% insulin were subcutaneously administered (125 U/kg) as a single dose to STZ-induced hyperglycemic rats. Plasma insulin levels were monitored. Mean ± SD, n = 5.
The second treatment seemed less effective, in particular, in males. The sensitivity in females seemed lowered during longer observation (). Rats can develop antibodies against human insulin after intermittent treatments, since rat and human insulin sequences differ by four amino acids (Cordell et al. Citation1979). In addition, there is a possibility that the immunogenicity of insulin released from the microcapsules had the potential for antiinsulin antibody formation. In fact, PLGA formulations of vaccines have been shown to display enhanced immunogenicity (Vordermeier et al. Citation1995; Partidos et al. Citation1997; Jabbal-Gill et al. Citation2001). (We confirmed that even intermittent treatment of human insulin solution induced immunological response). Taken together, the immunogenic activity of the insulin formulation can be more potent, but the incidence would be reduced in humans.
STZ-induced hyperglycemic rats receiving the formulation once a week had not only steady plasma insulin concentration, but also gained their body weight at a similar speed as seen in normal animals (Takenaga et al. Citation2002b). BB hyperglycemic rats receiving the formulation did not show a dramatic gain of weight, however, a significant increase was found in 200 U/kg/2 weeks-treated female BB rats. A slight decrease was observed before next lower-dose, treatment, probably reflecting decreased insulin level. Meanwhile, body weight in male animals was relatively stable. It should be noted that little change was seen even in 100 U/kg/2 weeks-treated male rats in spite of being hyperglycemia. This also supports the idea that it is quite important to provide the basal level of insulin.
This is the first report of PLGA microcapsules containing insulin were applied to BB pregnant rats. Two of them remained sterile during treatment. The number seems unlikely to be different from that of daily insulin-treated ones, because it is hard to get pregnant. In addition, the number of fetuses was not different from that in daily insulin-treated pregnant ones. The notable difference was that it was hard for born fetus to grow. Rats stopped keeping their fetuses, perhaps bewing to-lack of milk secretion. Thus, during pregnancy, blood glucose level should be much more strictly controlled by additional insulin treatment besides the basal insulin supply. Furthermore, our finding indicates a limitation of the formulation: not having a sensory function to release the insulin corresponding to glucose level.
We concluded that the formulation would be available for a basal insulin provider, but more insulin to control blood glucose level should be required for the birth and care of children. Gabbe (Citation2000) previously reported that subcutaneous insulin infusion or insulin pump therapy during pregnancy is necessary compared with multiple-dose insulin injections. This method more closely resembles that of physiological insulin release, a basal insulin infusion during the day and throughout the night with boluses given prior to meals. At least, the optimum insulin formulation can play a role in place of insulin pump. In conclusion, the formulation can contribute as a provider of basal insulin for IDDM patients and even pregnant women with IDDM.
CONCLUSION
The sustained-release insulin formulation enabled BB/Wor// Tky insulin-dependent rats to stay alive with intermittent treatment, although blood glucose concentration was altered. Some pregnant rats bore fetuses, although additional insulin therapy seemed necessary. These as a provider of basal insulin results indicate that the optimum insulin formulation can be a new tool for IDDM patients. It would be improve their general condition and reduce the incidence of diabetic complications.
We are very grateful to Akemi Hamaguchi, Natsumi Nakamura, Akiko Yamada, and Kayo Matsumoto for their excellent assistance.
REFERENCES
- Barichello J. M., Morishita M., Takayama K., Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev. Ind. Pharm. 1999; 25(4)471–476, [PUBMED], [INFOTRIEVE], [CSA]
- Chappel C. I., Chappel W. R. The discovery and development of BB rat colony. An animal model of spontaneous diabetes mellitus. Metabolism 1982; 32: 8, [CSA]
- Cordell B., Bell G., Tischer E., DeNoto F M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell 1979; 18(2)533–543, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Gabbe S. G. New concepts and applications in the use of the insulin pump during pregnancy. J. Matern. Fetal Med. 2000; 9: 42–45, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Haak T. New developments in the treatment of type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 1999; 107: S108–S113, [PUBMED], [INFOTRIEVE], [CSA]
- Jabbal-Gill I., Lin W., Kinstner O., Davis S. S., Illum L. Polymeric lammelar substrate particles for intranasal vaccination. Adv. Drug Deliv. Rev. 2001; 51: 97–111, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kawamori R. Practical concept for insulin therapy. Intern. Med. 1994; 73: 236–243, [CSA]
- Lam X. M., Duenas E. T., Daugherty A. L., Levin N., Cleland J. L. Sustained release of recombinant human insulin-like growth factor-I for treatment of diabetes. J. Control. Rel. 2000; 67: 281–292, [CROSSREF], [CSA]
- Like A. A., Butleer L., Williams R. M., Appel M. C., Weringer E. J., Rossini A. A. Spontaneous autoimmune diabetes mellitus in the BB rat. Diabetes 1982; 31: 7–13, [PUBMED], [INFOTRIEVE], [CSA]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951; 93: 265–275, [CSA]
- Ogawa Y. Injectable microcapsules prepared with biodegredable poly(alpha-hydroxy)acids for prolonged release of drugs. J. Biomater. Sci. Edn. 1997; 8(5)391–409, [CSA]
- Partidos C. D., Vohra P., Jones D., Farrar G., Steward M. W. CTL responses induced by a single immunization with peptide encapsulated in biodegradable microparticles. J. Immunol. Methods 1997; 206: 143–151, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am. J. Physiol. 1981; 240: E630–E639, [PUBMED], [INFOTRIEVE], [CSA]
- Takada S., Kurokawa T., Miyazaki K., Iwasa S., Ogawa Y. Sustained release of a water-soluble GPIIb/IIIa antagonist from copoly(DL-lactic/glycolic)acid microspheres. Int. J. Pharmaceut. 1997; 146: 147–157, [CROSSREF], [CSA]
- Takenaga M., Yamaguchi Y., Kitagawa A., Ogawa Y., Mizushima Y., Igarashi R. A novel sustained-release formulation of insulin with dramatic reduction in initial rapid release. J. Control. Rel. 2002a; 79: 81–91, [CROSSREF], [CSA]
- Takenaga M., Yamaguchi Y., Kitagawa A., Ogawa Y., Mizushima Y., Igarashi R. A novel insulin formulation can keep providing steady levels of insulin for much longer periods. J. Pharm. Pharmacol. 2002b; 54: 1189–1194, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Takenaga M., Yamaguchi Y., Kitagawa A., Ogawa Y., Kawai S., Mizushima Y., Igarashi R. Optimum formulation for sustained-release insulin. Inter. J. Pharm. 2004; 271: 85–94, [CROSSREF], [CSA]
- Vordermeier H. M., Coombes A. G., Jenkins P., McGee J P., O'Hagan D. T., Davis S. S., Singh M. Synthetic delivery system for tuberculosis vaccines:immunological evaluation of the M. Tuberculosis 38 kDa protein entrapped in biodegradable PLG microparticles. Vaccine 1995; 13(16)1576–1582, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Yamaguchi Y., Takenaga M., Kitagawa A., Ogawa Y., Mizushima Y., Igarashi R. Insulin-loaded biodegradable PLGA microcapsules initial burst release controlled by the hydrophilic additives. J. Control. Rel. 2002; 81: 235–249, [CROSSREF], [CSA]
- Zhang W., Lu D., Kawazu S., Komeda K., Takeuchi T. Adenoviral insulin gene therapy prolongs survival of IDDM model BB rats by improving hyperlipidemia. Horm. Metab. Res. 2002; 34: 577–582, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]