Abstract
Ciprofloxacin is mainly absorbed in the proximal areas of the gastrointestinal tract. The purpose of our study was production of floating-bioadhesive tablets to lengthen the stay of drug in its absorption area. Effervescent tablets were made using sodium carboxymethyl cellulose (CMC), hydroxypropyl methylcellulose (HPMC), polyacrylic acid (AA), polymetacrylic acid (MAA), citric acid, and sodium bicarbonate. Tablets with 5% effervescent base had longer lag time than 10%. The type of polymer had no significant effect on the floating lag time. All tablets floated atop the medium for 23–24 hr. Increasing CMC caused higher mucoadhesion than AA (p < 0.05). All formulations showed a Higuchi, non-Fickian release mechanism. Tablets with 10% effervescent base, 80% CMC/20% HPMC, or 80% AA /20% MAA seemed desirable.
Oral sustained-release technology provides oral delivery for 24 hr; however, in substances that cannot be well absorbed throughout the whole gastrointestinal tract, it may be disadvantageous (Baumgartner et al. Citation2000). Extended-release dosage forms with prolonged residence times in the stomach are highly desirable for drugs with narrow absorption windows, stability problems in the intestinal or colonic environments, locally acting in the stomach, and poor solubility in the intestine (Streubel, Siepmann, and Bodmeier Citation2003). Recent approaches to increase the gastric residence time of drug delivery systems include bioadhesive devices (Alvisi et al. Citation1996; Ponchel and Irache Citation1998), swelling devices that increase their size (CitationUrquhart and Theeuwes 1984; Mamajek Citation1980), low density devices (Streubel et al. Citation2003), floating systems (Deshpande et al. Citation1997), high density systems (Bechgaard and Ladefoged Citation1978; Davis et al. Citation1986), magnetic systems, unfoldable and expandable systems, magnetic systems, and superporous, biodegradable hydrogel systems (Singh et al. Citation2000).
The otherwise-excellent concept of floating system suffers from a disadvantage that it is effective only when the fluid level in the stomach is sufficient high; however, as the stomach empties and the tablet is at the pylorus, the buoyancy of the dosage form may be impeded (Chueh, Zia, and Rhodes Citation1995). This serious limitation can be overcome by using bioadhesive polymers to enable it to adhere to the mucous lining of the stomach wall (Chitnis, Malshe, and Lalla Citation1991). Floating and bioadhesive drug delivery systems offer the advantages of increased contact time with stomach mucusa, more effective absorption and bioavailability of drugs with absorption windows near proximal intestine and stomach, and low dosing frequencies (Chueh et al. Citation1995).
The various buoyant preparations include microballoons, granules, powders, capsules, tablets, and laminated films (Singh et al. Citation2000). Based on the mechanism of buoyancy, two distinctly different technologies, i.e., noneffervescent and effervescent systems have been utilized in the development of floating systems: 1. Noneffervescent systems that use commonly gel-forming or highly swellable cellulose-type hydrocolloids, polysaccharides, and matrix forming polymers such as poly-carbonate, polyacrylate, polymethacrylate, and polystyrene (Singh et al. Citation2000). 2. Effervescent systems that utilize matrices prepared with swellable polymers such as methocel® or chitosan and effervescent compounds, e.g., sodium bicarbonate and citric or tartaric acid (Rubinstein and Friend Citation1994) or matrices containing chambers of liquid that gasify at body temperature (Ritschel Citation1991).
Matrix tablets based on hydroxypropyl methylcellulose (HPMC K4M) have been developed by Li et al. (2000, Citation2003). Natural gums in combination with HPMC also have been evaluated for gel-forming properties (Dave, Amin, and Patel Citation2004). Different mass transport processes may occur during drug release from polymer-based matrix tablets, including water imbibition into the system, polymer swelling, drug dissolution, drug diffusion out of tablet, and polymer dissolution (Siepmann, Streuble, and Peppas Citation2002).
Ciprofloxacin, a broad spectrum antibiotic with 70% bioavailability, is absorbed mainly from the upper gastrointestinal tract, up to the jejunum (Harder et al. Citation1990; Rouge, Bari, and Doelker Citation1996). Thus, the drug may be delivered at stomach, the so-called absorption window, by increasing the gastric residence time of the dosage form. Different sustained–release formulations for this antibiotic are reported: extended (modified)-release formulation of ciprofloxacin (Cipro XR or Cipro XL) provides higher maximum plasma concentrations with lower interpatient variability than the conventional, immediate-release, twice-daily formulation. Therapeutic drug levels are achieved rapidly and maintained over the course of 24 hr, allowing once-daily dosing. Extended-release ciprofloxacin is at least as effective as the immediate-release formulation in cystitis and complicated urinary tract infections (UTIs) or acute uncomplicated pyelonephritis. It caused good tolerability and safety similar to the immediate-release formulation.
Therefore, extended-release ciprofloxacin is a convenient, well-tolerated, and effective therapy for UTIs that may improve patients' compliance with treatment and thus decrease the risk of treatment failure and the spread of antibiotic resistance (Talan et al. Citation2004a). Extended-release ciprofloxacin at 1,000 mg once daily was as safe and effective as conventional treatment with 500 mg ciprofloxacin twice daily, each given orally for 7 to 14 days in adults with complicated UTI or acute uncomplicated pylenphritis (AUP) (Talan et al. Citation2004b). A biodegradable polymer impregnated with ciprofloxacin and bacteriophages was used for treatment of severe local radiation injuries infected with Staphylococcus aureus. Purulent drainage stopped within 2–7 days. Clinical improvement was associated with rapid (7 days) elimination of the aetiologic agent, whereas wound healing was only moderately successful after 1 month therapy with traditional form of antibiotics (Jikia et al. Citation2005).
Cross-linked high amylose starch (CLHAS) matrix was used as a biodegradable drug delivery implant for the prevention and treatment of osteomyelitis. The results suggest that biodegradable ciprofloxacin-CLHAS implants are a safe and efficient modality for the prevention and treatment of osteomyelitis (Huneault et al. Citation2004). Combination therapy with liposomal formulations of ciprofloxacin and vancomycin for 7 days was more effective than either drug and showed much lower nephrotoxicity and a lower incidence of severe diarrhea than that induced by free drugs (Kadry et al. Citation2004)
The hypothesis for our research project is that if ciprofloxacin can be delivered in a controlled manner to the duodenum at a rate that does not exceed the maximum rate of its absorption, then the oral bioavailability of ciprofloxacin could be improved. Based on this hypothesis, the gastric floating and bioadhesive tablets were designed in such a way that they should be retained in the stomach for a prolonged period of time, thus maximizing the exposure of this drug to its absorption site.
MATERIALS AND METHODS
Ciprofloxacin HCl (TEMAD Co., Iran), hydroxypropyl methylcellulose (HPMC K4M), (Fluka Biochemika, Switzerland), sodium carboxy methylcellulose (NaCMC) (Merck, Germany), polyacrylic acid P934 (PAA) (BF Goodrich, Germany), polymethacrylic acid (PMA) (Fluka Chemika, Germany), citric acid (Merck, Germany), and sodium bicarbonate (Merck, Germany) were used.
Preparation of Bioadhesive and Floating Tablets
In the tablet formulation, HPMC/CMC or PAA/PMA were used as biadhesive agents. These polymers produce gel-forming matrices and, in contact with gastric fluid, possess sufficient structure to form a gel layer and achieve an overall specific gravity lower than that of gastric fluid. Citric acid and sodium bicarbonate were used as effervescent base to generate carbon dioxide and to enhance the buoyancy of the tablets. All powders except magnesium stearate were sieved through sieve of mesh size 20. The components of the formulation were mixed for 15 min in a cubic mixer. Magnesium stearate (40-mesh sieved) was added into powder blend as a lubricant and mixed for an additional 3 min before compaction process. Then 500 mg tablets containing 250 mg ciprofloxacin prepared by direct compression on an instrumented KS Kilian (Germany) single punch tableting machine, using 9 mm flat face punches and die adjusted to obtain 70 N hardness tablets, was measured with a hardness tester (TB 324, Erweka, Germany). The tablet formulations are shown in .
TABLE 1 Ingredients of floating-bioadhesive tablets of ciprofloxacin (mg)
Floating Behavior of Tablets
The in vitro floating behavior of the tablets was studied in 500 ml preheated 0.1 N HCl (pH 1.2, 37°C) and stirred at 50 rpm with a paddle (USP paddle method). The floating lag times (time period between placing the tablet in the medium and tablet floating) and floating durations of the tablets were determined by visual observation.
Bioadhesion Measurements
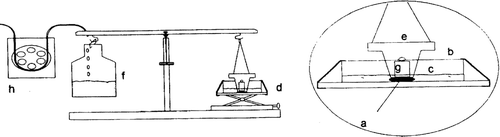
The force required to separate the sample disk from a model substrate was measured using a modified balance method as reported by Parodi et al. (Citation1996). As shows, the disk (a) was fixed to the base of the studying gt with a cyanoacrylate adhesive and came into contact with mucosal membrane of sheep tongue; the tablets were left for 5 min to hydrate. The beaker was placed on a moving platform (d). A flat polypropylene stopper (e) was suspended above the disk and counterbalanced with a plastic container (f). The beaker was then slowly raised until the substrate came in contact with the disk. A preload (g) of 50 g was placed into the stopper for 6 min so that adhesion bonding could be established. After this time, the preload was removed and water was added in the plastic container, by the peristaltic pump (h), at a constant rate of 90 mg/sec. The addition was stopped as soon as the detachment of the two surfaces was obtained. The equipment was located in an air-conditioned room at 22°C and 60% relative humidity.
FIG. 1 Schematic diagram of the device used for bioadhesion test (Parodi et al. Citation1996).
Density Measurements
The apparent densities of the tablets were calculated from their volumes and masses in triplicate. The volumes V of the cylindrical tablets were calculated from their heights h and radii r (both determined with a micrometer gauge) using the mathematical equation for a cylinder (V = π × r2× h). The tablets with ∼1 g/cm3 density or less were chosen for further studies (Chueh et al. Citation1995).
In Vitro Drug Release
In vitro drug release studies were conducted using the USP paddle method by placing the tablets in 900 ml preheated release medium (0.01 N HCl buffer solution, pH 1.2, 37°C) and stirred at 100 rpm. The dissolution samples were diluted and the concentration were determined on a spectrophotometer (UV mini 1240, Shimadzu, Japan) at 278 nm, the maximum absorbance of ciprofloxacin.
Analysis of Release Data
Dissolution efficiency (DE) (Banakar Citation1992) after 8 hr of release test was used to compare the results of dissolution tests of different formulations:
The other dissolution parameter used for comparing the different formulations was mean dissolution time or MDT that is calculated from the amount of drug released to the total cumulative drug. MDT is a measure of the rate of the dissolution process: the higher the MDT, the slower the release rate. The following equation was used to calculate the MDT from the mean dissolution data:
Where i is the dissolution sample number, n is the number of dissolution sample time, tmid is the time at the midpoint between i and i−1, and Δ M is the additional amount of drug dissolved between i and i−1 (Gohel and Panchal Citation2002).
RESULTS
shows the results of floating time and density of tablets. As this table shows increasing the effervescent base of tablets from 5% to 10% significantly lowers the lag time of floating from about 108 sec to 49 sec. In all studied formulations the density was ∼1 or less than 1 g/cm3.
TABLE 2 Physical properties of floating-bioadhesive tablets of ciprofloxacin (n = 3)
The results of bioadhesion studies are shown in . Tablets with 5% or 10% effervescent base in a cellulosic matrix (HPMC/CMC) and 10% effervescent base in acrylate matrix (PAA/PMA) are compared for the bioadhesion in this figure. and show the effect of different ratios of HPMC and CMC in tablets with two different percentages of effervescent base on drug release profiles. compares the effect of gas generating agent concentration on drug release rate of HPMC/CMC tablets. As this figure shows tablets with higher gas-forming agent facilitates drug release. compares the different ratios of PAA/PMA from a release characteristic point of view. In all formulations curve-fitting method was used to determine the drug release kinetics ().
FIG. 2 Bioadhesion strength of different floating tablets of ciprofloxacin measured by modified balance method (n = 3).
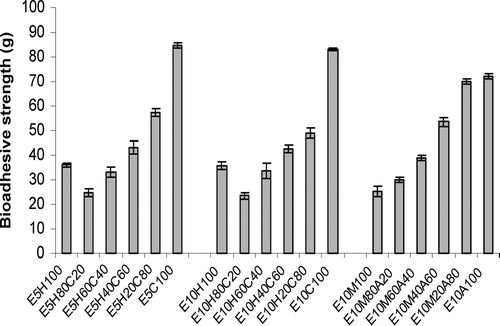
FIG. 3 Ciprofloxacin release profiles in HCl buffer solution (pH 1.2) from tablets containing 5% effervescent base and HPMC/CMC mixture (n = 3).
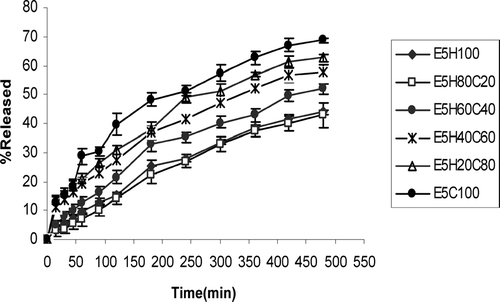
FIG. 4 Ciprofloxacin release profiles in HCl buffer solution (pH 1.2) from tablets containing 10% effervescent base and HPMC/CMC mixture (n = 3).
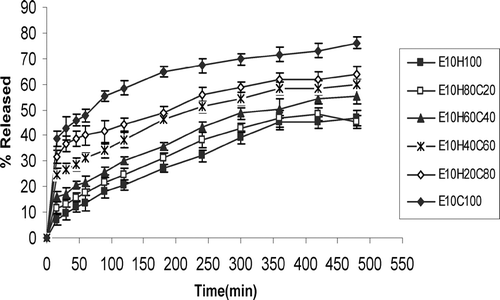
FIG. 5 Comparison between ciprofloxacin release profiles in HCl buffer solution (pH 1.2) from tablets containing 5% or 10% effervescent base and HPMC/CMC mixture (n = 3).
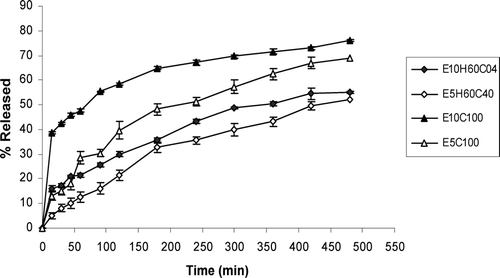
FIG. 6 Ciprofloxacin release profiles in HCl buffer solution (pH 1.2) from tablets containing 10% effervescent base and PAA/PMA mixture (n = 3).
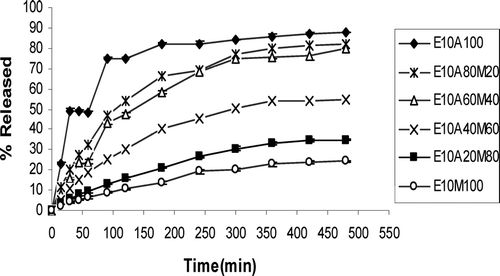
TABLE 3 Results of correlation coefficients of release data of floating bioadhesive tablets of ciprofloxacin by curve fitting method on zero-order, first-order, and Higuchi Kinetic models (n = 3)
Dissolution results were analyzed using the semiempirical equation:
where Mt/M∞ represents the fraction of drug released at time t, K is the diffusional constant characteristic of the drug/polymer system, t is the release time, and n is an exponent characterizing the mechanism of release of the drugs (Korsmeyer and Peppas Citation1983).
summarizes the range of values of the diffusional exponent n and the corresponding release mechanism. The n values are in the range of 0.45–0.85 representing a non-Fickian or anomalous transport. This table also represents the release parameters i.e., MDT, DE8%, and t50%.
TABLE 4 Results of mean dissolution time (MDT), dissolution efficiency after 8 hr (DE8%), time required for release 50% of drug (T50%), and diffusion exponent (n)
DISCUSSION
Studies show that some polymers like polyacrylic acid, polymethacrylic acid, sodium carboxymethyl cellulose, and hydroxypropyl methylcellulose are among the floating polymers that show bioadhesive properties more than other polymers and have been used in production of mucoadhesive tablets. As these polymers are well hydrated and can adhere to the mucosal membranes, specially if a combination of them is used, their properties are improved (Ahuja, Khar, and Ali Citation1997). Bioadhesive systems are used to localize a delivery device within the lumen and cavity of the body to enhance the drug absorption process in a site-specific manner (Chickering and Mathiowitz Citation1999). The approach involves the use of bioadhesive polymers that can adhere to the epithelial surface of the gastrointestinal tract. The proposed mechanism of bioadhesion is the formation of hydrogen and electrostatic bonding at the mucus polymer boundary (Wilson and Washington Citation1989).
Floating dosage forms are meant to remain buoyant on the gastric fluid when the stomach is full after a meal; however, as the stomach empties and the tablet is at the pylorus the buoyancy of the dosage form may be impeded (Muller-Lissnir and Blum Citation1981). It then becomes increasingly likely that the dosage form will pass through the pylorus into the small intestine. Thus, the buoyant ability of a floating drug delivery system in the stomach could be limited to only 3–4 hr. In a bioadhesive drug delivery system, it is quite likely that the system becomes dislodged from the stomach mucosa wall when the stomach is full and semiliquid contents are churning around under the influence of peristaltic movement (Chueh et al. Citation1995).
A synergism between a bioadhesive system and a floating system also has been explored. Chitnis et al. (Citation1991) synthesized a series of bioadhesive polymers that were cross-linked polymers of PMA and PAA. Floating tablets of isosorbide mononitrate were prepared and then coated with these polymers. The results showed good bioadhesion and low densities, indicating that the coat might confer buoyancy to these tablets. The results of bioadhesion test () shows that the bioadhesion was significantly higher in E10C100 tablets than other formulations (p < 0.05) and the following order is seen: E10M100 < E10H100 < E10A100 < E10C100.
Statistical analysis of bioadhesion between two groups of tablets containing HPMC/CMC or PAA/PMA shows that tablets with 80–100% and 60% of CMC have higher bioadhesion than tablets containing the comparable amounts of PAA (p < 0.05). However, tablets with 20–40% CMC, or those with pure HPMC, have less bioadhesion compared with tablets with similar amounts of PAA or without PAA (p < 0.05). Increasing the content of PAA in a series prepared with PAA/PMA increased the bioadhesion (p < 0.05). Chng et al. (Citation1985) also reported that PAA polymer adheres to the surface mucin of the epithelial cells and this cause a longer gastrointestinal transit time compared with PMA polymer. This is related to the charge of PAA and neutral nature of PMA (Chng et al. Citation1985).
In the design of ciprofloxacin tablets, the floatation was accomplished by incorporating gas-generating salts such as sodium bicarbonate and citric acid into a swellable hydrophilic matrix. The overall make-up of this particular matrix is of swellable hydrophilic polymers. As the dissolution medium was imbibed into the matrix, the interaction of fluid with effervescent base resulted in the formation and entrapment of carbon dioxide gas within the swollen gel, thus causing floatation as the matrix volume expanded and its density decreased. We observed that the amount of gas-generating effervescent base had a significant effect on the lag time of the system buoyancy (). However, statistical analysis of duration of floating time in HPMC/CMC and PAA/PMA tablets with 10% effervescent base showed no change (p < 0.05) in duration of system buoyancy by changing the percentage or the type of polymer mixtures (). In other words, the amount of gas-generating agent is just effective on the buoyancy lag time, but as the gas is generated at the early times of contact of fluid medium with effervescent base, the swelling of polymers is controlling the duration of system buoyancy.
Yang et al. (Citation1999) used a mixture of sodium bicarbonate and calcium carbonate to induce gas formation in intragastric floating tablets of tetracycline/metronidazole tablets. Li et al. (Citation2002, Citation2003) used citric acid as gas-generating agent in floating capsules of calcium carbonate. A 1:1 mixture of potassium bicarbonate:monobasic potassium citrate as effervescent base of verapamil floating capsules has been reported by Gan-Lin and Wei-Hua (Citation1998).
Drug release studies were made to determine whether the release of the drug is slow enough, i.e., which polymer percentage is enough to sustain the release of the drug for at least 8 hr. As and show, increasing the CMC content of tablets significantly increases the percentage of drug released at comparable times (p < 0.05). This is because of rapid swelling and erosion of CMC in contact with water (Dortung et al. Citation1998). Comparison of tablets with the same formulations but different effervescent base concentrations () shows faster release rate of drug and DE8% () in tablets with 10% of gas-generating agent than 5%. This is because of greater expansion of polymer matrix, better penetration of liquid medium into the tablet, and faster diffusion of drug. shows that increasing CMC content of tablets, reduces MDT and T50% while increasing the DE8% (p < 0.05). Comparison of T50% and MDT of tablets with the same ratio of HPMC/CMC but different effervescent bases shows a decrease in these parameters in tablets with 10% of gas-generating agent than 5% (p < 0.05) ().
In spite of more suitable sustained-release effect of tablets with 5% effervescent base (), but as their long lag-time of buoyancy (), tablets of E10H60C40 and E10H40C60 were chosen as optimum formulations ( and ). However, as there was some difficulties in flow rate of powder in preparation of these tablets, formulation E10H20C80 also seems optimum from floating lagtime, bioadhesion, and sustained-release point of view. Tablets composed of a polymeric matrix build a gel layer around the tablet core on contact with water, which controls the drug release. Drug release from HPMC matrices is controlled by diffusion through the gel layer for water-soluble drugs or by erosion of the outer polymer chains for poorly soluble drugs (Mitchell et al. Citation1993). The drug characteristics are as important as those of the gel. The size, shape, and ionization of the drug affect its diffusion through the gel layer (Peppas and Wright Citation1998).
In tablets prepared from acrylate series, increasing the PAA content, decreases the MDT and T50% but increases the DE8% significantly (p < 0.05) (). Considering MDT and DE8%, tablets of E10A80M20, E10A60M40, and E10A40M60 seem suitable for sustained–release of drug in the stomach ().
Curve fitting method according to zero-order, first-order, or Higuchi model for analysis of drug release kinetics are shown in . In all cases the Higuchi model is dominant and shows that the passage of ciprofloxacin, the water–soluble drug through the hydrated gel layer around the matrix tablet, is approximately dependent on the square root of time and can be described in the following form (Shah et al. Citation1993):
where Qt is the amount of the released drug in time t, k is the kinetic constant, and t is time.
To predict the mechanism of diffusional release, the following semiempirical equation of
was used to analyze data of controlled–release of this water-soluble drug from the studied polymer matrices (Peppas Citation1985; Yang and Fassihi Citation1997). In this equation Mt is amount of the released drug at time t, M∞ is the overall amount of the drug (whole dose), k is the constant incorporating structural and geometric characteristics of the controlled–release device, and n is the release exponent indicative of the drug release mechanism. For tablets of a known geometry (in this case a slab) n = 0.5 means Fickian diffusion, 0.5 < n < 1.0 non-Fickian diffusion, and n = 1.0 Case II diffusion (Peppas Citation1985). Considering the n values calculated for the studied tablets (), almost in most cases a non-Fickian mechanism is dominant.
The drug diffusion through most types of polymeric systems is often best described by Fickian diffusion, but other processes in addition to diffusion are important. In this case the non-Fickian or anomalous diffusion shows also a relaxation of the polymeric chains, and influences the drug release. Release from initially dry, hydrophilic glassy polymers that swell in contact of water and become rubbery show anomalous diffusion as a result of the rearrangement of macromolecular chains. The thermodynamic state of the polymer and the penetrant concentration are responsible for the different types of the diffusion. A third class of diffusion is case II diffusion, which is a special case of non-Fickian diffusion (Peppas Citation1985; Mitchell et al. Citation1993). The results of the calculated n () reveal a non-Fickian type of drug diffusion, which means that the process of diffusion and relaxation run at comparable rates.
CONCLUSIONS
In the current work, a matrix floating tablet incorporating a high dose of freely soluble active substance is described. The most successful tablets with the least lag time of buoyancy were those prepared with 10% of effervescent base but changing the polymer type of mixture ratio did not change the duration of buoyancy. Tablets containing 20% of HPMC and 80% CMC or 80% of PAA and 20% of PMA were optimum from both the bioadhesion and prolonged drug release rate point of view.
REFERENCES
- Ahuja A., Khar R. K., Ali J. Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 1997; 23(5)489–515, [CSA]
- Alvisi V., Gasparetoo A., Dentale A., et al. Bioavailability of a controlled release formulation of ursodeoxycholic acid in man. Drugs Exp. Clin. Res. 1996; 22: 23–29, [CSA]
- Banakar U. V. Pharmaceautical Dissolution Testing, 1st ed. Marcel Dekker, New York 1992; 191–194
- Baumgartner S., Kristl J., Vrecer F., Vodopivec P., Zorko B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int. J. Pharm. 2000; 195: 125–135, [INFOTRIEVE], [CSA], [CROSSREF]
- Bechgaard H., Ladefoged K. Distribution of pellets in the gastrointestinal tract. The influence on transit time exerted by the density or diameter of pellets. J. Pharm. Pharmacol. 1978; 30: 690–692, [INFOTRIEVE], [CSA]
- Chickering D. E., III, Mathiowitz E. Definitions, mechanisms and theories of bioadhesion. Bioadhesive Drug Delivery Systems. Fundamental, Novel approaches, and Development, E. Mathiowitz, D. E. Chickering, III, C. M. Lehr. Marcel Dekker, New York 1999; 1–8
- Chitnis V. S., Malshe V. S., Lalla J. K. Bioadhesive polymer-synthesis, evaluation and application in controlled release tablets. Drug Dev. Ind. Pharm. 1991; 17(6)879–892, [CSA]
- Chng H. S., Park H., Kelly P., Robinson J. R. Bioadhesive polymers as platforms for oral controlled drug delivery II: Synthesis and evaluation of some swelling water-insoluble bioadhesive polymers. J. Pharm. Sci. 1985; 74: 399–405, [CSA]
- Chueh H. R., Zia H., Rhodes C. T. Optimization of sotalol and bioadhesive extended-release tablet formulations. Drug Dev. Ind. Pharm. 1995; 21(15)1725–1747, [CSA]
- Dave B. S., Amin A. F., Patel M. M. Gastroretentive drug delivery system of ranitidine hydrochloride: formulation and in vitro evaluation. AAPS Pharm. Sci. Tech. 2004; 5(2), Article 34[CSA]
- Davis S. S., Stockwell A. F., Taylor M. J., et al. The effect of density on gastric emptying of single and multiple-unit dosage forms. Pharm. Res. 1986; 3: 208–213, [CSA], [CROSSREF]
- Deshpande A. A., Shah N. H., Rhodes C. T., Malick W. Development of a novel controlled-release system for gastric retention. Pharm. Res. 1997; 14: 815–819, [INFOTRIEVE], [CSA], [CROSSREF]
- Dortung B., Ozer L., Uyanik N. Development and in vitro evaluation of a buccoadhesive pindolol tablet formulation. Drug Dev. Ind. Pharm. 1998; 24(3)281–288, [CSA]
- Gan-Lin C., Wei-Hua H. In vitro performance of floating sustained-release capsule of verapamil. Drug Dev. Ind. Pharm. 1998; 24(11)1067–1072, [CSA]
- Gohel M. C., Panchal M. K. Novel use of similarity factors f2 and Sd for the development of diltiazem HCl modified-release tablets using a 3(2) factorial design. Drug Dev. Ind. Pharm 2002; 28(1)77–87, [INFOTRIEVE], [CSA], [CROSSREF]
- Harder S., Fuhr U., Beermann D., Staib A. H. Ciprofloxacin absorption in different regions of the human gastrointestinal tract: investigations with the hf-capsule. Br. J. Clin. Pharmacol. 1990; 30: 35–39, [INFOTRIEVE], [CSA]
- Huneault L. M., Lussier B., Dubreuil P., Chouinard L., Desevaux C. Prevention and treatment of experimental osteomyelitis in dogs with ciprofloxacin-loaded crosslinked high amylose starch implants. J. Orthop. Res. 2004; 22(6)1351–1357, [INFOTRIEVE], [CSA], [CROSSREF]
- Jikia D. Chkhaidze N., Imedashvili E., et al. The use of a novel biodegradable preparation capable of the sustained release of bacteriophages and ciprofloxacin, in the complex treatment of multidrug-resistant Staphylococcus aureus-infected local radiation injuries caused by exposure to Sr90. Clin. Exp. Dermatol. 2005; 30(1)23–26, [INFOTRIEVE], [CSA], [CROSSREF]
- Kadry A. A., Al-Suwayeh S. A., Abd-Allah A. R., Bayomi M. A. Treatment of experimental osteomyelitis by liposomal antibiotics. J. Antimicrob. Chemother. 2004; 54(6)1103–1108, [INFOTRIEVE], [CSA], [CROSSREF]
- Korsmeyer R. W., Peppas N. A. Macromolecular and modeling aspects of swelling controlled systems. Controlled Release Delivery Systems, T. J. Roseman, S. Z. Mansdorf. Marcel Dekker, New York 1983; 77–90
- Li S., Lin S., Daggy B. P., et al. Effect of formulation variables on the floating properties of gastric floating drug delivery system. Drug Dev. Ind. Pharm 2002; 28(7)783–793, [INFOTRIEVE], [CSA], [CROSSREF]
- Li S., Lin S., Daggy B. P., et al. Effect of HPMC and carbopol on the release and floating properties of gastric floating drug delivery system using factorial design. Int. J. Pharm. 2003; 253: 13–22, [INFOTRIEVE], [CSA], [CROSSREF]
- Mamajek R. C., Moyer E. S. Drug-dispensing device and method. US Patent 4, 207, 890, 1980
- Mitchell K., Ford J. L., Armstrong D. J., et al. The influence of concentration on the release of drugs from gels a matrices controlling Methocel. Int. J. Pharm. 1993; 100: 155–163, [CSA], [CROSSREF]
- Muller-Lissner S. A., Blum A. L. The effect of specific gravity and eating on gastric emptying of slow-release capsules. New Engl. J. Med. 1981; 304: 1365–1366, [INFOTRIEVE], [CSA]
- Parodi B., Russo E., Caviglioli G., et al. Development and characterization of a buccoadhesive dosage form of oxycodone hydrochloride. Drug Dev. Ind. Pharm. 1996; 22(5)445–450, [CSA]
- Peppas N. A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985; 60: 110–111, [INFOTRIEVE], [CSA]
- Peppas N. A., Wright S. L. Drug diffusion and binding in ionizable interpenetrating networks from poly(vinyl alcohol) and poly(acrylic acid). Eur. J. Pharm. Biopharm. 1998; 46: 15–29, [INFOTRIEVE], [CSA], [CROSSREF]
- Ponchel G, Irache J M. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Del. Rev. 1998; 34: 191–219, [CSA], [CROSSREF]
- Ritschel W. A. Targeting in the gastrointestinal tract: new approaches, Metods Find. Exp. Clin. Pharmacol. 1991; 13: 313–316, [CSA]
- Rouge N., Buri P., Doelker E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996; 136: 117–139, [CSA], [CROSSREF]
- Rubinstein A., Friend D. R. Specific delivery to the gastrointestinal tract. Polymeric Site-Specific Pharmacotherapy, A. J. Domb. Wiley, Chichester 1994; 283–285
- Shah N., Zhang G., Apelian V., Zeng, et al. Prediction of drug release from hydroxypropyl methylcellulose (HPMC) matrices: effect of polymer concentration. Pharm. Res. 1993; 10: 1693–1695, [INFOTRIEVE], [CSA], [CROSSREF]
- Siepmann J., Streuble A., Peppas N. A. Understanding and predicting drug delivery from hydrophilic matrix tablets using the “sequential layer” model. Pharm. Res. 2002; 19: 306–314, [INFOTRIEVE], [CSA], [CROSSREF]
- Singh B. N., Kim K. H. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J. Control. Rel. 2000; 63: 235–259, [CSA], [CROSSREF]
- Streubel A., Siepmann J., Bodmeier R. Floating matrix tablets based on low-density foam powder: effects of formulation and processing parameters on drug release. Eur. J. Pharm. Sci. 2003; 18(1)37–45, [INFOTRIEVE], [CSA], [CROSSREF]
- Talan D. A., Naber K. G., Palou J., Elkharrat D. Extended-release ciprofloxacin (Cipro XR) for treatment of urinary tract infections. Int. J. Antimicrob. Agents 2004a; 23: S54–S66, Suppl 1[INFOTRIEVE], [CSA], [CROSSREF]
- Talan D. A., Klimberg I. W., Nicolle L. E., et al. Once daily, extended release ciprofloxacin for complicated urinary tract infections and acute uncomplicated pyelonephritis. J. Urol. 2004b; 171: 2734–739, Pt 1[CSA], [CROSSREF]
- Urquhart J., Theeuwes F. Drug delivery system comprising a reservoir containing a plurality of tiny pills. U.S. patent 4, 434, 153, February 28, 1984
- Wilson C. G., Washington N. The stomach: its role in oral drug delivery. Physiological Pharmaceutics: Biological Barriers to Drug Absorption, M. H. Rubinstein. Ellis Horwood, Chichester 1989; 47–70
- Yang L., Fassihi R. Examination of drug solubility, polymer types, hydrodynamics and loading dose on drug release behaviour from a triple-layer asymmetric configuration delivery system. Int. J. Pharm. 1997; 155: 219–229, [CSA], [CROSSREF]
- Yang L., Eshraghi J., Fassihi R. A new intragastric delivery system for the treatment of Helicobacter pylori associated gastric ulcer: in vitro evaluation. J. Control. Rel. 1999; 57: 215–222, [CSA], [CROSSREF]
