Abstract
Polymeric micelles based on polyvinyl alcohol substituted with oleic acid were used as vehicles for progesterone and folic acid. The ability of this amphiphilic polymer to entrap lipophilic drugs and to generate stable micelles in aqueous neutral medium makes it a good candidate for drug delivery. The release of the loaded drugs in acidic environments represents another important property of these systems. Size of micelles, their stability, and their drug-loading capacity were evaluated, as well as the in vitro controlled-release profiles at pH 7.4 and 5.5.
Colloidal carrier systems (Lin and Paschalis Citation2000; Allen, Eisenberg and Maysinger Citation1999) recently have received great attention in the field of drug targeting due to their high loading capacity for poorly soluble drugs and to their unique disposition characteristics in the body. Polymer-based colloidal drug delivery carriers, such as micelles provide a set of unbeatable advantages: they can solubilize lipophilic drugs and thus increase their bioavailability; they have high stability in blood fluids; they can accumulate in body regions with leaky vasculature (EPR effect) due to their small size; and they can be prepared in large quantities easily and reproducibly. Being enclosed in micelles, the drug is well protected from possible inactivation under the effect of biological surroundings, it does not provoke undesirable side effects, and its bioavailability is usually increased (Kataoka et al. 2001; La et al. Citation1996; Yokoyama Citation1998; Yu et al. Citation1998; Jones Citation1999). The objective of our work was to find a new family of bioeliminable and biocompatible polymers whose solubility characteristics could allow preparation of systems stable in the aqueous environment to control drug release without protein adsorption and aggregation.
Polyvinyl alcohol, a water-soluble polyhydroxy polymer, was selected as a starting material for the preparation of such polymers due to its biocompatibility and the possibility for substitution through chemical linkage to its oxyresidues, which can modify its physicochemical properties (Martien Citation1986). We substituted polyvinyl alcohol with oleic acid to allow preparation of micellar carriers for folic acid and progesterone for parenteral use. The molecular weight selected for PVA was 10000 to gaurantee renal excretion after complete hydrolysis of the substituents (Seymour et al. Citation1987). We selected folic acid for its chemical structure similar to methotrexate and for its chemoprotective activity (Duthie Citation2001; Orienti et al. Citation1998); progesterone was selected as a well-known antitumoral drug (Grubbs and Whitaker Citation1988; Sivaraman et al. Citation1998). The physiochemical properties of the substituted polymer were evaluated in solutions at different pH values and correlated to the functional properties of the micellar carrier-drug system to establish the best conditions favoring the parenteral administration of folic acid and progesterone.
MATERIALS AND METHODS
Polyvinyl alcohol (PVA) (Mw = 10000, 20% acetylated), human albumin, progesterone, folic acid, 4-dimethylaminopyridine (DMAP), pyridine, N-methylpyrrolidone (NMP), ethyl alcohol, and oleyl chloride were purchased from Fluka (Milan, Italy). Phosphoric acid (85%, m/m), sodium hydroxide, and 2-propanol, all analytical grade, were purchased from Carlo Erba (Milan, Italy). HPLC grade acetonitrile and methanol were from Romil Ltd. (Cambridge, Great Britain). Water obtained from Milli-RX apparatus (Millipore, USA) was used to prepare solutions and mobile phases. The buffer solutions were filtered through a 0.45 μm membrane filter and degassed before their use for HPLC.
Synthesis of Substituted PVA
Substituted PVA was prepared as reported in a previous work (Luppi et al. Citation2002). Briefly, PVA (40 mmol of monomer) was dissolved in 50 ml of NMP. The solution was supplemented with pyridine (40 mmol), DMAP (4 mmol) and oleyl chloride (2 mmol) to obtain a 5% molar ratio (acyl chloride: hydroxyvinyl monomer). Immediately a precipitate of the pyridinium salt was observed. The mixture was stirred for 48 hr at room temperature. The precipitate of pyridinium salt was removed by filtration and the substituted polymer was separated by precipitation into water. The substituted polymer was characterized by FT-IR and 1H-NMR analysis. In particular, the substitution degree calculated from 1H-NMR sprectrum was 4.8%.
Preparation of the Polymeric Micelles
Micelles of average diameter of 200 nm were prepared by dissolving 100 mg of the substituted polymer in 2 ml ethanol and injecting this solution in 38 ml PBS (phosphate buffer solution at pH 7.4) under ultrasonication (5 min at 40% maximal amplitude at room temperature) by means of a Pasteur pipette. Polymeric micelles containing progesterone or folic acid were prepared by adding, respectively, 40 mg or 5 mg of the drugs to the polymer solution in ethanol and injecting this solution in 38 ml of PBS under ultrasonication in the same conditions. To obtain micelles of average diameter of 100 nm, the injection of polymer or drug-polymer solutions also was carried out by a syringe with a needle diameter of 0.33 mm. For better comparison, standard formulations were prepared with only 40 mg progesterone or 5 mg folic acid with the same preparative method.
Dynamic Light Scattering (DLS) Measurements
The size of the unloaded micelles in PBS or pH 5.5 was monitored at 37°C for 12 hr using a DLS instrument (Brookhaven 90-PLUS) equipped with a 50 mW He-Ne laser (532 nm). Measurements were carried out by fixing the scattering angle at 90°. Results were the combination of five 2 min runs for a total correlation function (ACF) accumulation time of 10 min. The diffusion coefficient was evaluated from the time autocorrelation function, g2 (τ) using the forced single-exponential fit (Equation 1):
[1]
[2]
[3]
where τ is the delay time, both A and B are constant, D is the translational diffusion coefficient, q is the scattering vector, n is the refractive index of pure solvent, λ0 is the wavelength of incident light in vacuum, and θ is the scattering angle. The hydrodynamic diameter, DH, was calculated using Stokes-Einstein equation:
[4]
where kB, T, and η are the Boltzmann constant, the absolute temperature, and the solvent viscosity, respectively.
Progesterone Content in Micelles
The progesterone content in the micelles was assessed by means of a HPLC method with ultraviolet (UV) detection recently developed by the authors for this purpose (Pucci et al. 2003). A suitable amount of polymeric micelles was dissolved in a water: 2-propanol mixture (1:1, v/v) to obtain a 1 mg ml−1 solution, then filtered through a cellulose acetate syringe filter (0.20 μm pore size). This solution was diluted with mobile phase (see below) and injected into the HPLC apparatus through a 20-μl loop. HPLC analysis was carried out using a C 8 reversed phase column (Res Elut, 150 × 4.6 mm I.D., 5 μm) as the stationary phase; the mobile phase was a mixture of 2-propanol and pH 2.5; 30 mM phosphate buffer (1:1, v/v) with an apparent pH of 3.0, at a flow rate of 1 ml min−1. The UV detector was set at 245 nm.
Folic Acid Content in Micelles
In a previous article, three alternative analytical methods for the determination of folic acid in micelles were reported: direct UV spectrophotometry after solid phase extraction, derivative UV spectrophotometry, and HPLC methods (Andrisano et al. Citation2003). For determining the folic acid content of the micelles, HPLC method was used as follows. The solvent delivery system was a quaternary HP 1050 Ti series pump, equipped with a Reodyne Model 7125 injector with a 20-μl sample loop. The eluents were monitored by a Multiwavelength HP 1050 Detector connected to a computer station (HP Chemstations, Vectra, VT, USA).
The chromatographic separations were carried out on a reversed-phase Phenomenex Luna C18 5 μm (I.D 150 × 2.0 mm) column, using a mobile phase consisting of methanol −0.01 M phosphate buffer (pH 5.0) containing 4 mM tetrabutylammonium hydrogensulfate (23:77) (v/v). The flow rate was 0.3 ml/min, and the detector wavelength was set at 270 nm.
Calibration Graph
Folic acid standard solutions (0.1–3.0 μg/ml) with a fixed concentration of benzoic acid (internal standard, 40 μg/ml) were prepared in the mobile phase. A 20-μl volume of each standard solution was injected in triplicate and the peak area ratios (analyte to internal standard) were plotted against the corresponding drug concentration to obtain the calibration graph.
Sample Analysis
A 60-mg quantity of micelle formulation was dissolved in a volumetric flask with 5 ml ammonium hydroxide (10%) and the volume adjusted with the mobile phase to 20 ml. An aliquot of 2 ml of this solution was transferred into a 20-ml volumetric flask, the amount of benzoic acid (internal standard) to obtain the concentration used for the calibration graph (40 μg/ ml) was added and the volume was adjusted with the mobile phase. This solution was injected in triplicate and the peak-to-area ratio (analyte to internal standard) used to determine the folic acid concentration in the analytical solution and in the micelles
Release Studies of Progesterone
Progesterone release from the suspensions prepared was monitored by an apparatus consisting of a release cell containing 2 ml of the micelle suspension in PBS separated by a dialysis membrane (Mw cut off = 14000 Daltons) from a receiving compartment containing 3 ml PBS or pH 5.5 buffer mixed with isopropyl alcohol (8:2; v/v), replaced at suitable time intervals to guarantee sink conditions throughout the runs. The apparatus was thermostatted at 37°C for 12 hr. Progesterone was analyzed in the receiving phase over time by an original spectrophotometric method (Pucci et al. 2003) already developed for the determination of progesterone content in commercial formulations, that has been now fully validated for this purpose (data not reported). The quantitative determination was carried out without any dilution on a double-beam spectrophotometer, using fresh receiving phase as the blank solution and reading the first derivative (dA/dλ) of absorbance. The reading corresponds to the difference between the relative maximum at λ = 254 nm and the relative minimum at λ = 227 nm.
Release Studies of Folic Acids
Folic acid release from the suspensions prepared was monitored by the same apparatus consisting of a release cell containing 2 ml of the micelle suspension in PBS and a receiving compartment containing 3 ml PBS or pH 5.5 buffer. Folic acid was analyzed in the receiving phase over time using the HPLC method described above. In particular, 1 ml of the receiving phase was added into a 10-ml volumetric flask, 1 ml of benzoic acid (internal standard) was added and the solution was diluted with the HPLC mobile phase.
Evaluation of Protein Adsorption
First, 2 ml of the unloaded micelles prepared in PBS by means of Pasteur pipette or syringe were suspended in 48 ml PBS containing 50 mg of human serum albumin. After 12 hr, the albumin concentration in the aqueous buffer was monitored by spectrophotometric determination at 278 nm to evaluate its decrease in concentration due to the adsorption on the micelles shell. Micelle suspensions in aqueous buffer without albumin and albumin solution in buffer were used as blanks.
RESULTS AND DISCUSSION
DLS Measurements of Micelles in Solution
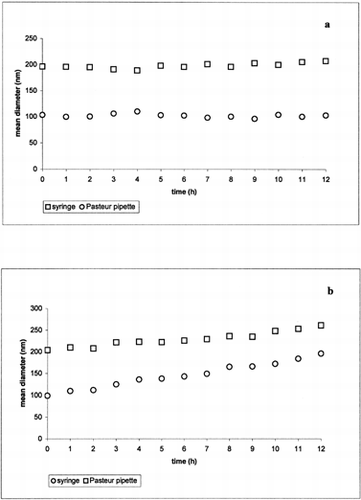
The size of the unloaded micelles prepared in PBS () with Pasteur pipette or with syringe was constant during the time experiment, indicating that no aggregation occurs over time. In the presence of the acidic medium () an increase in micelle size was observed. This behavior can be attributed to a protonation of the hydroxilic groups of the polymer allowing a micelles shell destabilization. In particular, at pH 5.5, the micelles prepared with syringe showed a more evident size increase than the micelles prepared with Pasteur pipette probably as a consequence of their higher surface area.
Progesterone Content
As reported (Pucci et al. 2003), the HPLC determination of progesterone content in the micelles gave clean chromatograms, without interference from the matrix, and the quantitative determination was precise and accurate. The progesterone loading of the micelles resulted near to 100% (mean result of six assays: 102.8%, relative standard deviation = 1.6%), thus testifying to the good and reproducible loading of this very lipophilic drug into the micelles.
Folic Acid Content
Under the described chromatographic conditions, linear relationships between the peak area ratios (analyte to internal standard) and folic acid concentration were obtained (peak area ratio = 2640.8 ± 29.6 [folic acid] − 0.026 ± 0.042; r2 = 0.9998; [folic acid] = 0.1–3.5 (μg/ml). Folic acid in the micelles was completely solubilized in ammonium hydroxide. The pH was quickly adjusted with the mobile phase dilution. Using the described HPLC method, no interference from micelles was observed (Andrisano et al. Citation2003). The method was applied to the analysis of the folic acid containing micelles and the results were in close agreement with the content declared (99.46%, relative standard deviation 1.9%).
Release Studies
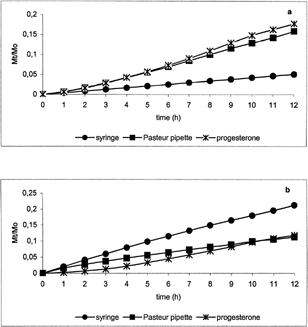
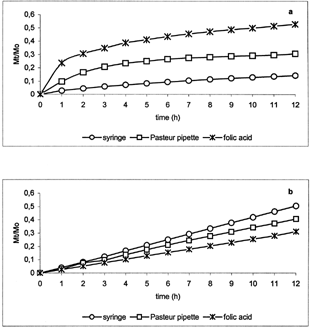
Release profiles of progesterone () and folic acid () from micelles prepared by means of syringe and Pasteur pipette showed significant differences at pH 7.4 (a) and 5.5 (b). In particular, at pH 7.4 the fractional amount of drugs alone was higher than that from corresponding micelle suspensions, whereas, at pH 5.5 the fractional amount of drugs alone was lower than that from corresponding suspensions. This can be explained by the destabilization of the polymeric micelles in acidic medium also observed during DLS measurements. This behavior was more evident for the micelles prepared by syringe. In particular, very low release at pH 7.4 and high release at pH 5.5 were observed from the micelle suspensions with respect to the pure drugs. This can be due to higher micelle surface area allowing greater stabilization at pH 7.4 and stronger destabilization at acidic pH
Evaluation of Protein Adsorption
No decrease in human serum albumin concentration was observed during the time experiment (); suggesting that no adsorption on the micelle shell occurred. This behavior can be due to the presence of hydroxyl groups in the polymer structure forming an hydrophilic shell.
Table 1 Amount (mg) of human serum albumin adsorbed on loaded and unloaded micelle prepared by means of Pasteur pipette and syringe in PBS
CONCLUSIONS
PVA substituted with oleic acid is an interesting material for the formulation of polymeric micelles able to entrap progesterone and folic acid. The amphiphilic nature of this substituted polymer yields micellar systems characterized by high loading ability toward lipophilic drugs and good stability in neutral aqueous environment over time without protein adsorption and aggregation. When used for intravenous route, they can accumulate in body regions with leaky vasculature due to their small size. Moreover, the destabilization observed in acidic enviroment represents another important property of these systems that can promote pH-dependent drug release.
REFERENCES
- Allen C., Eisenberg A., Maysinger D. Copolymer drug carriers: conjugates, micelles and microspheres. S. T. P. Pharma Sci. 1999; 9(1)139–151
- Andrisano V., Bartolini M., Bertucci C., Cavrini V., Luppi B., Cerchiara T. Analytical method for the determination of folic acid in a polymeric micellar carrier. J. Pharm. Biomed. Analysis 2003; 32: 983–989, [CROSSREF]
- Duthie S. J. Folic-acid-mediated inhibition of human colon-cancer cell growth. Nutrition 2001; 17(9)736–737, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Grubbs C. J., Whitaker L. M. Short-term hormone treatment as a chemopreventive method against mammary cancer initiation in rats. Anticancer Res. 1988; 8: 113–118, [PUBMED], [INFOTRIEVE]
- Jones M. C., Leroux J. C. Polymeric micelles—a new generation of colloidal drug carriers. Eur. J. Pharmaceut. Biopharmaceut. 1999; 48: 101–111, [CROSSREF]
- Kazunori K., Atsushi H., Nagasaki Yukio. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Del. Rev. 2001; 47: 113–131, [CROSSREF]
- La S. B., Okano T., Kataoka K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(beta-benzyl L-aspartate) block copolymer micelles. J. Pharmaceut. Sci. 1996; 85: 85–90, [CROSSREF]
- Lin Y., Paschalis A. Physicochemical aspects of drug delivery and release from polymer-based colloids. Curr. Opin. Colloid Interface Sci. 2000; 5: 132–143, [CROSSREF]
- Luppi B., Orienti I., Bigucci F., Cerchiara T., Zuccari G., Fazzi S., Zecchi V. Poly(vinylalcohol-co-vinyloleate) for the preparation of micelles enhancing retinyl palpitate transcutaneous permeation. Drug Deliv. 2002; 9: 1–6, [CROSSREF]
- Martien F. L. Encyclopedia of Polymer Science and Engineering. Wiley, New York 1986
- Orienti I., Gentilomi G., Bigucci F., Luppi B., Zecchi V. Substituted poly(methyl vinyl ether-alt-Maleic anhydride) for the release control and targeting of methotrexate. Archiv der Pharmazie—Pharmaceut. Med. Chem. 1998; 331: 347–351, [CROSSREF]
- Pucci V., Bugamelli F., Mandrioli R., Luppi B., Raggi M. A. Determination of progesterone in commercial formulations and in nonconventional micellar systems. J. Pharmaceut. Biomed. Anal. 1998; 30: 1549–1559, [CROSSREF]
- Seymour L. W., Duncan R., Strohalm J., Kopecek J. Effect of molecular weight of N-(2-idroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats. J. Biomed. Mat. Res. 1987; 21: 1341–1358
- Sivaraman L., Stephens L. C., Markaverich B. M., Clarck J. A., Krnacik S., Conneely O. M., O'Malley B. W., Medina D. Hormone-induced refractoriness to mammary carcinogeneisis in Wistar-Furth rats. Carcinogenesis 1998; 19: 1573–1581, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Yokoyama M. Novel passive targetable drug delivery with polymeric micelles. Biorelated Polymers and Gels, T. Okano. Academic Press, San Diego, CA 1998; 193–229
- Yu B. G., Okano T., Kataoka K., Kwon G. Polymeric micelles for drug delivery: solubilization and hemolytic activity of amphotericin B. J. Contr. Rel. 1998; 53: 131–136, [CROSSREF]