Abstract
Alendronate sodium is formulated into gels and evaluated for the treatment of bone resorptive lesions in periodontitis. Carbopol 934P was used for the preparation of gels in three different concentrations. The prepared gel was evaluated for various properties such as preformulation, content uniformity, viscosity, compatibility, sterility, in vitro diffusion, and in vivo studies. The drug and the polymer were found to be compatible and confirmed by Fourier transform infrared spectroscopy. Viscosity of the gels increased with the increase in the polymer concentration. The formulations were found to be sterile. In vitro release study revealed that drug released from the gel follows non-Fickian diffusion followed by first-order release. In vivo studies were carried out for 6 months in patients. The results revealed a significant improvement in the clinical parameters such as gingival index, probing pocket depth, clinical attachment level, and potent inhibitory effect on bone resorption by inhibition of osteoclasts. In addition, there was increase in the new bone formation.
Alendronate sodium, an aminobisphosphonate, is a synthetic analog of pyrophosphate (Merck Sharp and Dohme [Italy] Citation2000), and has a antiresorptive property (Yaffe and Miztkovich Citation1997). It binds to the crystals of hydroxyapatite in bone and binds preferentially to bone resorption surface particularly to those undergoing active osteoclastic resorption (Jiunn et al. Citation1991). It shows potent inhibitory effect on bone resorption by inhibition of osteoclasts and also has a direct effect on new bone formation (Jernberg Citation1997).
Periodontal disease is a general term for an inflammatory condition caused by specific microorganisms affecting the physiological structural organs supporting the teeth. In periodontal disease, inflammation of the gums around the tooth results in progressive destruction of periodontal ligaments and leaching of alveolar bone causing the tooth to become loose and eventually fall out.
Bacterial flora in periodontal pockets play an important role in the etiology of periodontal disease. Thus, the drug of choice must be given in higher doses to maintain the effective concentration of the drug at osseous defect. Therefore, this type of treatment requires sufficient caution against occasional side-effects, e.g., gastrointestinal disorders, and the development of resistant bacteria to prevent unpleasant or toxic effects as a consequence of the systemic regimen.
Because of these considerations, a variety of specialized local drug delivery systems were designed to maintain the concentration higher than that achieved by systemic administration (Binderman and Yaffe Citation2000). To overcome the disadvantage mentioned above, a controlled release injectable gel base has been investigated. Carbopo l934P was used in the gel formulation (Esoposital and Carotta Citation1996). Gels can be easily prepared and administered. Moreover, they pocess a higher biocompatibility, and they can be eliminated rapidly through the normal catabolic pathway and decrease the risk of irrigative or allergic host reactions at the application site. Hence, carbopol 934P gel formulation-containing Alendronate sodium were designed and administered to human dental resorptive lesions for the treatment of periodontitis.
MATERIALS AND METHODS
Alendronate sodium (mol.wt.325.12) was a gift sample from Sun Pharmaceuticals, Baroda, India; carbopol 934P (mol.wt. 3 × 106) was a gift sample from Zydus Cadila Pharmaceuticals, Ahmedabad, India; triethanolamine (mol.wt 149.19), methylparaben (mol.wt. 152.1), and propylparaben (mol.wt. 180.2) of analytical grade was purchased from Loba Chemicals, Mumbai, India. These ingredients were used as procured.
Preformulation Studies
For solubility, 1 gm of alendronate sodium was dispersed in the solvent and based on the amounts in , the solubility was determined. The pH of the prepared drug solution in water with 1% w/v concentration of alendronate sodium was tested by calibrated systronic pH meter (Elico, Hyderabad).
TABLE 1 Composition of gel formulations with excipients Ingredients of the alendronate sodium gels in percentages
Particle size measurement of alendronate sodium was carried out using optical microscope (Olympus, Japan). Compatibility between the drug and the excipients were evaluated by FTIR studies (Spectra 1000, Perkin-Elmer, Japan) by mixing the drug and polymer (1:1) ratio. FTIR spectrum of alendronate sodium was compared with that of the spectrum of polymers (Haydee Citation2002).
Preparation of Carbopol 934P Gel
Alendronate sodium was dissolved in a required amount of distilled water. Weighed quantity of carbopol 934P (1%, 1.5%, and 2% w/w) was taken and added to the distilled water. The mixture was stirred gradually and carbopol was allowed to soak for 2 hr. Triethanolamine was added to neutralize the carbopol solution and to form the gel. The pH was adjusted 6.8. Finally, the required amount of methyl paraben and propyl paraben were dissolved in ethanol and added to the gel.
Spectral Analysis
A sensitive ultraviolet visible spectrophotometric method was used to analyze alendronate sodium. The method was described by Meyyanathan and Ramasharma (Citation2001). Briefly, it consists of 10 mcg/ml solution of alendronate sodium in distilled water, and 0.3% w/v of sodium 1,2-napthaquinone-4-sulphonate reagent is added as chromophore. It was scanned between 400–600 nm in the visible range in ultraviolet visible spectrophotometer (Shimadzu UV1601A, Japan). The absorption maximum of alendronate sodium at 456.5 nm was selected and used for further studies.
In Vitro Studies
Content Uniformity
The prepared formulations were analyzed for drug content, by taking 1 g of formulation in 100 ml volumetric flask (Borosil, India). The drug was extracted in 0.1 N sodium hydroxide solution. Sodium 1,2-napthaquinone-4-sulphonate reagent was added and the drug concentration was determined by measuring the absorbance at 456.5 nm using the shimadzu spectrophotometer.
Determination of Viscosity
The viscosity of all the formulations was determined by using a Brookfield digital viscometer (Brookfield Model DV II+, USA). The measurements were carried out using spindle number 10 at 100 rpm and the temperature was maintained at 30° ± 1°C.
Microbiological Evaluation
The prepared gel formulation was sterilized by autoclaving (Thermocon, Bangalore, India) for 30 minutes at 121 ± 1°C and Sterility testing is done for aerobic bacteria used nutrient broth media and for anaerobic bacteria by using fluid thioglycollate media at 37 ± 1°C for 48 hr in incubator (Thermocon).
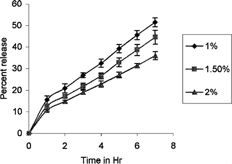
In Vitro Release Study
The dialysis membrane-50 (Himedia, Mumbai, India, mol. wt. cutoff 5,000–10,000)—with average flat width of 24.26 mm, average diameter of 14.33 mm, and capacity 1.61/ml/cm—was used for diffusion studies and was soaked overnight in 0.1 N NaOH solution. Then 1 gm of gel was kept in dialysis membrane sacs and was then placed in a glass beaker containing 20 ml of 0.1-N sodium hydroxide solution. The release studies were performed at 37 ± 1°C for 7 hr. Next, 5 ml of recipient solution was withdrawn at regular interval (every hour) and replaced with an equal amount of fresh 0.1 N sodium hydroxide solution. Samples were analyzed for alendronate sodium by adding 5 ml of sodium 1,2-napthaquinone-4-sulphonate reagent using the spectrophotometer at 456.5 nm.
In Vivo Studies
Clinical Evaluation
A total of 10 sites from 5 patients (age 25 to 50 years) diagnosed to have chronic adult periodontitis were selected for the study from outpatients in the Department of Periodontics, J.S.S. Dental College and Hospital, Mysore, India. The ethical approval was obtained for the study. Selection criteria included the presence of at least 3 or more infrabony pockets greater than 5-mm depth; radiographical defects showed vertical bone loss; no history of systemic disease that would contraindicate periodontal surgery; patient who were nonsmokers; no history of antibiotic intake for last 3 months; and patients who had taken any type of periodontal therapy prior to 6 months of initial examination.
Exclusion criteria for the study were patients who were allergic to alendronate sodium, and patients who had unacceptable oral hygiene following phase I therapy. Study design included selecting two sites in the patients, divided into experimental site and control site. The patient undergoes flap debridement with local drug delivery of alendronate sodium in the experimental site and only flap debridement at control site.
Surgical Procedure
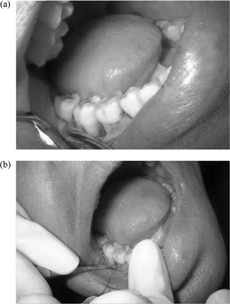
On completion of base line examination and thorough basic therapy of scaling and root planning, the selected sites were assigned randomly to either control or experimental site. The patients were reassessed 14 days after root planning for their oral hygiene compliance. The patient was seated comfortably in the dental chair and then asked to rinse the mouth with 10 ml of 0.2% chlorohexidine digluconate solution. The operative site was anesthetized with 2% xylocaine hydrochloride with adrenaline (1: 80,000); crevicular and interdental incisions were given on the facial and lingual sides using B.P knife with blade no.12 and no. 15, respectively. A full thickness mucoperiosteal flap was reflected using the periosteal elevator taking care that the interdental papillary tissue was retained as far as possible. After reflection of the flap and exposure of the osseous defects, thorough root planning was done. Osseous defects were curetted out and the surgical site was thoroughly irrigated with normal saline.
In the control site, the defect was left unfilled whereas in the experimental site, the defect was filled with alendronate sodium gel. The flap was sutured after placement of the material using black-braided (3-00 silk) interrupted sutures to obtain primary closures of the interdental papilla. The area was protected with a nonengeral dressing and the patient were instructed to rinse with chlorohexidine digluconate (0.2%) mouthwash daily for 2 weeks.
Postsurgical instructions given to the patient included avoiding hot foods, brushing over the pack, and too much rinsing frequently. The patient was informed to report to the clinic if other problems arise. In the postsurgical procedure, 1 week following surgery, the dressing and sutures were removed and the area was thoroughly irrigated with saline. Patients were questioned regarding symptoms related to discomfort, pain, and sensitivity. Dressing was replaced for another 1 week, if any sign of swelling, infection, particle migration, Flap displacement or necrosis was noted.
Patients were again instructed to rinse with chlorohexidine for 1 week. Recall appointments were made after 2 weeks, 1, 2, 3, and 6 months. During each visit, oral hygiene instruction was reinforced and supragingival plaque removal was carried out at the surgical sites.
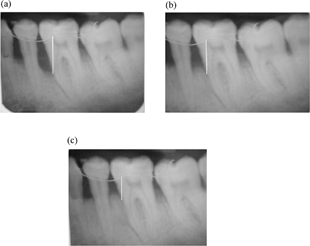
At the end of 3 months and 6 months, patients were evaluated clinically and radiographically at control and experimental sites. The clinical review recorded gingival index, probing pocket depth and clinical attachment level. Intraoral periapical radiographs were taken and measurements were repeated in a manner similar to the presurgical procedure at 3- and 6-month intervals following the operative procedure.
Criteria for Measuring
Metal wire was taken as a standard landmark for measuring the bone length. The deepest part of bone level was considered as the level of the bone, and on each X-ray film, bone level was measured and readings were tabulated and subjected to statistical analysis. Clinical recordings, assessed immediately before treatment (baseline) and after 3 months and 6 months were as follows:
Probing pocket depth (PPD) is defined as the distance from the gingival margin into the bottom of the pocket.
Clinical attachment levels (CAL) is the distance from the cement-enamel junction to the bottom of the pocket.
Gingival index (GI), defined as the distance from the cementoenemal to the gingival margin bone loss defect, is measured by the distance from the metal wire to the base of the defect. The distance between the two points was displayed in millimeters, and the average values were calculated and subjected to statistical analysis.
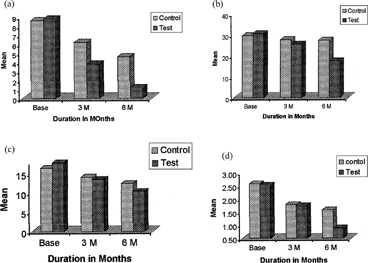
FIG. 4 Clinical parameters of central and experiments sites. (a) the mean difference in probing pocket depth of the control and test, (b) the mean improvement in osseous defect of the control and test, (c) the mean gain in clinical attachment level of the control and test, and (d) the mean difference in gingival score of the control, and test.
RESULTS AND DISCUSSION
The aim of the present investigation was to formulate and evaluate alendronate sodium gels for the treatment of bone resorptive lesions in periodontitis. Preformulation studies showed that alendronate sodium had optimum solubility, pH, and particle size to formulate into gel form. Content uniformity studies showed that the drug was distributed uniformly in all formulations. Viscosity studies showed that viscosity increased with increase in the polymer concentration. Viscosity of all formulations is enough to pass through a 23-gauge blunt needle. FTIR studies showed no chemical interaction between the drug and excipients. Sterility studies showed that formulations are free from microbes.
The in vitro release studies data were quantified using PCP Disso-v2.08 software to determine the percentage release of drug and also to determine the release mechanism. The data obtained were fitted into various models; the best fit model was found to be first-order (Paulocosta Citation2001). The “n” value calculated from Korsmeyer-Peppas model was between 0.5–1 indicating non-Fickian release mechanism followed by first order release.
The kinetic analysis of the release profile was carried out according to the Peppas general equation:
where Mt is the cumulative amount of drug released at time “t”; M∞ is the total amount of drug-incorporated; k is the proportionality constant the value of which depends on the structural and geometrical properties of the matrix; and “n” is the release exponent, its value depends on the mechanism of drug release. “R” regression coefficient also was calculated in a set of data; the model showing highest R value was taken as a best model.
A total of 10 sites in 5 patients were selected for the study. In each patient, the selected 2 sites were divided into experimental site and control site. The experimental site received flap debridement with local delivery of alendronate sodium gel whereas the control site received only flap debridement. The effect of the gels was monitored and recorded at the end of 3- and 6-month intervals. Gel preparation was found to be safe and easy in clinical handling. The gels were viscous enough to be placed into the osseous defect with 23-gauge blunt needle. The mean value of GI, PPD, CAL and osseous defect. Scores were measured at baseline at 3 months, and 6 months for both control and experimental sites, at which there was significant improvement. In all cases after treatment, the GI percentage should improvement after 6 months: control 37.59% and at experimental site 64.71% (p < .005). In the PPD, percentage improvement after 6 months was 46.51% in control and in experimental site 86.36%. The CAL percentage improvement after 6 months in control was 24.27% and in experimental site 41.80%. The osseous defect percentage improvement after 6 months was 7.43% in control and in experimental site 42.85%. However, little improvement were seen after 6 months was in control site were only flap debridement was done. Improvement was seen due to scaling and cleaning of the osseous defect area, and patients were instructed to rinse with chlorohexidine digluconate (0.2%) mouthwash daily for 4 weeks.
CONCLUSION
In this work, injectable gel formulation containing the drug alendronate sodium with carbopol 934P was developed. The in vitro release studies show the release mechanism was a non-Fickian process and followed by first-order release. The “n” value calculated from Korsmeyer-Peppas model was between 0.5–1. Clinical studies of the formulation with carbopol 934P showed a potent inhibition effect on bone resorption by inhibition of osteoclasts. In addition, there was an increase in bone formation, and statically significant improvement such in clinical parameters as gingival index, probing pocket depth, and gain attachment level.
The use of local drug delivery systems is a new addition in the management of periodontitis. By limiting the drug to the target site with little or no systemic uptake, we can avoid most of the problems associated with systemic therapy like oesophagus ulceration, irritation, and gastrointestinal irritation. This study revealed that the gel formulation is simple and comfortable, easy to administer, has less side effects, and has increased patient compliance. We conclude that gel-based drug delivery is a novel approach for the treatment of periodontitis.
The authors thank J.S.S. Mahavidyapeetha, the principals, and the vice principals of J.S.S. College of Pharmacy, Mysore, and J.S.S. Dental College & Hospital, Mysore, for providing the necessary research facilities to carry out clinical studies.
REFERENCES
- Binderman M. A., Yaffe A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. J. Periodon. 2000; 71: 1236–1240
- Esoposital E., Carotta V. Comparative analysis of tetracycline containing dental gels: Poloxamer and monoglyceride-based formulations. Int. J. Pharm 1996; 142: 9–23, [CROSSREF]
- Harvey, et al, United States Patent No. 6, 331, 533 (18 Dec. 2001)., 2001, Method for inhibiting dental resorptive lesions, 1–81
- Haydee B. F. Use of β-cyclodextrins to prevent modifications of the properties of carbopol gels due to carbopol-drug interaction. Chem. Pharm. Bulletin 2002; 50: 40–46, [CROSSREF], [CSA]
- Jernberg G. R., United States Patent No. 6, 123, 957 (16 July 1997), 1997, Delivery of agents and method for regeneration of periodontal tissue, 1–12
- Jiunn H., Lin, et al. Physiological disposition of alendronate, a potent anti-osteolytic bisphosphonate in laboratory animals. J. Drug Metabol. Dispos. 1991; 926–932, [CSA]
- Merck Sharp and Dohme (Italy). 2000; 1–14, Profile on alendronate sodium. Reg. No. Mal 1005100350A
- Meyyanathan S. N., Ramasharma G. V. S. Quantitative determination of alendronate sodium in pharmaceutical dosage form and in bulk drug by spectrophotometry. Ind. Drugs 2001; 38: 461–463
- Paulocosta J. M. S. L. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001; 13: 123–133, [CROSSREF], [CSA]
- Tenenbaum H. C. Bisphosphonates and periodontics: Potential applications for regulations of bone mass in the periodontium and other therapeutics/ diagnostic uses. J. Periodon. 2002; 73: 813–822, [CROSSREF]
- Yaffe A., Miztkovich. Local delivery of an amino bisphosphonate prevents the resorptive phase of alveolar bone following mucoperiosteal flap surgery in Rats. J. Periodon. Sep. 1997; 68: 884–889