Abstract
The aim of this study was to evaluate the ability of forming micelles from two types of synthesized diblock pegylated amphiphilic copolymers and their potential as a drug carrier. Two lactone monomers, ε -caprolactone (CL) and δ -valerolactone (VL), were copolymerized with methoxy poly(ethylene glycol) (MePEG), respectively. The properties of copolymers were investigated and their biocompatibility was tested through an in vitro cytotoxicity study. The influences of the type of lactone monomer (CL and VL) and the feed molar ratios of lactone/MePEG (50/1, 80/1, 160/1) on the performance and release behavior of drug-loaded micelles were investigated. The opening of CL and VL rings by MePEG was efficient, and the pegylation of poly(lactone)s allowed copolymers possessing amphiphilic property and efficiently self-assembled to form micelles with a low critical micelle concentration (CMC) in the range of 10 − 7–10− 8 M. The nano-sized micelles were able to incorporate hydrophobic drug and regulate drug release, and the release of drug was dominated by the hydrophobic poly(lactone) chain length. Although both amphiphilic copolymers exhibited similar controlled release character, the PCL/MePEG micelles possessed lower CMC, higher biocompatibility, and higher drug loading than PVL/MePEG micelles. These suggested that results choosing pegylated PCL as a drug carrier could be better than PVL/MePEG.
Poly(lactone) polymers were usually synthesized by ring-opening polymerization of lactone monomer through four different mechanisms including anionic, cationic, coordination, and radical polymerizations, where catalysts and/or initiators played an important role during synthesis (Brode and Koleske Citation1972). The ring-opening copolymerization of poly(lactone)s and poly(ethylene glycol) in the absence of external catalyst or initiator has been developed by Cerrai, Tricoli, and Andruzzi (Citation1989) and, Kim, Ha, and Lee (Citation2000) where the hydrogen atom of poly(ethylene glycol) end groups acted as an initiator to directly induce acyl-oxygen cleavage of lactone ring. The advantages of this method included the easy copolymerization process, no need of other catalysts (e.g., stannous octoate) or organic solvents, and no toxic materials residing in the final products. Therefore, modified ring-opening copolymerization method was applied in our study to synthesize two types of diblock copolymers, poly(ε -caprolactone)-co-methoxy poly(ethylene glycol) (PCL/MePEG) and poly(δ -valerolactone)-co-methoxy poly(ethylene glycol) (PVL/MePEG). ε -Caprolactone (CL) and δ -valerolactone (VL) possessed seven- and six-member ring in structure, respectively. We supposed that both could be synthesized via the same method due to similar lactone ring structure. It has been reported that the degradation of PCL was constrained by its hydrophobic character and high crystallinity. The reduction of carbon chain length would reduce polymer hydrophobicity and accelerate polymer degradation (Asano et al. Citation1990; Pitt et al. Citation1981). In addition, most of studies have focused on PCL polymer; the exploration and application of PVL to drug delivery was seldom investigated.
Micelle was a nano-sized drug carrier possessing hydrophobic core and hydrophilic shell structure, and it can be formed from diblock or triblock amphiphilic copolymers (Allen, Maysinger, and Eisenberg Citation1999; Torchilin Citation2001). Drugs can be incorporated inside the inner core of micelles via chemical conjugation, hydrophobic interaction, ionic interaction, hydrogen bonding, or physical entrapment to control drug release or protect drug from degradation. The critical micelle concentration (CMC) plays an important role in the stability of micelle system, where low CMC was favored to retain micelle structure after dilution with bulk volume of blood in the body. The CMC value of amphiphilic copolymers have reported to be lower than that of the small molecular surfactants, and this was one of the reasons why the amphiphilic copolymers have received much attention as a drug carrier (Kwon Citation1998).
The aim of our study was to evaluate the ability of forming core-shell structure of micelles from the synthesized diblock pegylated amphiphilic copolymers and their potential as the drug carrier. Two types of lactone monomers, ε -caprolactone (CL) and δ -valerolactone (VL), were copolymerized with methoxy poly(ethylene glycol) (MePEG), respectively. Theoretically, these copolymers should possess amphiphilic character as small molecular surfactants, where poly(lactone) block acted as the hydrophobic domain and MePEG block acted as the hydrophilic domain. The properties of nano-sized micelles formed from these amphiphilic copolymers were investigated and their biocompatibility was tested via an in vitro cytotoxicity study by using normal human fibroblast cells.
Indomethacin was selected as a model drug to be incorporated and delivered by micelles. The influences of the type of lactone monomer (CL and VL) and the feed molar ratios of lactone/MePEG (50/1, 80/1, 160/1) on the performance and release behavior of drug-loaded micelles were investigated and compared between two types of amphiphilic copolymers. The stability of micellar solution in terms of their size change was further evaluated in 5% dextrose solution at 4°C for 12 weeks.
MATERIALS AND METHODS
Methoxy poly(ethylene glycol) was from Fluka Chemical Company (Buchs, Switzerland); delta-valerolactone (δ -VL) and epsilon-caprolactone (ε -CL) were from Aldrich Chemical Company (WI, USA). MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide thiazolyl blue) and indomethacin were from Sigma Chemical Co. (Dorset, UK).
Synthesis and Characterization of Diblock Copolymers
The modified ring-opening copolymerization in the absence of external catalyst or initiator was applied to synthesize two types of diblock copolymers, PVL/MePEG and PCL/MePEG (Hseih and Wang Citation1985; Shin et al. Citation1998). The composition and number-average molecular weight (Mn) of copolymers were determined by 200 MHz 1H-NMR (Bruker DPX-200, USA). The molecular weight distribution in terms of polydispersity (Mw/Mn) of synthesized copolymers was determined by gel permeation chromatography (GPC) with a refractive index detector (Shimadzu RID-10A, Japan). The CMC of amphiphilic copolymers-induced micelle formation was determined with a fluorescence spectrophotometer (F-4500, Hitachi, Tokyo, Japan) using pyrene as a fluorescence probe.
In Vitro Cytotoxicity of Copolymers
Normal human fibroblast cells (IMR-90) was maintained in the minimum essential medium containing 10% fetal bovine serum in an atmosphere containing 5% CO2 at 37°C. First 5,000 cells in 100 μ L of culture medium were plated in a 96-well plate overnight. Cells were then incubated in an equal volume of various concentration of polymeric solution at four 10-fold dilutions ranging from 0.001 up to 1 mg/mL for 48 hr. Each polymer concentration was carried out in triplicate. The number of cells survived was evaluated using MTT assay and determined with a spectrophotometer (Spectra Max Plus, USA) at 550 nm. The ratio of cell survival after and before copolymer treatment was calculated.
Preparation of Drug-Loaded Micelles
Indomethacin and pegylated copolymers comprising various compositions of lactone/MePEG previously were dissolved in acetone, after that de-ionized water (Milli-Q plus, Waters, Millipore, USA) was added slowly. The solution was then placed in a dialysis bag, immersed in 1 L of de-ionized water, and dialyzed for 24 hr. The micelle solution was sonicated and centrifuged. The average particle size of micelles was measured with a particle sizer (Coulter® N4 Plus, Haieleah, FL, USA) at θ = 62.6°, and their morphology observed with a transmission electron microscope (Hitachi H-7100, Japan). The amount of indomethacin incorporated in micelles was determined with a validated ultraviolet spectrophotometer at 318 nm (Jasco model 7800, Tokyo, Japan). The percentage of drug loaded in micelles was defined as the amount of drug in micelles to the the amount of drug added initially.
In Vitro Release Study
Drug-loaded micelles were placed in a vial containing pH 7.2 phosphate buffer solution. The vial was then sealed with the dialysis membrane (Spectrum®, CA, USA, cut-off mw 6,000–8,000), and immersed in the same medium. The release of indomethacin from micelles was conducted at 37 ± 0.5°C, and the stirring speed was set at 50 rpm. Samples (1 mL) were withdrawn at specific time points for 14 days, and the release medium was replaced by the same volume of fresh medium. The sample solution was determined with a validated ultraviolet spectrophotometer at 318 nm. The cumulative amount of drug released at each sampling point was corrected with the volume of the release medium.
Stability Study
The micelles were stored in 5% dextrose solution at 4°C for 12 weeks. The particle size of micelles was measured at beginning and at 1, 2, 3, 4, 6, 8, 10, and 12 weeks after storage. The ratio of particle size of micelles after storage to their initial size was calculated.
RESULTS AND DISCUSSION
Characterization of Synthesized Copolymers
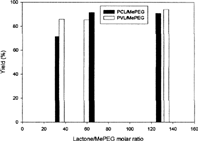
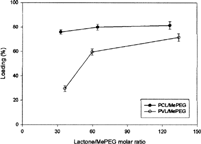
Two types of poly(lactone)/MePEG diblock amphiphilic copolymers, PVL/MePEG and PCL/MePEG, were synthesized in the absence of external catalysts or initiators to avoid toxic substances residing in the final products. This was very important especially for the material to be used as a drug carrier or a medical device in human body. shows the yields of copolymers that were synthesized with the initial feed molar ratio of lactone monomer to MePEG ranging from 50/1 to 160/1. Two types of copolymers had similar yields, and their corresponding values were higher than 70%. The copolymerization efficiency of CL and VL monomers into copolymers were 83.6% and 90.0%, respectively. This result indicated the rate of ring opening of lactones with seven- and six-member rings was similar and quite efficient under current copolymerization condition. The final compositions of synthesized copolymers were very close to their initial feed ratios.
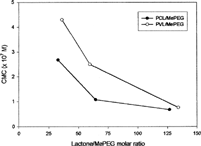
The Mn values of synthesized copolymers were in the range of 8,000 to 20,000 daltons, and the polydispersities of PCL/MePEG and PVL/MePEG copolymers were quite consistent in the range of 2.0-2.5 and 1.7–2.1, respectively. shows the CMC values of PCL/MePEG and PVL/MePEG copolymers. All the pegylated amphiphilic copolymers were spontaneously formed micelles with a low CMC value in the range of 10− 7–10− 8 M, and the micellization efficiency of PCL/MePEG was slightly higher than PVL/MePEG. The CMC of amphiphilic copolymers decreased as the number of lactone monomers increased.
In Vitro Cytotoxicity of Copolymers
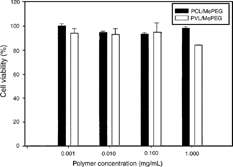
In vitro cytotoxicity of synthesized amphiphilic copolymers was evaluated in normal human fibroblast cells. The number of surviving cells after incubated with polymeric micelle solutions was determined by MTT assay. shows the ratio of survival cells after treated with 10− 3–1 mg/mL of polymeric solutions of PCL/MePEG and PVL/MePEG with molar ratios of monomer to MePEG of 127/1 and 134/1, respectively. Both types of copolymers showed similar in vitro cytotoxicity, and more than 93% of cells were viable except that treated with 1 mg/mL of PVL/MePEG where only 83.8 ± 0.6% of cells survived.
Properties of Drug-Loaded Micelles
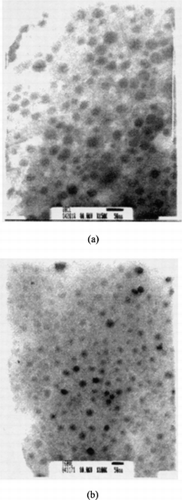
The average particle size of micelles was mostly ∼100–200 nm, and their morphology is shown in . shows the loading efficiency of indomethacin in micelles, and the corresponding values in PCL/MePEG micelles were higher than those in PVL/MePEG micelles. There were six and five carbons in each repeat unit of PCL and PVL, respectively, and the different hydrophobicity based on their chemical structures could be the reason for difference in interaction strength between indomethacin and poly(lactone). Although the hydrophilic MePEG block was extended in the aqueous environment and without effect of drug encapsulation, it has been reported that PEG can prevent liposomes and microparticles from uptake by reticular endothelium system, and prolong them in the blood circulation (Klibanov et al. Citation1990; Stolnik, Illum, and Davis Citation1995).
In Vitro Release Study
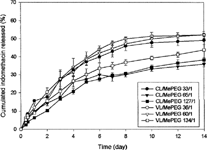
shows in vitro release of drug from two types of micelles formed by different compositions of copolymers in pH 7.2 buffer solutions. No significant burst release was observed in all these micelles, and the slowest release was from micelles with high molar ratio of lactone monomer. This result suggested that the release of drug was dominated by the hydrophobic poly(lactone) chain length of micelles however, the influence of the type of poly(lactone) on drug release was insignificant. Since the degradation of PCL/MePEG and PVL/MePEG was quite slow, the release of drug from current micellar delivery system was mainly controlled by partition and diffusion process.
Stability of Micelles
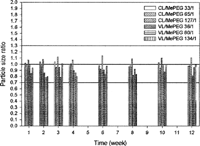
The stability of micelles was conducted in 5% dextrose solution at 4°C for 12 weeks. The average particle sizes of micelles before and after storage were measured. If the ratio of particle size of micelles after storage to its initial size was in the range of 1.0 ± 0.3 this indicated the stability of micellar system was retained. However, outside this range suggested a significant aggregation or dissociation had occurred (Saez et al. Citation2000). shows the change of particle size of PCL/MePEG and PVL/MePEG micelles following storage for 12 weeks. All the micelle solutions maintained their sizes within 1.0 ± 0.3 range at the end of study, irrespective of the type of lactone and the composition of copolymers.
CONCLUSIONS
Two types of diblock pegylated amphiphilic copolymers were successfully synthesized in the absence of toxic catalysts or initiators. The pegylation of polymers allowed copolymers possessed micellization potential to form nano-sized micelles, which were able to incorporate hydrophobic drug and regulate drug release. Although both amphiphilic copolymers showed similar controlled release character, the PCL/MePEG micelles possessed lower CMC, higher biocompatibility, and higher drug loading than PVL/MePEG micelles. This evidence suggested that choosing pegylated PCL as a drug carrier could be a better choice than PVL/MePEG.
This work was supported by National Science Council in Taiwan (NSC 91-2320-B-002-188). The authors thank Professor J. W. Chern and Professor J. H. Guh for their consultation and help.
REFERENCES
- Allen C., Maysinger D., Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloidal Surface B: Biointerfacial 1999; 16: 1–35
- Asano M., Yoshida M., Fukuzaki H., Kumakura M., Mashimo T., Yuasa H., Imai K., Yamanaka H. Effect of crystallinity on the in vivo degradation of poly(b-propiolactone) prepared by radiation-induced solid polymerization in organic solvent system at low temperature. Eur. Poly. J. 1990; 26: 29–33, [CROSSREF]
- Brode G. L., Koleske J. V. Lactone polymerization and polymer properties. J. Macromol. Sci.-Chem. 1972; A6: 1109–1144
- Cerrai P., Tricoli M., Andruzzi F. Polyether-polyester block copolymers by non-catalyzed polymerization of ε -caprolactone with poly(ethylene glycol). Polymer 1989; 30: 338–343, [CROSSREF]
- Hseih G. L., Wang I. W. Ring-Opening Polymerization. ACS, Washington, D.C. 1985
- Klibanov A. L., Maruyama K., Torchilin V. P., Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990; 268: 235–237, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kim S. Y., Ha J. C., Lee Y. M. Poly(ethyleen oxide)-poly(propylene oxide)-poly(ethylene oxide)/poly(ε -caprolactone) amphiphilic block copolymeric nanospheres II. Thermo-responsive drug release behaviors. J. Control. Rel. 2000; 65: 345–358, [CROSSREF], [CSA]
- Kwon G. S. Diblock copolymer nanoparticles for drug delivery. Critical Rev. Therap. Drug Carrier Sys. 1998; 15: 481–512, [CSA]
- Pitt C. G., Gratzl M. M., Kimmel G. L., Surles J., Schindler A. Aliphatic polyesters II. The degradation of poly(DL-lactide), poly(ε -caprolactone), and their copolymers in vivo. Biomaterials 1981; 2: 215–220, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Shin I. G., Kim S. Y., Lee Y. M., Cho C. S., Sung Y. K. Methoxy poly(ethylene glycol)/ε -caprolactone amphiphilic block copolymeric micelle containing indomethacin: I. Preparation and characterization. J. Control. Rel. 1998; 51: 1–11, [CROSSREF], [CSA]
- Stolnik S., Illum L., Davis S. S. Long-circulating microparticulate drug carriers. Adv. Drug Del. Rev. 1995; 16: 195–214, [CROSSREF], [CSA]
- Saez A., Guzman M., Molpeceres J., Aberturas M. R. Freeze-drying of polycaprolactone and poly(D,L-lactic-glycolic)nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur. J. Pharm. Biopharm. 2000; 50: 379–387, [PUBMED], [CROSSREF], [CSA]
- Torchilin V. P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Rel. 2001; 73: 137–172, [CROSSREF], [CSA]