Abstract
The aim of this research is the preparation of acryloylated bovine serum albumin microspheres and the evaluation of their employment in drug delivery. The influence of preparation parameters on albumin microspheres and the chemicophysical properties of loaded drugs were investigated. In particular, we focused our attention on acylation albumin degree, amount of acryloylated albumin against comonomer in the polymerization step, and finally the release profile. We considered on the interaction drug-matrix, the fuctionalization degree of albumin, and the water affinity of matrix.
Considerable interest in recent years has been shown in the use of microspheres as a carrier system for drug delivery (Truter, Santos, and Els Citation2001; Muzzalupo et al. Citation2001; Fundueanu et al. Citation2001; Miyata, Uragami, and Nakamae Citation2002; Madhan Kumar and Panduranga Rao Citation1998). Polymer-based microspheres have been widely studied for drug controlled release. We know that synthetic polymeric materials are applied for their mouldable properties, like molecular weight and cross-linked degree, but they do not exhibit biodegradability and biocompatibility. Naturally occurring polymers could avoid toxicity or biodegradability problems related to the use of synthetic materials (Majors and Friedman Citation1991; Kao et al. Citation2003; Gallo and Gupta Citation1989; Widder and Seneyei Citation1983). In particular, albumin is an attractive macromolecular carrier used to prepare microspheres in the large size range, extensively used for the sustained delivery of therapeutic agents and as drug carriers. These beads are used to realize site-specific delivery systems able to influence the remote site release of drugs.
By virtue of its ability to interact with a wide variety of drugs and its simple and low-cost preparation, albumin represents a very interesting material for therapeutic applications. A considerable number of strategies have been developed to obtain albumin microspheres. They can be achieved by thermal denaturation either by direct reaction between functional groups (usually carboxyl and amino goups) in the polypeptide side chains or by chemically cross-linking agents such as bifunctional carbonyl reagents (Merodio et al. Citation2001; MacAdam et al. Citation1997; Sahin et al. Citation2002). Microspheres also have been obtained from bovine serum albumin (BSA) not denatured (Longo et al. Citation1982; Katti and Krishnamurti Citation1999). All the techniques employed in the past used hydrophobic materials able to release the drug through an erosion mechanism.
In previous research we reported the preparation of hydrogels via radical copolymerization reaction employing with the starting material the copolymer PHEA-GMA obtained by partial derivatization of a polyaminoacid such as α, β-poly-(N-2-hydroxyethyl)-DL-aspartamide (PHEA) with glycidylmethacrilate (GMA) (Muzzalupo et al. Citation2001; Pitarresi Citation2001, Citation2004). More recently, we have reported the preparation of hydrogels, with the same procedure, from partially methacrylated albumin-based (BSA-Ma) (CitationIemma et al. 2004). The functionalization of PHEA with GMA and BSA with methacrylic anhydride (Ma), respectively, allowed us to introduce reactive acrylic groups in the side chain to facilitate radical reactions and to obtain biodegradable hydrogels.
The objectives of this study are two-fold the production and characterization of BSA-Ma microspheres designed for oral formulation and the release characteristics of drug from microspheres. For our purpose, we have chosen as a model drugs diflunisal (DF), β -propranolol (PP), and 5-fluorouracil (FU). Such drugs have been selected to evaluate their in vitro release profile under conditions mimicking gastrointestinal fluid in relation to their different chemical properties. Moreover, because of the chemical diversity of drugs there was careful selection of a loading technique with matching properties. All hydrogels obtained were characterized by particle size distribution analysis, scanning electronic microscopy, and swelling behavior. In vitro release studies, in simulated gastrointestinal fluids, showed the influence of the environmental pH, of beads composition, and of chemical nature of entrapped drug.
MATERIALS AND METHODS
All reagents used were of analytical grade, unless otherwise stated. N-hexane and carbon tetrachloride, purchased from Aldrich Chemical were purified by standard procedures. MA, 2,4,6-trinitrobenzensulphonic acid (TNBS), sorbitan trioleate (Span 85), polyoxyethylene sorbitan trioleate (Tween 85), N,N, N′,N′-tetramethylethylendiamine (TMEDA), and ammonium persulfate were purchased from Fluka Chemical; 5-FU, DF, and PP were provided by Aldrich Chemical.
BSA-Ma was prepared according to a procedure elsewhere reported. BSA fraction V (mw 68.000; pH 7.0 ± 0.2; grade ≥ 98%) was from Roche Diagnostics GmbH. DMAA. Derivatization of BSA with Ma to produce BSA-Ma (A and B) was carried out in distilled aqueous phase, under conditions of controlled pH and temperature (pH7 and 0°C), using a suitable amount of Ma and stirred for 1 hr, purified, and characterized following the procedure reported elsewhere (CitationIemma 2004). The derivatization degree (DD) of prepared BSA-Ma was determined in agreement with a procedure reported by Snyder (Citation1975). The beads were newly prepared to have fresh and greater amounts of materials.
Apparatus
The dialysis tubes used were 6-27/32″ (Medicell International Ltd.) Freezing-drying apparatus was from Micro Modulyo, Edwards. Ultraviolet spectra were recorded with a U-2000 Hitachi spectrophotometer using 1 cm quartz cells. The number of scans was 100. High-pressure liquid chromatography (HPLC) analyses were carried out using a Jasco PU-2080 liquid chromatography equipped with a Rheodyne 7725i injector (fitted with a 10 μl loop), a Jasco UV-2075 HPLC detector, and Jasco-Borwin1 integrator. A reversed-phase C18 column (μBondapak, 10 μm of 250 × 4.6 mm internal diameter obtained from Waters) was used. Particle size distribution was carried out using an image processing and analysis system, Leica DMRB equipped with a Leica Wild 3D stereomicroscope. This image processor calculates the particle area and converts it to an equivalent circle diameter. Scanning electron microscopy (SEM) photographs were obtained with a Leo stereoscan 420; the sample surface was made conductive by the deposition of a layer of gold on the samples in a vacuum chamber.
Microspheres Preparation
Microspheres BSA-Ma based were produced by radical copolymerization technique previously described. Briefly, a mixture of n-hexane and carbon tetrachloride was placed in a round-bottomed cylindrical glass reaction vessel fitted with an anchor-type stirrer and thermostated at 40°C, then treated, after 30 min of N2 bubbling, with a solution of BSA-Ma, comonomer (DMAA), and ammonium persulfate in water. The density of the organic phase was adjusted by the addition of CCl4 or n-hexane so that the aqueous phase sank slowly when stirring stopped. Under stirring at 1000 rpm, the mixture was treated with Span85 and Tween85; then after 10 min with TMEDA, stirring was continued for another 60 min. The amounts of all reagents used in these experiments are reported in . Each matrix so obtained was filtered; washed with 50 ml portions of 2-propanol, ethanol, and acetone; and dried overnight under vacuum at 40°C.
TABLE 1 Homopolymerizations and copolymerizations with DMAA of derivatizated bovine serum albumin
Water Content of Microspheres
The swelling characteristics of BSA-Ma (A1−2 and B1−2) microspheres were determined to check hydrophilic affinity of spherical microparticles. Typically, aliquots (40–50 mg) of the microparticles dried to constant weight were placed in a tared 5-ml sintered glass filter (10 mm; porosity, G3), weighted, and left to swell by immersing the filter plus support in a beaker containing the swelling media, i.e., double distilled water, HCl 0.1 N (simulated gastric fluid), and phosphate buffer pH 6.8 (simulated intestinal fluid). At a predetermined time, the excess water was removed by percolation at atmospheric pressure. Then, the filter was placed in a properly sized centrifuge test tube by fixing it with the help of a bored silicone stopper, then centrifuged at 3500 rpm for 15 min, and weighted. This operation was repeated at the different times (1, 4, and 24 hr). The filter tare was determined after centrifugation with only water. The weights recorded at the different times were averaged and used to give the water regain by the following equation:
where Ws and Wd are weights of swollen and dried spherical microparticles, respectively (Table 3). Each experiment was carried out in triplicate and the results were in agreement within ± 4% standard error.
Incorporation of Drug into Preformed Bsa-Ma Microspheres
Incorporation of drugs into preformed BSA-Ma microspheres was performed as follows: 150 mg of preformed empty microspheres (prepared as described above) were wetted with 2 ml in a concentrated drug solution (15 mg/ml). After 3 days, under slow stirring at room temperatures, the microspheres were freed of the solvent at reduced pressure in presence of P2O5 to constant weight. The weights of drugs are determined by the difference between loaded matrix weight and the empty matrix one, respectively.
In Vitro Drug Release at pH 1.0 and 6.8 from Microparticles
In vitro drug release profiles were obtained by HPLC. Aliquots (10 mg) of drug-loaded BSA-Ma microparticles were dispersed in flasks containing HCl 0.1 N (pH 1.0, simulated gastric fluid) and maintained at 37 ± 0.1°C in a water bath for 2 hr with magnetic stirring. After this time, a solution of 0.2 M tribasic sodium phosphate was added to raise the pH to 6.8 (simulated intestinal fluid), according to the method reported in USP XXII (drug release test, method A, for enteric-coated particles). Sink condition were maintained throughout the experiment. At suitable time intervals, samples were filtered and the solutions were analyzed by HPLC. Each experiment was carried out in triplicate and the results were in agreement within ± 5% standard error.
RESULTS AND DISCUSSION
The reaction of BSA with Ma in water at 0°C and neutral pH allowed us to obtain BSA methacrylated samples with 63% and 100% of acylated available amino groups, A and B. Also, they were able to take part in a radical polymerization for the preparation of microspheres. A and B, respectively, were cross-linked by radical polymerization through a reverse-phase suspension polymerization technique in the presence of a comonomer (DMAA) and using TMEDA and ammonium persulfate as initatior systems ().
A1−2 and B1−2 microparticles were perfectly spherical () and show a narrow size distribution (). To evaluate the affinity of prepared beads toward aqueous medium, the value of contained water percentage (WR%) was determined in aqueous media that simulate some biological fluids, such as gastric (pH 1) and intestinal (pH 6.8) liquid and also in distilled water. The swelling data, summarized in , demonstrate that albumin-based microspheres swell in an aqueous environment due to hydration.
FIG. 1 (a) SEM micrograph of A1, (b) SEM micrograph of A2, (c) SEM micrograph of B1, and (d) SEM micrograph of B2.
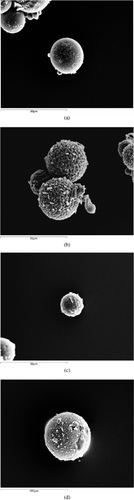
TABLE 2 Water regain percent of beads in various media
FIG. 2 (a) Size distribution profiles of A1, (b) size distribution profiles of A2, (c) size distribution profiles of B1, and (d) size distribution profiles of B2.
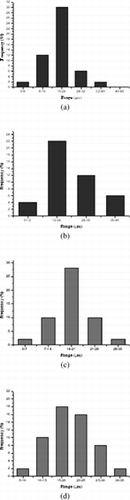
The extent of swelling process depends on composition of the reaction mixture. Therefore, at higher DMAA concentration, the beads are more hydrophilic. The values of WR% suggest that the swelling capacity decreases in the order A1 > A2, such as B1 > B2, in accord with the increase in the amount of DMAA in the sample. However, a remarkable difference varying the albumin derivatization degree was not noted. Collectively, all these results suggest that solvent penetrates easily through the pores, cavities, and/or channel of microparticles created. The fast swelling and the high value of water content percentage suggest a good ability of prepared matrices to release drug molecules in a physiological medium.
To estimate the ability of A/DMAA (A1, A2) and B/DMAA (B1, B2) matrices to release drug molecules, beads were loaded with various drugs. The incorporation of drugs during the cross-linking process has not been possible because of their inadequate solubility in the aqueous dispersed phase. The drugs have been loaded on microparticles by soaking procedure after the cross-linking reaction. The experiments have been carried out at 37°C at pH 1 (simulated gastric fluid) and pH 6.8 (simulated intestinal fluid) using the pH change method (see Methods section). The drug release was expressed as the percent of drug (related to the entrapped total dose) delivered as a function of time from A1−2 and B1−2 matrices. depict drug release of diflunisal, from microspheres with two different cross-linking degrees.
FIG. 3 (a) Release of diflunisal at pH 1 from 0 to 2 hr and at pH 6.8 from 2 to 20 hr (sample A1 and A2). (b) Release of diflunisal at pH 1 from 0 to 2 hr and at pH 6.8 from 2 to 20 hr (sample B1 and B2).
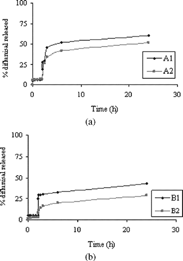
The experimental data showed a limited release of diflunisal from beads also after pH change because of the known strong interaction that is established between albumin and drugs with acid properties. Thus, an incomplete release was observed. This effect is more marked in the case of beads with greater content in albumin and with higher cross-linking degree.
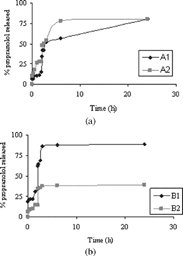
For β -propranolol, the release was greater regarding the diflunisal release (.). Moreover, it catches up approximately 80% for A1 − 2 and B1 beads. On the contrary the β -propranolol release from B2 microparticles is minor.
FIG. 4 (a) Release of β -propranolol at pH 1 from 0 to 2 hr and at pH 6.8 from 2 to 20 hr (sample A1 and A2). (b) Release of β -propranolol at pH 1 from 0 to 2 hr and at pH 6.8 from 2 to 20 hr (sample B1 and B2).
Finally, 5-FU is quickly released at pH 1 and it becomes complete within 3hr (a–5b).
FIG. 5 (a) Release of 5-fluorouracil at pH 1 from 0 to 2 hr and at pH 6.8 from 2 to 20 hr (sample A1 and A2). (b) Release of 5-fluorouracil at pH 1 from 0 to 2 hr and at pH 6.8 from 2 to 20 hr (sample B1 and B2).
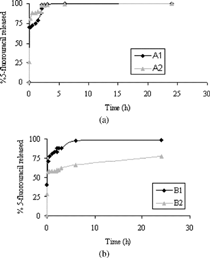
It is possible to observe a remarkable variation in the amount of drug released for B2 beads. For diflunisal, the variation in the amount of drug released at pH 6.8 is caused by salification of acid drug and by greater ability to swell to pH 6.8 of microparticles, although the release remains incomplete because of the strong interaction between the drug and albumin-based matrices. With a basic drug, like β -propranolol, the release profile shows a greater percentage at pH 1 within 2 hr, since in these conditions the ionized form of the drug prevails. However, it is interesting observing the different profile release of drug in enviromental pH values in which these drugs are in undissociate form (diflunisal pH < pKa; β -propranololo pH > PKb). A particular performance has been observed for B2 particles loaded with β -propranolol. In this case about 40% of the drug is released at pH 6.8 and it remains incomplete within 24 hr. Most probably, we can assume that great interactions are established between the β -propranolol and the polymeric network and then reduce the drug release.
Finally for 5-FU that does not undergo salification in aqueous medium, no different release is noted for A1, A2, and B1 by varying the pH values. Only for B2 was lower release observed and attributed to swelling properties and cross-linking degree of this matrix.
CONCLUSIONS
BSA derivatized and DMAA were used for preparing materials cross-linked by a reverse phase suspension polymerization method. All obtained microparticles showed a spherical shape, porous surface, and narrow size distribution. Swelling studies revealed a pH-dependent behavior in media that simulate gastrointestinal fluids. The applicability of these materials as drug delivery systems has been evaluated by loading drugs with different chemical properties by a soaking procedure. In particular, the drug release features depend principally on cross-linking degree, ratio among albumin and DMAA, and interactions of “loaded drug beads.” In fact for diflunisal and β -propranolol, the parameter most influential is the interaction with matrix, strong for the former and weak for the latter, and less important the cross-linking degree and composition of matrix. Only for the β -propranolol system, the cross-linking degree becomes important. The 5-FU release decreases when the cross-linking degree is very high and it is poorly influenced by others parameters.
This work was financially supported by Italian MURST and University Founds.
REFERENCES
- Fundueanu G., Mocanu G., Constantin M., Carpov A., Bulacovschi V., Esposito E., Nastruzzi C. Int. J. Pharm. 2001; 218: 13, [PUBMED], [INFOTRIEVE]
- Gallo J. M., Gupta P. K. J. Pharm. Sci. 1989; 78: 190, [PUBMED], [INFOTRIEVE]
- Iemma F., Spizzirri U. G., Muzzalupo R., Puoci F., Trombino S., Picci N. Colloid Polym. Sci., 2004
- Kao W. J., Burmania J., Li J., Einerson N., Witte R., Stevens K., Nelson D., Martinez-Diaz G. Mater. Sci. Forum 2003; 426–4: 3145
- Katti D., Krishnamurti N. J. Microencap. 1999; 16: 231, [CROSSREF], [CSA]
- Longo W. E., Iwata H., Chindheimer T. A., Goldberg E. P. Pharm. Sci. 1982; 71: 1323
- MacAdam A. B., Shafi Z. B., James S. L., Mariot C., Martin G. P. Int. J. Pharm. 1997; 151: 47, [CROSSREF]
- Kumar Madhan, A. B., Panduranga Rao K. Biomaterials 1998; 19: 725, [CROSSREF], [CSA]
- Majors K. R., Friedman M. B. Animal testing of polymer based systems. Polymer for Controlled Drug Delivery, P. J. Tarcha. CRC Press, Boca Raton, FL 1991; 231–239
- Merodio M., Arredo A., Renedo M. J., Irache J. M. Eur. J. Pharm. Sci. 2001; 12: 251, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Miyata T., Uragami T., Nakamae K. Adv. Drug Del. Rev. 2002; 54: 79, [CROSSREF], [CSA]
- Muzzalupo R., Iemma F., Picci N., Pitarresi G., Cavallaro G., iammona G. Colloid Polym. Sci. 2001; 279: 688, [CROSSREF]
- Pitarresi G., Pierro P., Giammona G., Iemma F., Muzzalupo R., Picci N. Biomaterials 2004; 25: 4333–4343, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Pitarresi G., Pierro P., Giammona G., Muzzalupo R., Trombino S., Picci N. Drug Del. 2001; 9: 97–104, [CROSSREF]
- Sahin S., Selek H., Pronchel G., Ercan M. T., Sargon M., Hincal A. A., Kas H. S. J. Contr. Rel. 2002; 82: 345, [CROSSREF], [CSA]
- Snyder S. L., Sobocinski P. Z. Anal. Biochem. 1975; 64: 284, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Truter E. J., Santos A. S., Els W. J. Cell. Biol. Int. 2001; 25: 51, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Widder K. J., Seneyei A. E. Pharm. Ther. 1983; 29: 377, [CROSSREF]