Abstract
α,β-poly(asparthylhydrazide) (PAHy), a water soluble synthetic polymer, was functionalized by using EDCI chemistry with 3-(carboxypropyl)trimethyl-ammonium chloride (CPTACl) obtaining carboxypropyltrimethyl ammonium copolymers (PAHy-CPTA). Three PAHy-CPTA copolymers at increasing derivatization degrees (38%, 48%, 58%) were chosen for subsequent investigations. The capability of these copolymers to bind, neutralize, and protect DNA against degradation by DNase II was evalued by gel retardation assay and DNA degradation test at pH 5.5. Zeta potential measurements show that all studied polymers are able to neutralize the anionic charge of DNA at polymer/DNA weight ratio in the range of 0.8/1–5/1. Polyplex dimensional distribution analyses in bistilled water, saline solution NaCl 0.9%, and HEPES pH 7 show that polyplex size is strongly affected by both presence and type of electrolyte and with time incubation.
The opportunity to insert into a specific cell new genetic material to substitute defective genes or induce a new function in a defecting tissue has reached great scientific interest in the last decade. For gene therapy purposes, is fundamental to choose the appropriate carrier to condense, protect, and direct the genomic material into the specific target cell. Some viral vectors are very efficient to carry genes into cells, but they are often toxic and not easy to manage. Nonviral gene delivery systems, including cationic liposomes and polymers, have generated great interest as an alternative because of their lower toxicity and easiness to produce and to use. The so-called “polyplex” are obtained for spontaneous strong interaction between gene material and nonviral vectors mimicking the ability of viruses to package DNA and to deliver their genomic material into the cells (Kabanov and Kabanov Citation1995). It is possible to enhance the efficacy of these systems by incorporating different kinds of functional groups to direct them to specific target tissues and/or to properly modify pharmacokinetic profiles (Hwang and Davis Citation2001; Wiethoff and Middaught Citation2003).
One of most popular and more widely studied polymer for gene delivery is polyethyleneimine (PEI) (Godbey, Wu, and Mikos Citation1999). This polymer shows a high gene delivery efficiency and, in particular, 25 kDa PEI has become a benchmark for other polymers. At physiological pH PEI has about 20% of its amine functions in the cationic form. Inside the endosomal compartment, because of lowering pH from 7.2 to ∼5.5, there is a high increase of percentage of protonation. This makes PEI able to work like a buffering system, causing swelling and bursting of the endosomes and causing a lysosomic escape (Abdallah et al. Citation1996). However, PEI is a very cytotoxic polymer for many cell lines and at PEI concentrations used for the transfection experiments, cell metabolic activity may be highly reduced (Lim, Kim, and Park Citation2002).
Our research group recently has proposed the cationic derivative of α, β -poly(asparthylhydrazide) (PAHy) with glycidyltrymethylammonium chloride (GTA) as a potential polymeric vector for genomic material (Pedone et al. Citation2001). PAHy is a polyasparthylhydrazide that has been proposed by our group as a plasma expander and carrier for macromolecular prodrugs (Giammona et al. Citation1989, Citation1994). It is a hydrophilic polymer, nontoxic and nonantigenic in vivo. The PAHy-GTA copolymers have shown to be quite biocompatible, not cytotoxic and hemolytic. Unlike many others polycations, they have no organotrophism for liver and they showed a high transfection efficiency in the polymer/DNA weight ratio 12/1–15/1. However, the reaction between PAHy and GTA, performed in aqueous medium at pH 8.5, was not efficient enough because high positive charge percentages were not introduced into the polymeric structure without a significant decrease of molecular weight of resulting copolymers. We attempted to obtain polycations based on PAHy containing different and easy modulable positive charge amounts and at the same time maintain a proper control of product molecular weight.
In this article we describe the synthesis and the characterization of new PAHy-polycation derivatives obtained by functionalization of PAHy backbone with (3-carboxypropyl)trimethyl-ammonium chloride using a water soluble carbodiimide. The hydrazide groups of PAHy are must reactive toward the carboxy group activated in acidic environment with 1-ethyl3-(dimethylamino)propyl)carbodiimide (EDCI) (Nakajima and Ikada Citation1995); the reaction is very fast, selective, and easily modulable. We tested the versatility of this chemical approach and obtained copolymers (PAHy-CPTA) at different positive charge amount properly changing reaction conditions.
Moreover, we tested the capability of PAHy-CPTA copolymers to condense, compact, and protect the DNA using the gel retardation assay and the degradation test with DNAse II. Also, the dimensional distributions of the hydrated diameter and surface charge of the complexes polycation/DNA in different media were studied.
MATERIALS AND METHODS
1-Ethyl-3-(dimethylamino)propyl)carbodiimide (EDCI) and 3-(carboxypropyl)trimethyl-ammonium chloride (CPTACl) were purchased from Aldrich (Milano, Italy), λ Hind III DNA digest, calf thymus DNA, and DNAse II were all supplied from Sigma (Milano, Italy). α,β -poly(asparthylhydrazide) (PAHy) was prepared and purified as elsewhere reported (Giammona et al. Citation1989). The molecular characterization of PAHy and PAHy-CPTA was performed using a SEC system consisting of Water 600 pump with a Water 2410 refractive index detector equipped with two ultraydrogel columns from Water (1000 and 250 Armstrong). The following eluation conditions were used: flux, 0.8 ml/min (PBS pH 7.8 with 0.1 N NaNO3); temperature 25 ± 0.1°C.
The size and zeta potential measurements were determined by a Malvern Zetasizer 3000 HS (Malvern Instrument, Herrenberg, Germany). Electrophoresis experiments were performed using Consort E 143 system.
Synthesis of PAHy-CPTA Copolymers
A proper amount of CPTACl was added to a 20 ml solution of PAHy 1% w/v. The solution pH was increased to 4.75 with NaOH 0.1 N, then the suitable amount of EDCI was added under mixing. The moles of CPTACl and EDCI added for each copolymers were expressed, respectively, as X and Y ratios: where X was defined as ratio of moles of CPTACl and moles of PAHy repeating units and Y as ratio of moles EDCI and moles of CPTACl; three different reactions were performed maintaing constant X value equal to 1 and changing Y value (Y = 0.5, 1.0 or 1.5).
The pH mixture was adjusted to 4.75 with HCl 0.1 N and the reaction was kept for 2 hr at 25 ± 0.1°C. After this time the reaction was stopped increasing the pH value to 7 with NaOH 0.1 N and the solution was exhaustively dialyzed using Visking dialysis tubing with cut-off of 12000–14000, then lyophilized. All PAHy-CPTA copolymers were obtained with a yield higher than 90% (w/w) based on starting amount of PAHy (). PAHy-CPTA copolymers were characterized by FT-IR, 1H-NMR, and SEC analyses.
TABLE 1 [Values of Y (moles of EDCI/moles of CPTACl)], molecular weight, polydispersity index, and derivatization degree of PAHy-CPTA copolymers and starting batch of PAHy
FT-IR spectra (KBr disks) showed a broad band centered at 3300 cm− 1 (─NH, NH2) and bands at 1655 cm−1 (broad amide I), 1540 cm− 1 (amide II) with a shoulder at 1495 cm− 1 (symmetric bending ─CH3).
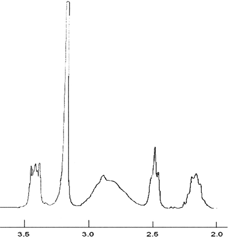
1H-NMR (D2O): δ 2.2 [─CH2C H2CH2N(CH3)]; δ 2.5 [─CH2CH2C H2N(CH3)3]; δ 2.9 [CH─C H2─CO─NH─]; δ 3.2 [─CH2CH2CH2N(C H3)3]; δ 3.4 [─C H2CH2CH2N(CH3)3], 4.75 [─C H─CH2─CO─NH─].
The degree of derivatization, indicated as percentage of positive charged groups in comparison with repeating units of PAHy, were calculated by 1H-NMR by comparing the integral of each peak attributable to linked CPTACl residues at δ 2.2, 2.5, 3.2, and 3.4 with that at 2.9 attributable to PAHy.
Chloride Determination of PAHy-CPTA Copolymers
First, 10 mg of each PAHy-CPTA copolymer were transferred on to a piece of filter paper, wrapped, placed in platinum basket, and subjected to Schoniger decomposition in a flask filled with oxygen. An absorption medium consisting of 10ml of NaOH 0.01 M, with some drops of a 30% solution of H2O2 placed in the flask before combustion, was used. Then the flask was washed with 95% ethanol, and the solution was transferred in a beaker. The chloride was then determined with a potentiometer instrument Crison 2002 (Sconigher Citation1995).
Gel Retardation Assay
PAHy-CPTA copolymers obtained from reaction with a value of X = 1 and Y 1.5-1.0-0.5 were mixed with DNA λ Hind III with a weight ratio PAHy-derivatives/DNA of 5/1, 3/1, 2/1, 1/1, and 0.8/1. (Diethylamino)ethyl dextran (DEAE-dextran) at polymer/DNA weight ratio in from 1/1–5/1 was used as positive control. The polyplex formation was performed in 0.9% sterile saline solution at room temperature and complexes left to stand for a half-hour prior to analysis. The polyplexes were run into an agarose gel (1%, w/v) containing ethidium bromide (0.25 μ g/ml) in Tris-Acetate/EDTA (TAE) buffer at 80 V for a half-hour and the pattern of banding was visualized by ultra violet transillumination and photographed using a Polaroid land camera (Maniatis, Fritsch, and Sambrook Citation1986).
Polyplex Size and Zeta Potential Measurements
Polyplexes, obtained by mixing PAHy-CPTA copolymer solutions () and DNA calf thymus solution at the polymer/DNA weight ratio of 1/1, 5/1, and 10/1 in bistilled water, in 0.9% NaCl solution and HEPES 25 mM (), were subjcted to size and zeta potential analyses. The polyplexes formed at room temperature were left to stand at room temperature for half hour prior to analysis. Size and zeta potential analyses also were performed on the formed polyplexes after subsequent dilution with the same medium and as a function of time.
TABLE 2 Hydrated diameters of polyplexes PAHy-CPTA/DNA calf thymus at different weight ratios after dilution in HEPES solution 25 mM, pH 7.0, after 0.5 and 48 hr
DNase II Degradation Assay
Degradation of calf thymus DNA using DNase II was assayed using the method previously reported (Barret, Heath, and Dingle Citation1977). Calf thymus DNA and PAHy-CPTA/DNA polyplexes were incubated with DNase II (300 units/ml) in a sodium acetate–acetic acid buffer (0.2 M, pH 5.5) with potassium chloride (0.2 M) at 37°C ± 0.1°C. At different times up to 1 hr, 0.5 ml of sample solutions was precipitated with 10% (w/v) perchloric acid (0.5ml). After 20 min at 4°C, the samples were centrifuged (12000× g for 20 min) and the absorbance of the supernatant was measured at 260 nm. Results were expressed as percentage of control degradation at 60 min.
RESULTS AND DISCUSSION
Synthesis and Characterization of PAHy-CPTM Copolymers
Polycations/DNA complexes represent one very promising approach for the nonviral delivery of therapeutic DNA. The synthesis of successful polycations in the gene therapy seems to be based on the optimization of two main aspects such as chemical and biological aspects. The synthesis of copolymers shoud be fast, cheap, and easily modulable to modify the amount of positive charge into the polymeric structure; the resulting polycations should be biocompatible, able to complex DNA at low polymer/DNA ratio, and have no particular organotrophism (e.g., for liver).
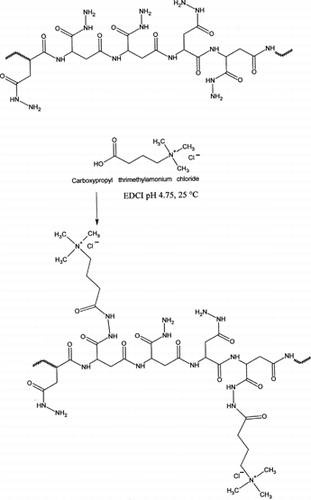
In this article, we focused our attention on the synthesis and the characterization of new polycations based on PAHy, a nontoxic, nonantigenic, nonimmunogenic polymer synthesized in our laboratory. We proposed it as a plasma expander, a carrier for polymeric prodrugs, and a starting material to obtain hydrogels with or without previous derivatization. PAHy is a polymer with protein-like structure bearing hydrazido reactive groups and able to covalent bind molecules with a carboxilyc group properly activated by a water soluble carbodiimide (Giammona et al. Citation1994). For these reasons, the reagent chosen to obtain polycations based on PAHy and bearing permanent positive charge was CPTACl. The synthesis of PAHy-CPTA copolymers is reported in the Diagram.
We used the EDCI chemistry to easily activate the carboxylic groups of CPTACl and allow the coupling with the hydrazido group of PAHy. The reaction of a hydrazido group in aqueous media at acidic pH with a carboxylic group activated by EDCI has been widely investigated (Pouyani, Harbison, and Prestwich Citation1994). The acidic environment is fundamental to a fast activation of the carboxylic groups; with the consumption of EDCI the pH of the reaction mixture begins to increase so that it is necessary to stabilize pH value by continuously adding HCl drops. In the 4–5 pH range, the hydrazide groups are already sufficiently nucleophilic to be able to attach the activated carboxylic group producing a new amidic bond and ureidic derivatives as secondary products. However, the reaction at pH 4.75 is quite fast, occurring almost 30 min after adding EDCI, and the reaction seems to be complete in ∼1 hr, characterized only by small pH increases after this time.
Copolymers obtained after dialysis and lyophilisation were characterized by FT-IR, 1H-NMR, and SEC analyses. FT-IR spectrum shows beside typical amide I and II bands of PAHy, a shoulder at 1495 cm− 1 due to the symmetric bending of CH3 groups. As an example a typical 1H-NMR spectrum is reported in .
The degree of derivatization, indicated as percentage of positive charged groups in comparison with repeating units of PAHy, was calculated by 1H-NMR by comparing the integral of each peak attributable to linked CPTACl residues at δ 2.2, 2.5, 3.2, and 3.4 with that at 2.9 attributable to PAHy. PAHy-CPTA copolymers were characterized from the molecular point of view by size exclusion chromatography, to evidence the eventual effects of experimental reaction conditions on the resulting molecular weight and polydispersity index of copolymers.
The main molecular characterization parameters of obtained PAHy-CPTA copolymers are reported in . As we can see from , the degree of derivatization became higher with increased amount of EDCI; no increase of derivatization degree was detected increasing reaction time (data not shown), indicating that 2 hr of reaction in this condition are sufficient to reach the reaction completation.
We see from that only a slight decrease of molecular weight was detected on PAHy-CPTA copolymers in comparison with starting PAHy molecular weight indicating a slight degradation process occurred during the derivatization reaction; no significant differences in the copolymer molecular weights were observed in increasing Y values in the range of 0.5 to 1.5. Therefore, considering the narrow obtained polydispersity of all copolymers and the dialysis molecular weight cut-off of 12000–14000, it seems probable that an exclusion effect operated by the dialysis membrane tubes.
Chloride determination of PAHy-CPTA copolymers (Sconigher Citation1995) showed the presence of ∼85% of chloride ions in comparison with estimated values through degree of derivatization.
Gel Retardation Assay
The interaction between the PAHy-CPTA copolymers and DNA was investigated by retardation of the electrophoretic mobility of DNA (Kabanov and Kabanov Citation1995). The complexes were formed in NaCl 0.9% sterile solution for almost half an hour, mixing fixed amounts of DNA with increasing amounts of PAHy-CPTA at different degree of derivatization using different ratios polycation/DNA (w/w).
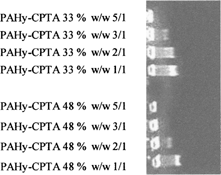
As shown in all PAHy-CPTA copolymers have great capacity to retard DNA migration in agarose gel. In particular PAHy-CPTA Y = 1.5 (D.D. 58%) shows no DNA migration at all used weight ratios; at 5/1 ratio the retardation is complete and a slight exclusion of ethidium bromide also is evident. At ratios 1/1 and 0.8/1, there is complete inhibition of DNA migration. PAHy-CPTA (48%) and PAHy-CPTA (33%) show inhibition of DNA migration only at weight ratios 5/1. To discriminate between the relative activity of PAHy-CPTA (48%) and PAHy-CPTA (33%), we analyzed the complexing ability of two copolymers in the polymer/DNA weight ratio of 1/1 to 5/1. As shown in , PAHy-CPTA (48%) is able to retard the migration of DNA at ratio 2/1 and to block the migration at ratio 3/1; elsewhere PAHy-CPTA (33%) is able to retard the migration to ratio 3/1 and to block only a ratio 5/1.
FIG. 2 Gel retardation assay PAHy-CPTA (58%), PAHy-CPTA (48%), PAHy-CPTA (28%)/DNA λ Hind III at different ratios w/w 0.8/1, 1/1, 5/1.
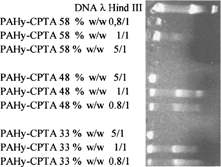
FIG. 3 Gel retardation assay of PAHy-CPTA (48%), PAHy-CPTA (33%) at polymer/DNA λ Hind III weight ratios 1/1, 2/1, 3/1, 5/1.
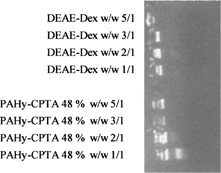
Moreover, the complexing ability of PAHy-CPTA copolymer 48% compared with that of DEAE-dextran (Richardson Kolbe, and Duncan Citation1999; Somayarac and Danna Citation1981), was used as positive control, showed that the retardation ability of this copolymer is comparable to that of positive control at polymer/DNA weight ratio of 2/1 (). On the other hand, polycationic PAHy copolymers obtained by reaction with glycidylthrimethyl ammonium chloride (PAHy-GTA) had a much lower DNA complexing ability in comparison with PAHy-CPTA copolymers. PAHy-GTA 46% was able to inhibit DNA migration at polymer/DNA weight ratio of 75/1 under the same experimental conditions.
FIG. 4 Gel retardation assay confronting retardation capacity of PAHy-CPTA (48%) and DEAE-Dex ratios w/w 1/1, 2/1, 3/1, 5/1.
These results confirm the good capacity of our new PAHy copolymers to complex DNA and neutralize anionic charge; all the polycations tested, at three different percent of functionalization, are very efficient to complex and compact the genomic material. The trend of efficiency varied between a polymer/DNA weight ratio of 0.8/1 to 5/1 going from a PAHy-CPTA at higher derivatization degree to a lower derivatization degree.
Size and Zeta Potential Measurements
The hydrated diameters of the polyplexes are very important to predict the effective capability of the conjugate to transfect because it complexes greater than 150–200 nm are not efficiently endocytosed by the target cells (Pouton and Seymour Citation1998). shows values of hydrated diameter and zeta potential in water of polyplexes PAHyCPTA/DNA at different derivatization degrees and different weight ratios. In all cases and in the polymer/DNA weight ratio from 1/1 to 10/1, the diameter is quite small in the 100 nm–200 nm range.
TABLE 3 Hydrated diameters and zeta potential of polyplexes PAHy-CPTA/DNA calf thymus at different weight ratios in water
These results are in good agreement with the literature because it is reported that under low ionic strength, such as in water polyplexes, small sizes are obtained. Moreover, no variations of polyplex size was detected in water as a function of time until 48 hr (storage at 25°C) and after dilution with water (1:16 v/v) (data not shown). The trend of zeta potential measurements in water shows that increasing the used weight amount of PAHy-CPTA copolymer in the polyplex formation decreases zeta potential values. For all the PAHy-CPTA derivatives, a weight ratio equal to 5/1 is sufficient to make the zeta potential positive.
On polyplexes formed in 0.9% p/v NaCl solution, size measurements showed in all cases diameters bigger than 300nm increasing polycation/DNA weight ratios (). This result can be explained considering that at higher ionic strength than water, such as in NaCl 0.9% p/v, aggregation phenomena among polyplexes occur (Ogris et al. Citation1998). On the other hand, polyplexes formed in water and diluted with fixed amount of NaCl 0.9% p/v (1:16, v/v) showed a rapid increase of size as a function of time until 24 hr (data not shown). Finally, polyplexes formed in water and diluted with a fixed volume of HEPES 20 mM pH 7 (low ionic strength) () showed quite small size ∼140 and 200 nm, and remained the same after 48 hr at room temperature.
TABLE 4 Hydrated diameters of polyplexes PAHy-CPTA/DNA calf thymus at different weight ratios in 0.9% NaCl p/v solution
These results confirm that the ionic strength of complex incubation strongly affects the size of resulting polyplexes. Considering the critical role of polyplex size in obtaining an efficient transfection in vivo, the choice of a proper medium of formation and administration of polyplexes is a crucial step in the preformulation studies of gene therapy.
DNase II Degradation Assay
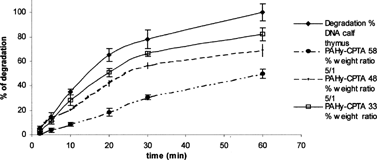
The degradation of DNA plasmid by serum enzymes (Hashida et al. Citation1996) or by nucleases is an important barrier that can prevent the successful transfection of genetic material; for these reasons, an efficient polymeric vector should be able to stabilize DNA and to decrease DNA rate degradation. To detect the capability of our polymers to stabilize DNA, we used the method of Barret et al. (Citation1977). The DNase II was selected as model enzyme for the degradation study. It is known that this enzyme is able to cleave of both strands of DNA leaving the 3′phosphate end group associated to the nucleotide (Barret et al. Citation1977). We started testing our copolymers at different derivatization degrees, PAHy-CPTA 58%, PAHy-CPTA 48% and PAHy CPTA 33% using the same polymer/DNA weight ratio (5/1), and results are reported in . An evident increase of protection ranging from ∼20% to ∼55% was detected going from PAHy-CPTA 33% to PAHy-CPTA 58%.
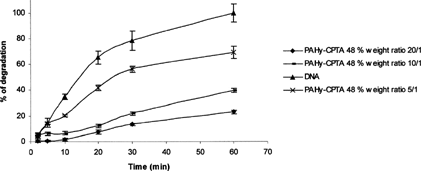
Also considering the ability to protect DNA from degradation, PAHy-CPTA copolymers work better than PAHy-GTA copolymers (Pedone et al. Citation2001): for PAHy-GTA 46% and PAHy-CPTA 48% a comparable protection effect (∼70%) at polymer/DNA weight ratio of 15/1 and 5/1, respectively. Moreover, increasing the amount in weight of polymer used to complex the DNA (from 5/1 to 20/1) (), it is possible to dramatically decrease the percent of degradation of DNA.
CONCLUSIONS
New cationic derivatives of PAHy have been synthesized using EDCI chemistry by coupling the hydrazido group of this polymer with CPTACl. The carbodimide chemistry is very efficient to easily functionalize the polymeric backbone of PAHy, and copolymers at different derivatization degrees have been obtained by only varying the amount of EDCI. Polycations at different percents of functionalization have shown to be very efficient to compact DNA and to reduce the DNA degradation rate by DNAse II even at low weight ratios. Polyplex dimensional distribution analyses in various media show that polyplex size is strongly affected by both presence and type of electrolyte and with time incubation.
In particular, in low ionic strength media (water, HEPES pH 7) all the studied complexes have sizes in the range of 100–200 nm. They are quite stable as a function of time until 48 hr evidencing the ability of PAHy-CPTA copolymers to form in this medium with DNA interpolyelectrolytes with proper dimension for gene delivery purposes. In general, PAHy-CPTA copolymers showed better complexing and protecting ability than PAHy-GTA copolymers previously synthesized. In vitro and in vivo toxicity and transfection studies are in progress.
The authors thank MIUR for funding.
REFERENCES
- Abdallah B., Hassan A., Benoist C., Goula D., Behr J. P., Demeneix B. A. A powerful non viral vector for in vivo gene-transfer in the adult mammalian brain: polyethylenimine. Hum. Gene Ther. 1996; 7: 1947–1954, [PUBMED], [INFOTRIEVE], [CSA]
- Barret A. J., Heath M. F., Dingle J. T. Lysosomes: A Laboratory Handbook, 2nd ed. North Holland Publishing Oxford Company, Amsterdam, New York 1977; 19–147
- Giammona G., Carlisi B., Cavallaro G., Pitarresi G., Spampanato S. A new water soluble synthetic polymer, α,β -Poly(asparthylhydrazide), as potential plasma expander and drug carrier. J. Control. Rel. 1994; 29: 63–72, [CSA], [CROSSREF]
- Giammona G., Carlisi B., Palazzo A., Palazzo S. Polimeri carrier sintesi di una nuova poliaspartidrazide. Boll. Chim. Farm. 1989; 281: 62–64, [CSA]
- Godbey W. T., Wu K. K., Mikos A. G. Size matters:molecular weight affects the efficiency of poly-(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999; 45: 268–275, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hashida M., Mahado R. J., Kawabata K., Myao M., Nishikawa Y., Tokamura Y. Pharmacokinetics and targeted delivery of proteins and genes. J. Control. Rel. 1996; 41: 91–97, [CSA], [CROSSREF]
- Hwang S. J., Davis M. E. Cationic polymers for gene delivery: designs for overcoming barriers to systemic administration. Curr. Opin. Mol. Ther. 2001; 3: 183–191, [PUBMED], [INFOTRIEVE], [CSA]
- Kabanov V., Kabanov V. A. DNA complexes with polycation for the delivery of genetic material into cell. Bioconjugate Chem. 1995; 6: 7–20, [CSA], [CROSSREF]
- Lim Y. B., Kim S. M., Park J. S. Biodegradable, endosome disruptive, and cationic network-type polymer as higly efficient and nontoxic gene delivery carrier. Bioconjugate Chem. 2002; 13: 952–957, [CSA], [CROSSREF]
- Maniatis T., Fritsch E., Sambrook J. Molecular Cloning:A Laboratory Manual, 2nd ed. Cold Spring Harbour Press, New York 1986; 1824–1825
- Nakajima N., Ikada Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjugate Chem. 1995; 6: 123–130, [CSA], [CROSSREF]
- Ogris M., Steinlein P., Kursa M., Mechtler K., Kircheis R., Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998; 5: 1425–1433, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pedone E., Cavallaro G., Richardson S. C. W., Duncan R., Giammona G. α,β -Poly(asparthylhydrazide)-glycidyltrimethylammonium chloride copolymers (PAHy-GTA):novel polymers with potential for DNA delivery. J. Control. Rel. 2001; 77: 139–153, [CSA], [CROSSREF]
- Pouton C. W., Seymour L. W. Key issues in nonviral gene delivery. Adv Drug Delivery Rev. 1998; 34: 3–19, [CSA], [CROSSREF]
- Pouyani T., Harbison G. S., Prestwich G. Novel hydrogels of hyaluronic acid: synthesis, surface morphology and solid-state NMR. J. Am. Chem. Soc. 1994; 116: 7515–7522, [CSA], [CROSSREF]
- Richardson S. C. W., Kolbe H. V. J., Duncan R. Potential low molecular mass chitosans as DNA delivery system:biocompatibility, body distributionand ability to complex and to protect DNA. Int. J. Pharm. 1999; 178: 231–243, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sconigher W. Rapid microanalytical determination of halogen in organic compound. Microkim. Acta 1995; 123–129, [CSA]
- Sompayrac L., Danna K. Efficient infection of monkey cells with DNA of simian virus 40. Proc. Natl. Acad. Sci. USA. 1981; 12: 7575–7584, [CSA]
- Wiethoff C. M., Middaught C. R. Barriers to nonviral gene delivery. J. Pharm. Sci. 2003; 92: 203–217, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]