Abstract
Solid lipid nanoparticles (SLNs) containing tamoxifen, a nonsteroidal antiestrogen used in breast cancer therapy, were prepared by microemulsion and precipitation techniques. Tamoxifen loaded SLNs seem to have dimensional properties useful for parenteral administration, and in vitro plasmatic drug release studies demonstrated that these systems are able to give a prolonged release of the drug in the intact form. Preliminary study of antiproliferative activity in vitro, carried out on MCF-7 cell line (human breast cancer cells), demonstrated that SLNs, containing tamoxifen showed an antitumoral activity comparable to free drug. The results of characterization studies and of in vitro antiproliferative activity strongly support the potential application of tamoxifen-loaded SLNs as a carrier system at prolonged release useful for intravenous administration in breast cancer therapy.
In the recent years solid lipid nanoparticles (SLNs) have received increasing interest among colloidal particulate drug delivery systems (Lim and Kim Citation2002). These systems, composed of a high melting point lipid as a solid core coated by surfactants such as lecithin, represent an attractive alternative to polymeric nanoparticles both for hydrophobic and hydrophilic drugs (Weyhers Citation1995).
Preparation methods of SLNs include essentially high pressure homogenization techniques, the use of microemulsions and precipitation methods (Müller, Mader, and Gohla Citation2000 and references therein). The first method, although able to give SLNs with good reproducibility, involves a preparatory step for drug incorporation into the bulk lipid by drug dissolving or dispersing in the melted lipid and then exposition of drug to high temperature that cannot be always recommended. Then, large-scale production of SLNs with homogeneous and easily controlled physicochemical properties by the microemulsion technique appeared interesting (Gasco Citation1997), whereas the very simple method of precipitation by solvent evaporation needs the use of a organic solvent that has to be carefully removed (Siekmann and Westesen Citation1996).
Basically SLNs can be used for all parenteral applications suitable for polymeric nanoparticles ranging from intrarticular to intravenous administration (Yang et al. Citation1999). For the latter a limited uptake by mononuclear fagocytic system (MPS) can be achieved by the presence of polyethylens glycol on nanoparticles surface, in addition besides to a proper lipidic composition that reduces the hydrophobic surface exposed to blood components. This pharmacokinetic behavior can be exploited properly to have a larger accumulation of SLN in other tissues at high lipidic component such as brain (Fundarò et al. Citation2000).
With increasing interest in this research field and the very attractive perspectives of these systems, as well as the experience of our research group on colloidal drug delivery devices including polymeric nanoparticolate systems, we have addressed the possibility of preparing SLNs by using tamoxifen as model drug.
Tamoxifen, trans-2-[4-(1,2-diphenyl-1-butenyl)phenoxyl]-N,N-dimethylethylamine, is an highly hydrophobic drug (water solubility 0.04 μ g/ml at 37°C) (Gao and Singh Citation1998) with a nonsteroidal structure, an estrogen antagonist with partial agonist properties, and at the moment the endocrine treatment of choice for breast cancer (MacGregor and Jordan Citation1998). The use of tamoxifen, after surgery resection of tumor, improves clinical trials both in pre- and postmenopausal women (Fisher et al. Citation1989; Fornander et al. Citation1989; Ziegler and Budzar Citation1991; Early Breast Cancer Trialists Collaborative Group Citation1992; Love Citation1992; Jaiyesimi et al. Citation1995). Moreover, many studies showed that tamoxifen treatment lowers the risk of contralateral breast cancer by 40% (Love Citation1992; Jaiyesimi et al. Citation1995; Bush and Helzlsouer Citation1993); because of its antiestrogenic activity out of the mammary tissue this drug has side effects such as vaginal hemorrhage, liquid retention, blazes (Mourits et al. Citation2001).
The aim of our investigation was the preparation and characterization of tamoxifen-loaded SLNs with regard to particle size distribution, zeta potential, drug release rate under different condition, and subsequently in vitro evaluation of cellular antiproliferative activity with respect to free tamoxifen. We investigated a possible use of a chosen colloidal carrier as controlled delivery system in breast cancer therapy. The methods used for the SLNs preparation were the microemulsion technique (Gasco Citation1997) because it has easily controlled process parameters and a modified precipitation method.
MATERIALS AND METHODS
Palmitic acid was obtained from Fluka Chemie (Buchs, Switzerland). Epicuron 200 (soya phospatidylcholine 95%) was a kind gift from Lucas Meyer (Hamburg, Germany); taurocholate sodium salt and tamoxifen were obtained from Sigma Aldrich (Milan, Italy). Other used chemicals were of analytical grade (Carlo Erba, Milan, Italy). Human plasma was obtained from voluntary healthy blood donors.
Preparation of Tamoxifen-Loaded Nanoparticles
Tamoxifen-loaded SLNs were prepared either by microemulsion (SLN-m) and precipitation methods (SLN-p). By SLN-m, a warm oil-in-water (o/w) microemulsion was prepared containing palmitic acid (purity 99%, 181.3 mg, 0.7mmol) as a internal phase, Epikuron 200 (purity 95%, 110 mg, 0.14 mmol) as a surfactant, taurocholate sodium salt (purity 97%, 382.5 mg, 0.69 mmol) as a cosurfactant, and distilled filtered water (4 g, 222.2 mmol) as continuous phase. In the first step, tamoxifen (150 mg, 0.4 mmol) were added to the melted palmitic acid; successively a suspension of Epikuron and the aqueous solution of taurocholate sodium salt were rapidly added under mechanical stirring obtaining a clear microemulsion at 70°C ± 1°C (Gasco Citation1997).
SLNs-m were obtained by dispersing the warm o/w microemulsion in distilled cold water (3°C ± 1°C) at a ratio 1:10 (microemulsion-water, v/v), under mechanical stirring (Gasco Citation1997). The obtained aqueous dispersion of SLNs was purified by dialysis against water at room temperature for 5 days, using Visking tubes (18/32 in.) with a molecular weight cut-off 12000–14000. After dialysis the dispersion was added of mannitol (5% w/w) and freeze-dried (Edwards Freeze Dryer Moduloyo, equipped with an Edwards high vacuum pump serial E 2M8 42810) (−40°C, 45 mbar, 5 days). The obtained nanoparticles were stored and refrigerated at +4°C.
SLNs also were prepared through precipitation technique (SLN-p). A solution of the Epikuron 200 (110 mg, 0.14 mmol) in ethanol (2 ml) at 70°C was added to melted palmitic acid (181.3 mg, 0.7 mmol) and kept at the same temperature (70°C). Subsequently tamoxifen (150 mg, 0.4 mmol) was added to this solution, under mechanical stirring, within 10 min. SLNs were obtained by dispersing the warm solution in cold water (100 ml at 3°C ± 1°C) containing taurocholate sodium salt (382.5 mg, 0.69 mmol), under mechanical stirring. The aqueous dispersion of SLN-p was purified by dialysis as described for SLNs-m. After dialysis the dispersion was added of mannitol (5% w/w) and freeze-dried (−40°C, 45 mbar, 5 days). The obtained nanoparticles were stored at +4°C. Ethanol is completely removed from the aqueous dispersion during dialysis and freeze-drying steps. For the subsequent characterizations, a proper amount of dried material was resuspended in bidistilled water under sonication.
Particle Size Determination
The average diameter and width of distribution (polydispersity index) of SLNs were determined by photon correlation spectroscopy (PCS) using a Malvern Zetasizer 3000 HS (Malvern Instrument, Herrenberg, Germany) at a fixed angle of 90°C, a temperature of 25°C, and aqueous solution of NaCl (0.9% w/v) as a suspending medium. Each suspension, properly diluted, was kept in a cuvette and analyzed. Each reported value is the average of 5 measurements.
Zeta Potential Measurements
Zeta potential was measured on dispersion (0.005% w/v) of each lyophilized nanoparticle batch by using an aqueous solution of NaCl (0.9% w/v) or filtered double-distilled water (through 0.2 μ m filter) or phosphate buffer 0.01 M at pH 7.4 as dispersing media using a Malvern Zetasizer 3000 HS. Each sample was analyzed in triplicate.
Analysis of Tamoxifen
An adequate HPLC method was developed to study tamoxifen-loading capacity, drug release profiles from drug-loaded SLNs, and stability of drug. A reversed-phase C18 column (μBondpak, 5 μm, 250 × 46 mm i.d., Waters) was used as a stationary phase and CH3OH/0.1 M K2HPO4 (pH 8.7) (90/10, v/v) as a mobile phase, with a flow rate of 1.5 ml min− 1. The chromatographic apparatus consisted of a Varian LC Star 9012 solvent delivery system equipped with a 7125 Rheodyne Injector (fitted with a 10-μl loop), a Kontron model 432 variable wavelength UV-VIS detector, and Hewlett-Packard HP 3394 integrator.
Mobile phase was monitored at the wavelength of 250 nm. Quantification of drug was performed using a calibration curve obtained from tamoxifen solutions at known concentrations.
The straight-line equation was y = 1.2793x + 0.038317 (y = peak area × 10− 6; x = drug concentration μ g ml− 1). The linear regression value was r = 0.99837. The linearity of the method was studied in the range 0.1–1 μg ml− 1.
Drug Content in Tamoxifen-Loaded SLNs
The procedure to evaluate the amount of tamoxifen loaded in the nanoparticles was as follows 5 mg of freeze-dried nanoparticles were solubilized in 20 ml of CH3OH. The organic solutions were filtered through Millex SR 0.45 μm filter unit (Millipore Corporation, MA, USA) and analyzed by HPLC to determine the amount of drug. No interaction was observed between drug molecules and filter membrane. Results were expressed as the percentage of the drug amount contained in 100 mg of dried material.
Tamoxifen Stability
The stability of tamoxifen was investigated in buffer solutions at pH 7.4 and pH 1.1. Samples of tamoxifen (10 mg) were incubated at 37°C in phosphate buffer/CH3OH (100 ml, 50/50 v/v) at pH 7.4 and in HCl-glycine-NaCl buffer/CH3OH (100 ml, 50/50 v/v) at pH 1.1. At scheduled time intervals, samples were withdrawn and assayed by HPLC using the above described method to measure tamoxifen concentration. Experiments were carried out for 24 h at pH 7.4 and for 4 h at pH 1.1.
Tamoxifen Stability Studies in Human Plasma
To verify tamoxifen stability in human plasma, a saturated solution of tamoxifen in buffer phosphate pH 7.4 (0.09 ± 0.02 μg/ml) was prepared. Aliquots (1 ml) of this solution were added to samples of preheated plasma (500 μl) and keep at 37 ± 0.1°C. At scheduled time intervals, 500 μl of trifluoroacetic acid (10% v/v) were added and samples were centrifuged at 10,000 rpm, for 10 minutes, then filtered on 0.45 μm cellulose filter, and assayed by HPLC.
Drug Release at pH 7.4
Drug release was assayed at 7 prefixed time intervals. For this purpose, 7 suspensions, each containing 5 mg of tamoxifen-loaded nanoparticles, prepared by precipitation technique (SLN-p) were dispersed in 150 ml of pH 7.4 phosphate buffer (0.01 M) and kept at 37°C ± 1°C by a water bath under mechanical stirring. At suitable time intervals, samples were centrifuged at 110,000 × g for 30 min at 4°C, to remove the SLNs and analyzed by HPLC. Each experiment was carried out in triplicate.
Drug Release in Human Plasma
Drug release was assayed at 7 prefixed time intervals. For this purpose, 7 suspensions, each containing 5 mg of tamoxifen-loaded nanoparticles, prepared by precipitation technique (SLN-p) in 10 ml of human plasma were prepared and kept at 37°C ± 1°C by a water bath under mechanical stirring. At fixed time intervals, each sample was centrifuged at 110000 × g for 30 min at 4°C to remove the SLNs. Then, 3.2 ml of trifluoroacetic acid (10% v/v) were added to 0.8 ml of supernatant to precipitate plasma proteins. The obtained suspension was centrifuged at 13,000 × g for 5 min at 5°C. Then supernatant was assayed by HPLC. Each experiment was carried out in triplicate.
Drug Release at pH 1.1
Drug release was assayed at 7 prefixed time intervals. For this purpose, 7 suspensions, each containing 5 mg of tamoxifen-loaded nanoparticles, prepared by precipitation technique (SLN-p) were prepared in flasks containing 10 ml of HCl-glycine-NaCl buffer pH 1.1 and kept at 37°C ± 1°C in a water bath under magnetic stirring (100 rpm). At fixed time intervals, samples were withdrawn and, after neutralization with 0.1 M phosphate buffer at pH 7.2, centrifuged at 110,000 × g for 30 min at 4°C and analyzed by HPLC to determine the amount of drug released. Also, each time the drug fraction entrapped in the nanoparticles at various time intervals was determined. For this purpose residual nanoparticles, after centrifugation at 110,000 × g for 30 min at 4°C, were solubilized in CH3OH. After filtration through a 0.45-μ m nylon filter membrane (Whatman International Ltd, England), the filtrate was analyzed by HPLC. Each experiment was carried out in triplicate.
Drug Release in Octanol/Water System
Drug release was assayed at 7 prefixed time intervals. For this purpose, 7 suspensions, each containing 5 mg of tamoxifen-loaded SLN-p dispersed in a mixture composed by 10 ml of distilled water and 5 ml of octanol, were prepared and kept at 37°C ± 1°C by a water bath under mechanical stirring. At fixed time intervals, organic and aqueous phases were separated. Both phases were centrifuged at 110,000 × g for 30 min at 4°C, then the supernatants were assayed by HPLC. Each experiment was carried out in triplicate.
Determination of Antitumoral Activity
The in vitro antiproliferative activity was assayed on MCF-7 cell line (human breast cancer cells). Cytotoxicity of compounds was evaluated by MTT method (Mosmann Citation1983; Hansen, Nielsen, and Berg Citation1989). The experiment was carried out as follows: 50 μl of cell culture medium (RPMI 1640 without red phenol, supplemented with 10% fetal serum and with antibiotics), containing 1 × 104 cells, were added to 50 μl of cultured ground in each well in a 96-well plate. Then, 1 μl of DMSO was added to controlled wells to consider any solvent activity. The same experiment was carried out incubating in culture medium 1 μ l of tamoxifen DMSO solution (at 10− 4, 10− 5, 10− 6 molar concentrations) with MCF-7 cells or 1 μ l of SLN-p dispersion in buffer phosphate at pH 7.4 (0.01 M), with a tamoxifen concentration equal to 10− 4, 10− 5, 10− 6 molar concentration.
After 4 days of incubation at 37°C in moistured atmosphere, enriched by CO2 at 5%, 10 μl of MTT solution (5 mg/ml) were added to each well and the plate was incubated at 37°C for another 4 h. After this time to each well 100 μl of a mixture of 2-propanol, triton X-100 (10%) and HCl 0.1 N were added, and the optical density at 570 nm was measured after background correction at 690 nm by a multiwell plate reader ELX-800 BIO-TEK.
Values of percentage of growth inhibition were obtained comparing the average OD (optical density) of controlled wells with OD of sample wells as follows:
Statistics
Statistical comparison of the results was performed by variance analysis. Significance of the differences between the means of experimental data was determined by Student's t-test and Mann-Wthitney U-test.
RESULTS AND DISCUSSION
For the preparation of tamoxifen-loaded SLNs, palmitic acid was selected as a solid lipid component that ably constitutes the matrix of SLNs. Epikuron 200 and taurocholate sodium salt were chosen as surfactant and cosurfactant, respectively, because they are acceptable components in parenteral administration.
Tamoxifen-loaded SLNs were prepared by two different methods based on microemulsion and precipitation technique; two batches of nanoparticles were prepared using the same amount of tamoxifen, respectively, SLNs-m and SLNs-p. Since some physicochemical properties (dimension, surface charge, dispersity) are quite critical for biopharmaceutical behavior of SLNs, these systems were characterized in terms of particle size, zeta potential, drug loading capacity, as well as drug release rate.
Particle Size Analysis
shows the dimension and polidispersity index (PI) of SLNs, prepared by two different methods, obtained by PCS technique. As can be seen, nanoparticles prepared by precipitation technique were smaller than those prepared using microemulsion method.
TABLE 1 Dimensional analysis by PCS and drug-loading capacity of tamoxifen-loaded SLN prepared by warm oil-in-water microemulsion (SLN-m) and by precipitation method (SLN-p)
Considering that fat emulsions for parenteral nutrition can present mean particle size ranging from 200 to 400 nm and a PI ranging from 0.05 to 0.13, SLNs seem to have dimensional properties proper for parenteral administration (Müller and Heinemann Citation1992). Moreover, such SLNs can be easily sterilized by filtration on 0.2 μ m or by aseptic preparation (Mehnert and Mader Citation2001).
These systems, thanks to their small size, could minimize the uptake of macrophages of MPS (Schwarz Citation1995; Zur Muhlen Citation1996; Zur Muhlen, Schwarz, and Mehnert Citation1998). This results in a prolonged circulation time that enhances the probability for SLNs to release drug into lipidic tissues.
Studies of Loading Capacity
The evaluation of the drug loaded into nanoparticles was performed dissolving known amount of drug-loaded SLNs into methanol and analyzing obtained solution by HPLC. reports loading data of tamoxifen in SLNs. Experimental results show that the amount of drug loaded into nanoparticles prepared by different methods is quite high (ranging from 28 and 31% w/w) and it is practically independent of preparation technique.
Studies of Zeta Potential
shows the zeta potential of SLN batches prepared by two methods in 3 different solutions: distilled water, NaCl solution, and phosphate buffer at pH 7.4. For two batches, SLNs-m and SLNs-p zeta potential values in water were equal to −39.6 ± 1.6 mV and −44.8 ± 1.0 mV, respectively, and the presence of electrolytes caused a diminution of surface charge. Moreover, since the same dispersing agent is used, no statistically significant difference was found between two examined batches except SLN-p in water.
TABLE 2 Zeta potential values (mV) of the tamoxifen-loaded SLN prepared by warm oil-in-water microemulsion (SLN-m) and by precipitation method (SLN-p)
Studies of Tamoxifen Stability at pH 7.4, pH 1.1, and in Human Plasma
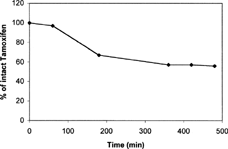
To evaluate the stability of tamoxifen in aqueous media used for in vitro release studies, known amounts of this drug were dissolved in aqueous buffered solution/methanol mixture (Materials and Methods section) at pH 1.1 and 7.4 and incubating these solutions at 37°C ± 0.1°C. Experimental results indicated that tamoxifen is stable at both pH values in considered time range (not showed data). Stability of tamoxifen in human plasma also was evaluated. As reported in , we observed a rapid decrease in tamoxifen plasma concentration within 6 h, when about 50% of tamoxifen in the free and intact form was detected; moreover, no degradation peaks were found. These results can be explained considering that tamoxifen, as reported in literature (Paterson, Lim, and Smith Citation2003), seems to have a high-binding affinity to plasma proteins.
Tamoxifen Release at pH 7.4 and in Human Plasma
To evaluate the possibility of drug-loaded SLNs to release tamoxifen in a prolonged way as a function of time after parenteral administration, drug release kinetics were investigated by in vitro studies under experimental conditions mimicking extracellular fluids (pH 7.4) and in human plasma.
shows tamoxifen release from SLNs prepared by precipitation technique at pH 7.4 and in plasma. All release times are referred to the beginning of centifugation step. As it can be seen, drug at pH 7.4 release rate is considerably low at just 6.6% w/w of the entrapped drug, the amount of released drug within 4 h. Interestingly, drug release is limited to low solubility of tamoxifen in buffer solution at pH 7.4. The obtained drug release profile is in good agreement with that reported for several drugs in SLNs, such as doxorubicin (Cavalli, Capulo, and Gasco Citation1993).
FIG. 2 Release profiles of tamoxifen at pH 7.4 and in plasma from nanoparticles prepared by precipitation technique (SLN-p). Each value is the mean of three experiments. All calculated SE were less than 3% of the mean values and then they were not reported.
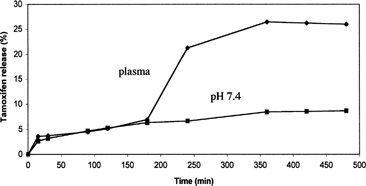
also shows tamoxifen release in human plasma. Preliminary experiments showed that no interfering peaks were in blank plasma chromatogram and that the method used (trifluoroacetic acid to deproteinize plasma) allowed a plasma recovery for free drug of 98 ± 0.5%. In plasma, drug release shows a different profile in respect to the one observed in buffer solution at pH 7.4. In fact, the release rate is quite low until 3 h after the beginning of the experiment (being actually superimposible to that obtained at pH 7.4) and then the successively release rates increase quickly and the amount of drug released reaches 21% after 4 hours and 26% after 6 hours. The different kinetics of drug release is probably due to enzymatic reactions that start to degrade nanoparticle system and to a higher drug solubility in plasma.
Moreover, to evaluate if the amount of drug not released after 6 h is still incorporated into nanoparticles, the amount of tamoxifen entrapped in SLNs after 6 h was evaluated. Obtained results evidenced that the amount of not-released tamoxifen remains incorporated into nanoparticles (data not shown).
Therefore, considering that experiments performed in the presence of only tamoxifen in plasma supported evidence that the bioavailability of drug is strongly affected by its strict bound to albumin and other plasma proteins, tamoxifen loaded nanoparticles, able to release free and intact drug in plasma in a prolonged way, can constitute a good drug delivery system that improves drug bioavailability. Analogous tamoxifen release profiles were obtained from drug-loaded SLNs prepared by microemulsion technique both at pH 7.4 and in plasma. This confirms that the two chosen different preparation methods of drug-loaded nanoparticles produce SLNs with very similar biopharmaceutical properties.
Tamoxifen Release at pH 1.1
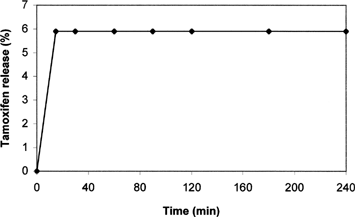
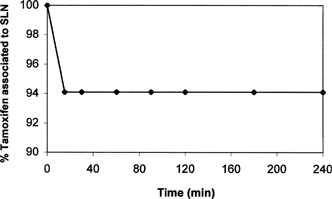
To evaluate the potential of SLNs as drug delivery system for oral administration, in vitro drug release studies were performed in solution mimicking gastric medium (pH 1.1). Drug release profile from SLNs-p at pH 1.1 is reported in . As can be seen, after ∼15 min only 6% w/w was quickly released, and no further drug release was detected as function of time. Because, at these conditions, probably only tamoxifen adsorbed on SLN surface was released, while the most part of drug remained firmly incorporated inside the nanoparticles system. To confirm this hypothesis the amount of tamoxifen entrapped in SLNs at each time after incubation at pH 1.1 was evaluated. The results of these experiments are shown in . Almost 94% of tamoxifen remained incorporated in SLNs; moreover, no degradation occurs in the considered time intervals. Comparable results were obtained using batches of SLNs-m confirming the analogous release behavior of nanoparticles prepared by two different methods.
Tamoxifen Release in Octanol/Water
To gain information on the release of tamoxifen from SLNs in lipid tissues, a simple in vitro model was used in which solid lipid nanoparticles were suspended in a mixture of water/octanol; aqueous phase represents extracellular fluids while octanolic phase mimics a lipid tissue e.g., mammary tissue.
Release studies also were carried out in octanol/phosphate buffer system. The results were similar to that obtained in octanol/water and are not reported.
Since in previous in vitro release studies no significant differences in release behavior were detected in SLN preparation method, just SLNs-p were used, and after incubation at 37 ± 0.1°C two phases (octanol and water phases) were centrifuged and analyzed by HPLC at fixed time intervals. Results are shown in . The amount of tamoxifen released in organic phase is quite high. After 15 min it reaches 68% and increases continuously during the experiment until 73.5% after 4 h.
FIG. 5 Release profile of tamoxifen into octanol phase from nanoparticles prepared by precipitation technique (SLN-p). Each value is the mean of three experiments. All calculated SE were less than 3% of the mean values and then they were not reported.
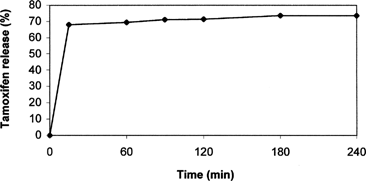
On the contrary in the aqueous phase, only traces of dissolved tamoxifen were found whereas undissolved SLNs, containing the remaining drug amount, were still recovered. These data, compared with those obtained in buffer solution at pH 7.4, showed that tamoxifen, due to its apolar character, is completely released in octanolic phase mimicking a lipidic tissue from prepared SLNs. These results seem particularly interesting in realizing a drug targeted toward lipidic tissues and organs having a high lipidic composition (e.g., breast).
Antitumoral Activity
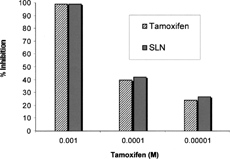
In vitro antiproliferative activity studies, carried out on MCF-7 cell line (human breast cancer cells), showed that SLNs-m containing tamoxifen maintain an antitumoral activity comparable to free drug. These results, showed in , demonstrate that tamoxifen activity is not reduced in the presence of nanoparticle carrier. They also suggest that biological availability of drug is not negatively affected when it is incorporated into SLNs.
CONCLUSION
This article describes the experimental conditions to prepare tamoxifen-loaded SLNs based on palmitic acid by warm oil-in-water microemulsions and by precipitation technique. Nanoparticles prepared by precipitation technique were smaller than those prepared using the microemulsion method. The SLN dimensional data, obtained by PCS measurements, seem to suggest the use of prepared nanoparticles as colloidal delivery systems even for parenteral administration, previous sterilization obtained by filtration on 0.2 μ m or by aseptic preparation.
Drug release study showed that at pH 7.4 the amount of drug released in function of time is quite low, similar to that reported in the literature for analog systems under the same experimental conditions. Even the study at pH 1.1 (gastric fluid conditions) showed a low release of tamoxifen.
On the contrary, release study in plasma showed a drug release rate comparable to that obtained at pH 7.4 at 3 h, followed by rapid increase of drug release rate probably due to the action of plasmatic enzymes that start to degradate SLN system enhancing liberation of drug. Moreover, a quite high release of tamoxifen was showed in octanol/water system mimicking lipid tissue. This result seems to be interesting for possibly drug targeting to mammary tissue since tamoxifen is particularly active against breast cancer. This property of tamoxifen-loaded SLNs could be a therapeutic advantage in respect to free drug administration.
Finally, the in vitro antiproliferative study, showed that tamoxifen incorporated in SLN maintains an unchanged antitumoral activity. In conclusion, taking into account that prepared tamoxifen-loaded SLNs, due to their small size, could minimize the uptake of macrophages of MPS, the nanoparticles could be a long-circulating carrier system that contain tamoxifen potentially useful for breast cancer targeting by intravenous administration.
We thank MIUR for financial support.
REFERENCES
- Bush T., Helzlsouer K. Tamoxifen for the primary prevention of breast cancer: a review and critique of the concept and trial. Epidemiol. Rev. 1993; 15: 233–243, [PUBMED], [INFOTRIEVE], [CSA]
- Cavalli R., Caputo O., Gasco M. R. Solid lipid lipospheres of doxorubicin and idarubicin. Int. J. Pharm. 1993; 89: R9–R12, [CSA], [CROSSREF]
- Early Breast Cancer Trialists Collaborative Group. Systemic treatment of early breast cancer by hormonal cytotoxic or immune therapy. Lancet 1992; 339: 1–15, 339:71–85[CSA]
- Fisher B., Costantino J., Redmond C., Poisson R. R., Bowman D., Counture J., Dimitrov N., Wolmark N., Wickerham D., Fisher E., Margolese R., Robidoux A., Shibata H., Terz J., Paterson A., Feldma M., Farrar W., Evans J., Lickeley H., Ketner M. Randomized clinical trial evaluating tamoxifen in the treatment of patient with node-negative breast cancer who have oestrogen receptor positive tumors. N. Engl. J. Med. 1989; 320: 479–494, [PUBMED], [INFOTRIEVE], [CSA]
- Fornander T., Cedermark B., Mattsson A., Skoog L., Askergren J., Rutqvist L., Glas U., Silfverswäed C., Somell A., Wilking N., Hjalmar M. Adjuvant tamoxifen in early breast cancer occurrence of new primary cancers. Lancet 1989; I: 117–119, [CSA], [CROSSREF]
- Fundarò A., Cavalli R., Bargoni A., Vighetto D., Zara G. P., Gasco M. R. Non-stealth and stealth solid lipid nanospheres carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol. Res. 2000; 42: 337–343, [CSA], [CROSSREF]
- Gao S., Singh J. In vitro percutaneous absorption enhancement of a lipophilic drug tamoxifen by terpenes. J. Control. Rel. 1998; 51: 193–199, [CSA], [CROSSREF]
- Gasco M. R. Solid lipid nanospheres from warm microemulsions. Pharm. Techonol. Eur. 1997; 9: 52–58, [CSA]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989; 119: 203–210, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jaiyesimi I., Buzdar A., Decker D., Hortobagyi G. Use of tamoxifen for breast cancer: twenty-eight years later. J. Clin. Oncol. 1995; 13: 513–529, [PUBMED], [INFOTRIEVE], [CSA]
- Lim S. J., Kim C. K. Formulation parameters determining the physocochemical characteristics of solid lipid nanaoperticles loaded with all-trans retinoic acid. Int. J. Pharm. 2002; 243: 135–146, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Love R. Tamoxifen in auxiliary node-negative breast cancer: multisystem benefits and risks. Cancer Invest. 1992; 10: 587–593, [PUBMED], [INFOTRIEVE], [CSA]
- MacGregor J., Jorda V. Basic guide to the mechanisms of antiestrogen action. Pharmacol. Rev. 1998; 50: 151–196, [PUBMED], [INFOTRIEVE], [CSA]
- Mehnert W., Mader K. Solid lipid nanoparticles. Production, characterization and applications. Adv. Drug Del. Rev. 2001; 47: 165–196, [CSA], [CROSSREF]
- Mosmann T. Rpid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983; 65: 55–63, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mourits M. J. E., De Vries E. G. E., Willemse P. H. B., Ten Hoor K. A., Hollema H., Van Der Zee A. G. J. Tamoxifen treatment and gynecologic side effetcs: a review. Ostet. Gynecol. 2001; 97: 855–866, [CSA], [CROSSREF]
- Müller R. H., Heinemann S. Fat emulsions for parenteral nutrition I: evaluation of microscope and laser light scattering methods for the determination of the physical satbility. Clin. Nutr. 1992; 11: 223–272, [CSA], [CROSSREF]
- Müller R. H., Mader K., Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur. J. Pharm. Bipharm. 2000; 50: 161–177, [CSA], [CROSSREF]
- Paterson S. C., Lim C. K., Smith K. D. Analysis of interaction between alpha-1-acid glycoprotein and tamoxifen and ist metabolites. Biomed. Chromatogr. 2003; 17: 143–148, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schwarz C. Feste lipid nanopartkel: Herstellung, Charakterisienrung, Arzneistoffink und Freisetzung, Sterilisation und Lyophilisation. Ph.D. Thesis, Free University of Berlin. 1995
- Siekmann B., Westesen K. Investigation on solid lipid nanoparticles prepared by precipitazion in o/w emulsions. Eur. J. Pharm. Biopharm. 1996; 43: 104–109, [CSA]
- Weyhers H. Nanopartikel (SLN) fur die gewebsspezifische Arzneistofapplikation. Ph.D. Thesis, Free University of Berlin. 1995
- Yang S., Lu Y., Cai Y., Zhu J., Liang B., Yang C. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Rel. 1999; 59: 299–307, [CSA], [CROSSREF]
- Ziegler L., Budzar A. Current status of adjuvant therapy of early breast cancer. Am. J. Clin. Oncol. 1991; 14: 101–110, [PUBMED], [INFOTRIEVE], [CSA]
- Zur Muhlen A. Feste Lipid Nanopartikel mit prolongierter Wirkstoffliberation, Herstellung, Langzeitstabilitat, charakterisierung Freisetzungsverhalten und mechanismen. Ph.D. Thesis, Frei Universitat Berlin. 1996
- Zur Muhlen A., Schwarz C., Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery-drug release and release mechanism. Eur. Pharm. Biopharm. 1998; 45: 149–155, [CSA], [CROSSREF]