Abstract
Chitosan phthalate polymer was synthesized and its microspheres were prepared by emulsion phase separation technique. The characterization of microspheres was determined by means of FTIR spectroscopy, electron microscopy, particle size, and zeta potential. The insulin was loaded to the microspheres by passive absorption technique. The peptic and tryptic enzymes degradation of insulin in microspheres was investigated. The in vitro release behavior of the microspheres was investigated under different pH conditions (pH 2.0 and pH 7.4). The degree of phthalate substitution in the synthesized polymer was 20%. The prepared microspheres were spherical with an average diameter 46.34 μ m. The insulin-loading capacity was 62%. Chitosan phthalate microspheres protect the insulin from gastric enzymes degradation that may enhance the oral stability of insulin. The encapsulated insulin was quickly released in a phosphate buffer saline (pH 7.4), whereas a small amount of insulin was released under acidic condition (0.1N HCl; pH 2.0) because under acidic conditions, carboxylic groups present in the system exist in nonionized form and are poorly hydrophilic. However, in alkaline conditions, it exists in ionized form and is considerably hydrophilic. The results suggest that chitosan phthalate microspheres may be used as a potential carrier for oral insulin delivery.
To date, more than 30 million people around the world suffer from insulin-dependent diabetes mellitus. The most common therapy is twice-daily subcutaneous injection of insulin. This type of treatment is painful and inconvenient, resulting in poor patient compliance (Pillai and Panchagnula Citation2001). Therefore, other routes of administrations have been developed: nasal, rectal, and pulmonary (Trehan and Ali Citation1998). Among the alternative routes for administration, the oral route is the most convenient and physiological because insulin undergoes a first hepatic bypass, thus ensuring a primary effect by inhibiting hepatic glucose output (Cournarie et al. Citation2002). However, oral delivery of insulin is limited due to acid and enzymatic degradation in the gastrointestinal tract, low epithelial permeability, and instability under formulation condition (Lee et al. Citation2000). Many research studies address various approaches such as alternative routes, absorption enhancers, protease inhibitors, chemical modification, and dosage forms that have been examined to overcome the delivery problem of insulin via gastrointestinal tract (Lee and Yamamoto Citation1990; Mackay, Phillips, and Hastewell Citation1997). On the other hand, there has been increasing interest in developing a new carrier to protect insulin from acid and enzymatic degradation in the gastrointestinal tract and to facilitate its transport from gut lumen to the blood compartment.
Chitosan [poly (1,4 −β-D-glucopyranosamine)] is a polysaccharide derived from naturally occurring chitin in crab and shrimp shells by alkaline deacetylation. This polymer has been investigated extensively for applications in the pharmaceutical field such as in various delayed drug delivery systems. It has appealing intrinsic characteristics that, include biodegradability, biocompatibility, bioadhesiveness and an ability to open epithelial tight junction to allow an increase in paracellular transport macromolecular drugs (Kotze et al. Citation1999; Thanou, Verhoef, and Junginger Citation2001). Notable studies have investigated oral delivery of insulin by using chitosan microspheres (Pan Citation2002a), microparticles (Van der Lubben et al. Citation2001), and nanoparticles (Pan Citation2002b). However, chitosan suffers from high solubility in gastric pH 2.0 and low solubility in physiological pH 7.4, which limits its use as a carrier in oral delivery of insulin. Recently, chitosan derivatives, chitosan phthalate, have attracted more attention in oral drug delivery system because of its low solubility in acidic pH and high solubility in basic pH. Aiedeh and Taha (Citation1999) reported that chitosan phthalate polymer protects the drug molecule from acidic pH 2.0, and most of the drug molecule releases in alkaline pH 7.4.
In the present study, we have prepared chitosan phthalate microspheres from chitosan phthalate polymers by emulsion phase separation technique, and their application toward oral insulin delivery was evaluated. The structure and characterization of microspheres were studied. Insulin loading onto the microspheres was performed by passive absorption method and in vitro release profile at pH 2.0 and pH 7.4 were compared.
MATERIALS AND METHODS
Chitosan phthalate polymer was prepared according to the method reported by Aiedah and Taha (Citation1999). The degree of substitution was calculated to be 20%. Porcine insulin (23 IU/mg) was obtained from Abbott Laboratories. Pepsin and trypsin were purchased from Sigma (St. Louis, MO, USA). Light liquid paraffin, glutaraldehyde, and Span 80 were procured from Merck (USA). Micro BCA kit was purchased from Pierce (USA). All other reagents were of analytical grade.
Preparation of Microspheres
Microspheres were prepared from chitosan phthalate by cross-linking with glutaraldehyde on the surface of emulsion droplets. Chitosan phthalate was dissolved in 15 mL of distilled water. The aqueous phase was extruded through a syringe (no. 20) in 100 mL of the external oily phase of liquid paraffin containing 0.2% Span 80; stirring was carried out using propeller stirrer (Remi, India) at 500 rpm. After 15 min, 1 mL of glutaraldehyde (25% v/v aqueous solution) was added drop-by-drop to the emulsion and stirring was continued. The emulsion was left stirring for 1 hr and then was centrifuged (Remi, India) at 2000 rpm for 15 min. The microspheres were separated by filter paper and washed with petroleum ether. The microspheres were then kept at 4 to 8°C.
Infrared Spectra Analysis
The chitosan and chitosan phthalate microspheres were mixed with KBr and samples were pressed to disk. Infrared (IR) spectra of the microspheres were recorded on a Fourier-transform infrared spectrometer (FTS-135, Biorad, USA).
Morphology, Particle Size Analysis, and Zeta Potential
The shape and surface characteristics of the microspheres were studied by electron microscopy. The size of the microspheres was determined using a particle size analyzer CIS 80 (Ankersmid). The zeta potential of the chitosan phthalate microspheres was determined in distilled water, using Malvern 3000 HS zetasizer (Malvern Instruments, UK).
Insulin Loading of Microspheres
The insulin loading of microspheres was performed by passive absorption technique (Jameela, Misra, and Jayakrishnan Citation1994) by incubating 1% (w/v) chitosan phthalate microspheres and 0.5–2.0% (w/v) insulin in PBS pH 7.4 under shaking at 25°C. After incubation for 12 hr, suspension was centrifuged (12,000 rpm for 15 min) to remove the unloaded insulin. The degree of loading was determined by quantifying nonbound insulin in supernatant with Micro BCA technique. The loading efficiency and loading capacity were determined according to the method established by Fernandez-Urrusuno et al. (Citation1999).
Polyacrylamide Gel Electrophoresis
Polyacrylamide gel electrophoresis (Hoefer™ SE 260, Amershan Biosciences, USA) was performed to determine possible protein degradation by loading and release processes. For electrophoresis, the protein samples were boiled for 5 min in presence of 10% SDS and 0.1% bromophenol blue. An aliquot of the samples, equivalent to 10 μ g/mL of protein, was electrophoresed on a 12% SDS polyacrylamide gel for 1 hr at 300 V. Proteins were visualized using Comassic blue staining. Standard insulin and plain microspheres were used as control.
Peptic and Tryptic Enzyme Degradation of Insulin
Insulin-loaded chitosan phthalate microspheres were incubated with equivolumetric peptic and tryptic solution 0.05 g/L in 0.1N HCl (pH 2.0) at 37°C while shaking. Aliquot volumes of 200 μ L were taken at predetermined time points and the reaction was terminated by adding 200 μ L of 0.05N NaOH. The samples were stored at −10°C until HPLC analysis.
HPLC Analysis
The HPLC system consisted of a Shimadzu LC-10AT liquid chromatograph, a Shimadzu SPD-10A UV-Vis monitor, and a Shimadzu CR 6A chromatopac. The column is 250 mm× 4.6 mm, Lichrospher ODS-C18, 5 μ m. The mobile phase was a mixture of acetonitrile and sulfate buffer solutions (Na2SO4 0.025 mmol/L+NaH2PO4 0.05 mmol/L, pH value was adjusted to 3.0 with H3PO4 [28:72]). The flow rate was 1.0 mL/min. The temperature of column was 40°C. Detection wavelength was at 214 nm.
In Vitro Insulin Release
The in vitro release of insulin from microspheres was carried out in 0.1N HCl (pH 2.0) and PBS (pH 7.4) using shaker (Lab-Therm, Kuhner, Switzerland) under physiologic conditions at 37°C with a shaking frequency of 50 rpm. A sample of chitosan phthalate microspheres was suspended in 100 mL buffer solution. At scheduled time intervals, the release medium was withdrawn and replaced with the same volume of fresh medium. Samples were centrifuged at 12,000 rpm for 10 min and supernatant insulin concentrations was analyzed by Micro BCA technique.
RESULTS AND DISCUSSION
IR Spectra Analysis
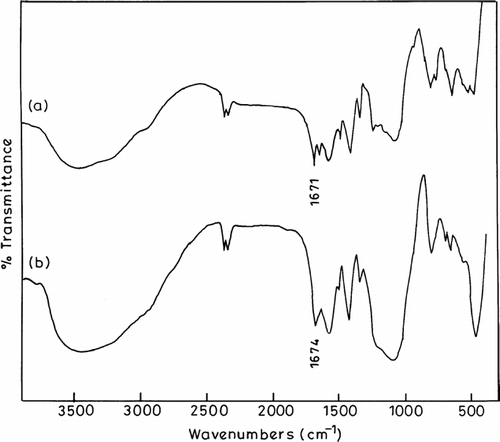
shows the IR spectra of chitosan and chitosan phthalate microspheres. From the IR spectrum of chitosan microspheres, we found that distinctive absorption peaks appear at 1662 cm−1-amide I, 1598 cm−1-NH2 bending and 1380 cm−1-amide III (Zhang et al. Citation2004). IR spectrum of the chitosan phthalate microspheres shows characteristic peak at 1689 cm−1 (amide carbonyl) due to formation of amide links between (−NH2−) of chitosan and (−COOH−) of phthalate groups. The results confirm that the microspheres containing phthalate moieties are linked to the chitosan backbone chain.
Morphology, Particle Size Analysis and Zeta Potential
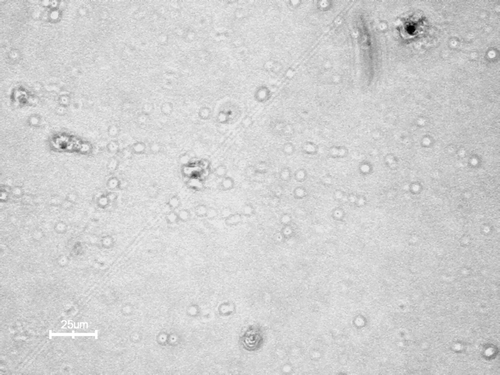
Electron microscopy of chitosan phthalate microspheres is shown in . The microspheres are spherical and regular in shape. The surface of the chitosan phthalate microspheres was slightly rough due to modification of chitosan polymer chain. The figure also indicates that microspheres cross-linking with glutaraldehyde are excellent, which leads to uniform formation of spheres. The average particle size of the chitosan phthalate microspheres was found 46.34 μ m. The chitosan phthalate microspheres were charged positively and average zeta potential was +13 mV. The prepared chitosan phthalate polymer has only 20% of phthalate substitutions. The unreacted amino groups may be responsible for this positive zeta potential.
Insulin Loading of Chitosan Phthalate Microspheres
Because the peptide and protein drug such as insulin would not be exposed to the harsh process involved in microspheres preparation, the loading process was performed using different concentrations of insulin loading solution (0.5, 1, 1.5, and 2% w/v). The results show that the loading capacity (LC) and loading efficiency (LE) of the microspheres were not significantly different. When insulin-loading concentration increased, more concentration of the insulin remains unbound. Therefore, 0.5% (w/v) of insulin in the loading solution was selected as the optimal concentration because a small amount of insulin was unbound during the loading process compared with other insulin-loading concentrations. The LC and LE of insulin were ∼62% and 88%, respectively.
Polyacrylamide Gel Electrophoresis
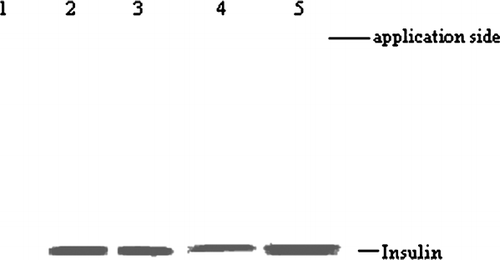
shows SDS-polyacrylamide gel electrophoresis of released samples, supernatant after insulin-loading process and standard insulin. No difference in molecular weight was observed between the released, supernatant, and standard insulin. Hence, we assumed that the loading and release process did not affect the structural integrity of insulin.
Peptic and Tryptic Enzyme Degradation of Insulin
Peptic and tryptic enzyme degradation of pure insulin and insulin encapsulated in chitosan phthalate microspheres are shown in . The insulin in microspheres was strongly protected from peptic degradation compared with pure insulin (88 ± 3.0% and 53± 2.5%, respectively after 2 hr). The chitosan phthalate polymer also strongly protected the insulin from tryptic degradation compared with pure insulin (86 ± 1.8% and 49 ± 1.5%, respectively after 2 hr). The reason may be that pepsin and trypsin are positive charged and cannot bind to chitosan phthalate microspheres that also have the positive charge. Therefore, insulin encapsulated in chitosan phthalate microspheres could be protected from gastric enzymes like pepsin and trypsin.
TABLE 1 Degradation of insulin and insulin-loaded chitosan phthalate microspheres in pepsin and trypsin solution
Insulin Release from Microspheres
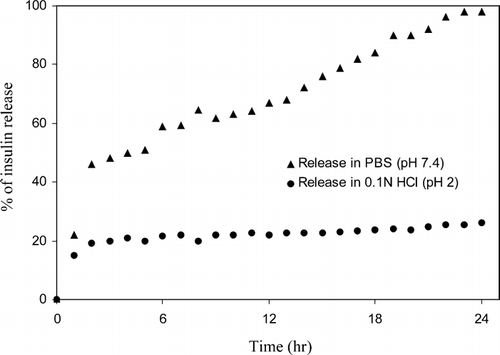
In vitro releases of insulin from microspheres were carried out at different pH environments (pH 2.0 and pH 7.4) and the results are shown in . Fully 98% of the insulin release was observed from microspheres at pH 7.4, whereas only 27% of the insulin release was observed at pH 2.0 within 24 hr. The result suggests that in the present system, a major factor controlling insulin release is pH of the medium. The result was attributed to the fact that under acidic conditions (pH 2.0), carboxylic groups present in the system exist in nonionized form and are poor hydrophilic. When microspheres are kept in alkaline condition (pH 7.4), carboxylic groups exist in ionized form and are considerably hydrophilic. Therefore, chitosan phthalate microspheres acted as expected under acidic condition and resisted hydration and the subsequent release of insulin. The fast release of insulin at pH 7.4 was attributed to the higher permeability of buffers in the microspheres, which resulted in more swelling of the polymers. At pH 7.4, the swelling was much higher than at pH 2.0; this resulted in a large amount of insulin release from the microspheres via diffusion.
CONCLUSION
We prepared chitosan phthalate microspheres by emulsion phase separation technique. The microspheres were spherical and regular shape. The loading capacity of insulin was ∼62%. Chitosan phthalate microspheres could protect the insulin from gastric enzymes which may enhance the oral stability of insulin. The insulin-loaded microspheres retard the release at low pH and release the insulin at physiological pH. The chitosan phthalate microspheres might serve as a potential carrier in oral insulin delivery system.
The authors gratefully acknowledge the facilities provided at the National Institute of Immunology, New Delhi, India. We also thank Dr. Yogesh K. and Prof. V. Loganathan for their helpful discussions throughout the investigation.
REFERENCES
- Aiedeh K., Taha M. O. Synthesis of chitosan succinate and chitosan phthalate and their evaluation as suggested matrices in orally administered, colon specific drug delivery systems. Arch. Pharm. Pharm. Med. Chem. 1999; 332: 103
- Cournarie F., Auchere D., Chevenne D., et al. Absorption and efficiency of insulin after oral administration of insulin-loaded nanocapsules in diabetic rats. Int. J. Pharm. 2002; 242: 325
- Fernanedez-Urrusuno R., Calvo P., Remunan-Lopez C., et al. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999; 16: 1576
- Jameela S. R., Misra A., Jayakrishnan A. Cross-linked chitosan microspheres as carriers for prolonged delivery of macromolecular drugs. J. Biomater. Sci. Polym. Ed. 1994; 6: 621
- Kotze A. K., Lueben H. L., Thanou M. M., et al. Chitosan and chitosan derivatives as absorption enhancers for peptide drugs across mucosal epithelia. Bioadhesive Drug Delivery System: Fundamentals, Novel Approaches and Development, E. Mathiowitz, D. E. Chickering, C. M. Lehr. Marcel Dekker, New York 1999; 341–387
- Lee V. H. L., Kashi S. D., Grass G. M., Rubas W. Oral route of protein and peptide drug delivery. Peptide and Protein Drug Delivery, V. H. L. Lee. Marcel Dekker, New York 2000; 691–740
- Lee V. H. L., Yamamoto A. Penetration and enzymatic barriers to peptide and protein drug delivery. Adv. Drug. Del. Rev. 1990; 4: 171–207
- Mackay M., Phillips J., Hastewell J. Peptide drug delivery: colonic and rectal absorption. Adv. Drug. Del. Rev. 1997; 28: 253–273
- Pan Y., Li Y. J., Zhao H. Y., et al. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002a; 249: 139–147
- Pan Y., Zheng J. M., Zhao H. Y., et al. Relationship between drug effects and particle size of insulin loaded bioadhesive microspheres. Acta Pharmacol. Sin. 2002b; 23: 1051–1056
- Pillai O., Panchagnula R. Insulin therapies- past, present and future. Drug Discov. Today 2001; 6: 1056
- Thanou M., Verhoef J. C., Junginger H. E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug. Del. Rev. 2001; 50: 91–101
- Trehan A., Ali A. Recent approaches in insulin drug delivery. Drug. Dev. Ind. Pharm. 1998; 90: 589
- Van der Lubben I. M., Verhoef J. C., Van Aelst A. C., et al. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine peyer's patches. Biomaterials 2001; 22: 687–694
- Zhang C., Ping Q., Ding Y., et al. Synthesis, characterization, and microsphere formation of galactosylated chitosan. J. App. Poly. Sci. 2004; 91: 659–665