Abstract
The objectives of this study were to solubilize oxytetracycline hydrochloride (HCl) in reverse micelles to prepare poly-d,l-lactide-co-glycolide (PLGA) microspheres and to explore parameters affecting its encapsulation efficiency. Oxytetracycline HCl was dissolved in the reverse micelles consisting of cetyltrimethylammonium bromide, water, and ethyl formate. A PLGA polymer was then dissolved in the reverse micellar solution, and a modified solvent quenching procedure was carried out to prepare PLGA microspheres. Encapsulation efficiencies of oxytetracycline HCl ranged from 2.3 ± 0.2 to 24.9 ± 4.6%, depending on experimental conditions. Important parameters affecting its encapsulation efficiency included the amounts of water used to prepare the reverse micelles and PLGA polymer. With regard to microsphere morphology, the reverse micellar process produced the microspheres with smooth and pore-free surfaces. In particular, their internal matrices did not possess hollow cavities that were frequently observed when a typical double emulsion technique was used to make microspheres. In summary, it was possible to encapsulate oxytetracycline HCl into PLGA microspheres via the ethyl formate-based reverse micellar technique. We also anticipate that the use of ethyl formate could avoid environmental and human toxicity issues associated with methylene chloride.
INTRODUCTION
Encapsulation of pharmaceuticals into poly-d,l-lactide-co-glycolide (PLGA) microspheres provides their controlled release up to several months (Kostanski, Thanoo, and DeLuca Citation2000). PLGA microspheres are prepared by a number of encapsulation processes including single emulsion, double emulsion, phase separation, and spray drying (Benoit et al. Citation1996; Jain Citation2000). In particular, an oil-in-water (o/w) emulsion–based solvent evaporation is commonly used to encapsulate hydrophobic drugs into PLGA microspheres. Methylene chloride is the most frequently used organic solvent, since it is easily removed by evaporation over a period of several hours. Alternatively, a water-in-oil-in-water (w1/o/w2) double emulsion method is used for encapsulation of hydrophilic drugs. In this system, their aqueous solution is first emulsified in methylene chloride in which a PLGA polymer is dissolved. This primary water-in-oil (w1/o) emulsion is emulsified in an external aqueous phase (w2) to prepare a double emulsion that transforms into a microsphere suspension over hardening.
Surfactants find a variety of applications in controlling wetting, stability, and bioavailability of pharmaceutical dosage forms. Another important property of micelles is related to their ability to increase the solubility of hydrophobic substances in water (Rangel-Yagui, Pessoa, and Tavares Citation2005). In contrast to such normal micelles, reverse micelles can be used to dissolve water-soluble compounds in apolar solvents. A typical reverse micellar system consists of water, bis(2-ethylhexyl)sodium sulfosuccinate (AOT), and isooctane or hexane. Nano-sized AOT reverse micelles contain about 23 molecules per aggregate (Mallick et al. Citation2001). Certain amounts of water are pooled inside the reverse micellar cavity to make room for water-soluble compounds. Sometimes, reverse micelles allow the sustained drug release: upon contact with body fluids the reverse micellar solutions transform into liquid crystalline phases or vesicle dispersions, thereby reducing drug release rate (Mueller-Goymann and Hamann Citation1993). Previously, a reverse micellar solution was utilized in loading proteins into PLGA microspheres (Hayashi et al. Citation1994). Their reverse micellar systems were made of AOT, sucrose ester of fatty acids, chloroform, and methylene chloride. Their reverse micellar solutions were subjected to a conventional solvent evaporation process to make PLGA microspheres.
The objective of our study was to develop a new reverse micellar system that could be used in encapsulating a water-soluble compound into PLGA microspheres via a modified encapsulation method. The reverse micelles consisted of cetyltrimethylammonium bromide, water, and ethyl formate. Oxytetracycline hydrochloride (HCl) was used as a model drug, whereas ethyl formate was chosen as an organic solvent. The choice of ethyl formate was based on its feasibility as a dispersed solvent for preparing PLGA microspheres as reported elsewhere (Sah Citation2000).
MATERIALS AND METHODS
Sigma Chemicals (St. Louis, MO, USA) was the supplier of cetyltrimethylammonium bromide (CTAB), oxytetracycline hydrochloride (OTC), and trifluoroacetic acid (TFA). They were used as received without further purification. Ethyl formate of analytical grade was obtained from Aldrich (Milwaukee, WI, USA). PLGA with a lactide:glycolide ratio of 75:25 was purchased from Birmingham Biopolymers (Birmingham, AL, USA). The polymer had an inherent viscosity of 0.44 dLg−1 in chloroform at 30°C. Hereinafter, it was abbreviated as PLGA75:25. Polyvinyl alcohol with a molecular weight of 25,000 (88% hydrolyzed) was purchased from Polysciences (Warrington, PA, USA).
Preparation of Reverse Micelles
OTC (10–40 mg) and CTAB (20 mg) were put into a vial containing ethyl formate (3 ml) and water (0.2–0.4 ml). The mixture was kept still at 37°C inside an oven until a clear, transparent solution was produced. Since reverse micelles were formed spontaneously, any mixing devices such as a magnetic stirrer and a sonicator were not used in this experiment.
Measurement of Reverse Micellar Solubilization of OTC
At predetermined time intervals, the mixture described above was taken out of the oven and centrifuged at 2,500 rpm for 2 min. Then 50 μl of the clear ethyl formate phase were taken and diluted with 5 ml of water. Aliquots (20 μl) of the diluted solutions were subjected to HPLC analysis to measure the amount of OTC dissolved in the reverse micelles.
Preparation of OTC-Loaded PLGA75:25 Microspheres
The clear reverse micellar solution was used to dissolve 0.35, 0.45, 0.6, or 0.7 g of PLGA75:25. The polymeric solution was then poured into 20 ml of a 1% aqueous polyvinyl solution presaturated with ethyl formate. During this addition, the aqueous phase was stirred at 600 rpm using a 400 HPS magnetic plate stirrer (VWR Scientific, West Chester, PA, USA). After 5 min, 60 ml of a 0.5% aqueous polyvinyl alcohol solution was added to the emulsion to quickly extract ethyl formate in the polymeric phase into the aqueous external phase. Afterward, the microsphere suspension was stirred for 40 min, collected by filtration, and redispersed in 80 ml of a 0.5% aqueous polyvinyl alcohol solution. After 1 hr stirring, microspheres were obtained by filtration and dried under vacuum overnight.
Measurement of OTC Encapsulation Efficiency
A known amount of dried PLGA75:25 microspheres was dissolved in 3 ml of methylene chloride. The 9 ml of 20 mM phosphate buffer at pH 7 were added into the organic solution. The mixture was vortexed to extract OTC back into the aqueous phase. After dilution, aliquots of the diluted solutions were subjected to HPLC analysis. The percentage encapsulation efficiency of OTC was calculated as 100 × (actual OTC loading/theoretical OTC loading).
HPLC Analysis
Measurements of OTC concentrations were performed using the Shimadzu HPLC system. The instrument consisted of two pumps (LC-10ATVP), an autosampler (Sil-10ADVP), an ultraviolet detector (SPD-10AVP), and a data processing system (Class-Vp version 6.10 integrator). The Luna C8(2) column (150 × 4.6 mm, 5 μm; Phenomenex Inc., Torrance, CA, USA) fitted with a security guard cartridge was used as a stationary phase. A mobile phase consisting of 1% TFA-containing water and acetonitrile (3:1, by v/v) was used at a flow rate of 1 ml/min. The elution of OTC out of the column was detected at 254 nm. A series of known concentrations of OTC was used to construct a standard calibration curve.
Data Report
Each set of the experiments described above was repeated at least three times. Results were expressed as the mean ± standard deviation in text.
Scanning Electron Microscopy
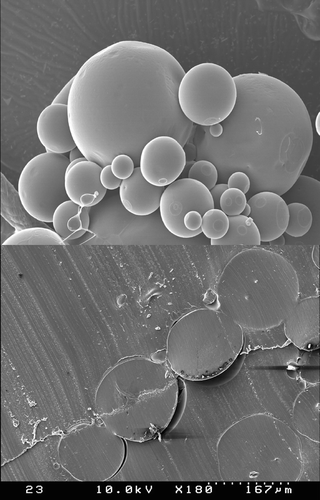
The external and internal morphology of PLGA75:25 microspheres was observed using a Hitachi scanning electron microscope (SEM; Model S-4100, Hitachi High-Corp., Ibaraki-Ken, Japan). To observe their internal morphology, the PLGA75:25 microspheres were embedded in epoxy resin and then sliced with a blade. Prior to SEM observation, microsphere samples were coated to a thickness of 100 nm by an ion sputter (model E-1030, Hitachi High-Corp.)
RESULTS AND DISCUSSION
Previously, CTAB was dissolved in n-hexanol/isooctane to make reverse micelles (Costas-Costas et al. Citation2004). Because such organic solvents were unsuitable for the preparation of PLGA microspheres, new formulations using ethyl formate were developed in this study. Under our experimental conditions, it was possible to dissolve 10 to 40 mg of OTC in the CTAB/water/ethyl formate reverse micelles, without the aid of any mixing devices. The HPLC chromatogram of OTC remained unchanged, before and after encapsulation into the reverse micelles. illustrates HPLC chromatograms obtained after the OTC/CTAB/water/ethyl-formate mixture was incubated to prepare reverse micelles. As incubation proceeded, there were continual increases in the degree of reverse micellar solubilization of OTC. Regardless of variations in formulation, OTC was fully dissolved into the reverse micelles after the mixture was incubated for at least 20 hr ().
FIG. 1 HPLC chromatograms demonstrating the amount of OTC dissolved inside the reverse micelles as a function of incubation time. The peak area standing for OTC rises with ongoing incubation.
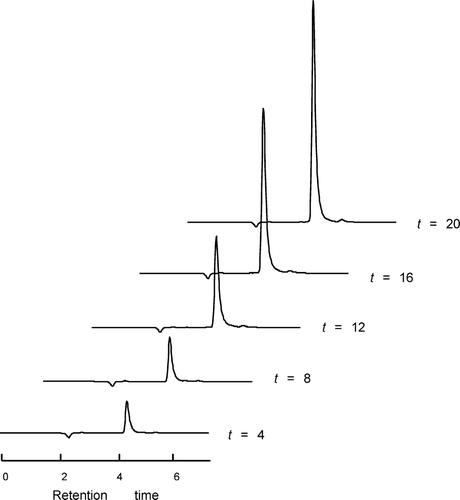
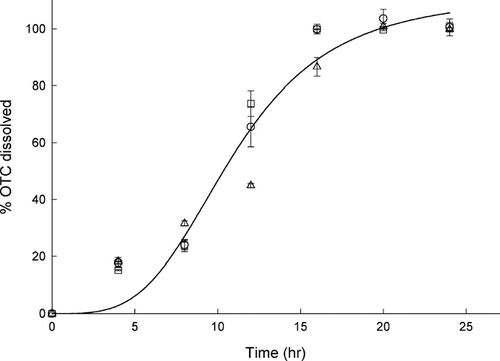
FIG. 2 Changes in the magnitude of reverse micellar solubilization of OTC as a function of incubation time (hr). The OTC/CTAB/water/ethyl formate compositions (mg/mg/ml/ml) were ˆ = 20/20/0.2/3, □ = 20/20/0.4/3, and ▵ = 10/20/0.2/3.
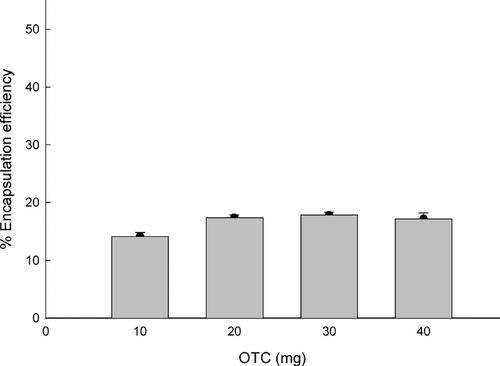
When the reverse micelles consisted of 0.2 ml of water, 3 ml of ethyl formate, and 10 to 40 mg of OTC, its encapsulation efficiency observed at 0.6 g of PLGA75:25 ranged from 14.1 ± 0.7 to 17.1 ± 1.1% (). Under our experimental conditions, changes in the amount of OTC did not affect its encapsulation efficiency to great extents. These encapsulation efficiencies are quite comparable to those observed with typical w1/o/w2 double emulsion processes. For instance, the efficiency of pentamidine encapsulation into PLGA microspheres varied from 7.9 to 23.7%, depending on formulations (Graves et al. Citation2004). With salbutamol sulfate, encapsulation efficiencies of 2.06–22.0% were attained under a variety of experimental conditions (Celebi, Erden, and T-rkyilmaz Citation1996). Such relatively low encapsulation efficiencies are attributed to the diffusion of water-soluble drugs in the w1 phase across the oil phase into the w2 phase (Pays et al. Citation2002). It is also likely that water-soluble drugs may leach into the w2 phase when embryonic microspheres are stirred for complete hardening.
FIG. 3 Effect of OTC quantity on its encapsulation efficiency. OTC (10 to 40 mg) was dissolved in the CTAB (20 mg)/water (0.2 ml)/ethyl formate (3 ml) reverse micelles, and microspheres were prepared by use of 0.6 g PLGA75:25.
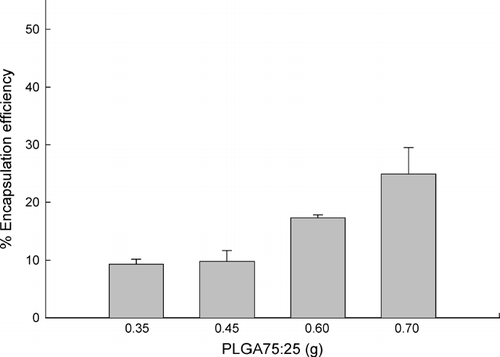
Based on these considerations, an increase in the amount of PLGA75:25 was expected to improve OTC encapsulation efficiency. As anticipated, an increase in the polymer amount from 0.6 to 0.7 g enhanced OTC encapsulation efficiency from 17.3 ± 0.5 to 24.9 ± 4.6%. By sharp contrast, decreases to 0.45 and 0.35 g resulted in significant declines in its encapsulation efficiency ().
FIG. 4 Effect of PLGA75:25 quantity on OTC encapsulation efficiency. Various amounts of PLGA75:25 were dissolved in the OTC (20 mg)/CTAB (20 mg)/water (0.2 ml)/ethyl formate (3 ml) reverse micelles to prepare microspheres. An increase in PLGA75:25 content enhances its encapsulation efficiency.
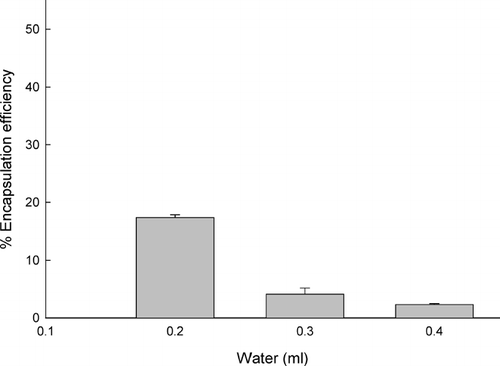
Interestingly, OTC encapsulation efficiency was influenced by the amount of water used to prepare the reverse micelles (). For instance, when 0.4 ml of water was used, almost all OTC seemed to escape from the reverse micelles into the aqueous external phase. As a result, only 2.3 ± 0.2% of an initial OTC concentration was encapsulated into PLGA microspheres. Decreases in water content to 0.2 ml improved its encapsulation efficiency to a degree that 17.3 ± 0.5% of the initial amount of OTC was loaded into PLGA microspheres. In the literature, researchers reported that the reverse micelles of AOT/water/isooctane swelled to deformed spherical micelles when water content increased. The addition of more and more water made it impossible to confine a larger water pool inside the reverse micelles (Bohidar and Behboudnia Citation2001). We can infer from these results that OTC tends to diffuse from the reverse micelles to the aqueous external phase during encapsulation, and high water content exacerbates such a propensity.
FIG. 5 Dependence of OTC encapsulation efficiency on the amount of water used to prepare reverse micelles. The amounts of OTC, CTAB, ethyl formate, and PLGA75:25 used in this experiment were 20 mg, 20 mg, 3 ml, and 0.6 g, respectively.
A conventional methylene chloride-based double emulsion process usually leads to the formation of the microspheres with a honeycombed internal structure (Yang, Chung, and Ng Citation2001). The presence of many hollow cavities inside the microsphere matrix could be traced to the water droplets dispersed in an organic phase by the first emulsification step (Ruan, Feng, and Li Citation2002). However, our reverse micellar microencapsulation process resulted in the formation of the microspheres distinguished from the previous ones. Their internal matrices did not possess numerous hollow cavities, and their external structure was smooth and pore-free (). Such morphology is understandable if we consider that the reverse micellar solution containing PLGA75:25 is a single phase.
CONCLUSION
The present study provides for formulations that could solubilize a water-soluble OTC in the CTAB/water/ethyl formate reverse micelles. The reverse micellar system presents a potential in encapsulation of OTC, thereby contributing to the development of microsphere dosage forms. The use of ethyl formate would overcome environmental and human toxicity issues associated with the chlorinated solvent methylene chloride. Furthermore, in comparison to a double emulsion process, the reverse micellar solubilization avoids the step of making a primary water-in-oil emulsion by use of static or dynamic mixers. As a result, PLGA75:25 microsphere attributes including internal morphology are clearly distinct from those observed with the microspheres prepared by a double emulsion process.
This work was supported by the Ewha Womans University Research Grant of 2005.
REFERENCES
- Benoit J. P., Marchais H., Rolland H., Velde V. V. Biodegradable microspheres: advances in production technology. Microencapsulation: Methods and Industrial Applications, S. Benita. Marcel Dekker Inc., New York 1996; 35–72
- Bohidar H. B., Behboudnia M. Characterization of reverse micelles by dynamic light scattering. Colloids Surfaces A-Physicochem Eng Aspects 2001; 178: 313–323
- Celebi N., Erden N., Türkyilmaz A. The preparation and evaluation of salbutamol sulphate containing poly(lactic acid-co-glycolic acid) microspheres with factorial design-based studies. Int. J. Pharmaceut. 1996; 136: 89–100
- Costas-Costas U., Bravo-Díaz C., Chaimovich H., Cuccovia I. M. Solvolysis of Tris-p-nitrophenyl-phosphate in aqueous and reverse micelles. Colloids Surfaces A-Physicochem Eng Aspects 2004; 250: 385–394
- Graves R. A., Pamujula S., Moiseyev R., Freeman T., Bostanian L. A., Mandal T. K. Effect of different ratios of high and low molecular weight PLGA blend on the characteristics of pentamidine microcapsules. J. Control. Rel. 2004; 270: 251–262
- Hayashi Y., Yoshioka S., Aso Y., Po A. L. W., Terao T. Entrapment of proteins in poly(l-lactide) microspheres using reversed micelle solvent evaporation. Pharmaceut. Res. 1994; 11: 337–340
- Jain R. A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glcolide) (PLGA) devices. Biomaterials 2000; 21: 2475–2490
- Kostanski J. W., Thanoo B. C., DeLuca P. P. Preparation, characterization, and in vitro evaluation of 1-and 4-month controlled release orntide PLA and PLGA microspheres. Pharmaceut. Dev. Technol. 2000; 5: 585–596
- Mallick K., Jewrajka S., Pradhan N., Pal T. Micelle-catalyzed redox reaction. Current Sci. 2001; 80: 1408–1412
- Mueller-Goymann C. C., Hamann H. J. Sustained release from reverse micellar solutions by phase transformation into lamellar liquid crystals. J. Control. Rel. 1993; 23: 165–174
- Pays K., Giermanska-Kahn J., Pouligny B., Bibette J., Leal-Calderon F. Double emulsion: how does release occur?. J. Control. Rel. 2002; 79: 193–205
- Rangel-Yagui C. O., Pessoa A., Jr., Tavares L. C. Micellar solubilization of drugs. J. Pharm. Pharmaceut. Sci. 2005; 8: 147–163
- Ruan G., Feng S. S., Li Q. T. Effect of material hydrophobicity on physical properties of polymeric microspheres formed by double emulsion process. J. Control. Rel. 2002; 84: 151–160
- Sah H. Ethyl formate-alternative dispersed solvent useful in preparing PLGA microspheres. Int. J. Pharmaceut. 2000; 195: 103–113
- Yang Y. Y., Chung T. S., Ng N. P. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 2001; 22: 231–241