Abstract
Two dipeptide drugs are synthesized utilizing an acetylsalicylic acid or nicotinic acid molecule for the framework. A D-alanine-D-alanine dipeptide moiety is attached to the carbonyl carbon of acetylsalicylic acid (I) and nicotinic acid (II). Dipeptide derivatives (I) and (II) showed significant reduction of Escherichia coli (E. coli) bacterial growth and colony-forming units. A mixture of (I) and (II) induced growth inhibition of 8%, 17.5%, 28%, and 42.5% at concentrations of 100, 200, 300, and 400 μg/mL, respectively. Ampicillin demonstrated much less growth inhibition of this penicillin-resistant E. coli bacteria. Derivatives (I) and (II) showed significant reduction of colony-forming units at concentrations higher than 200 μg/mL, whereas ampicillin showed no significant affect on colony-forming units. Both (I) and (II) produced no violations of the Rule of 5, indicating favorable characteristics for bioavailability. Molecular properties were determined and showed (I) to have a Log Kow of −0.22 with aqueous solubility of 683.8 mg/L, whereas (II) had a Log Kow of −1.00 and aqueous solubility of 6859 mg/L. A mixture of the parent compounds acetyl salicylic acid and nicotinic acid demonstrated some antibacterial activity.
INTRODUCTION
Escherichia coli is an enteric bacteria (family Enterobacteriaceae) that is a facultatively anaerobic Gram-negative rod. The Enterobacteriaceae are among the most important bacteria medically and E. coli has been linked to diseases found in many parts of the body. E. coli is the most encountered bacteria in the clinical laboratory and is the number one cause of urinary tract infections, but it is also responsible for pneumonia, meningitis, and diarrhea (Davis et al. Citation1990).
Antibiotic resistance expressed by bacterial pathogens is a global problem.The various mechanisms of antibiotic resistance include alteration/modification of the target site, degradation of antibiotic molecule, and reduced intracellular concentration due to decreased permeability and energy-dependent efflux (Kumar, Herbert, and Schweizer Citation2005). The synergism between reduced uptake and drug efflux results in intrinsic or acquired resistance in many clinically important Gram-negative bacteria (Kumar et al. Citation2005).
Clinical isolates of diarrheogenic E. coli have high levels of multidrug resistance in previous studies (Estrada-Garcia et al. Citation2005). E. coli can induce persistent diarrhea that has been associated with malnutrition, growth impairment, and death in developing countries (Nataro and Kaper Citation1998). Gram-negative bacteria (inclusive of E. coli) have shown high levels of resistance to beta-lactam antibiotics and in particular to ampicillin (Al Sweih, Jamal, and Rotimi Citation2005). E. coli isolates obtained from fecal polluted waters showed multidrug resistance to even high concentrations of 14 antibiotics, that included ampicillin (Edge and Hill Citation2005). Isolates of E. coli showed high frequency of resistance to ampicillin from Tanzania (Blomberg et al. Citation2005), Vietnam (Nguyen et al. Citation2005), northern Palestine (Adwan et al. Citation2004), Korea (Hong, Chun, and Lee Citation2004), and Taiwan (Lau, Peng and Chang Citation2004). Hospital sewage has been found associated with the high incidence of E. coli resistance to antibiotics ampicillin, piperacillin, cefalothin, nalidixic acid, and tetracycline (Reinthaler et al. Citation2003).
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been applied in cotreatment of bacterial infections and they have been shown to play a role in the effective struggle against infections (Katler and Weissmann Citation1977). Many NSAIDs, inclusive of acetylsalicylic acid, have shown significant inhibition of bacterial clinical isolates (Fouda Citation2001). Acetylsalicylic acid has been utilized beneficially with vancomycin to treat E. faecalis and methicillin-resistant S. aureus endocarditis (Marangos et al. Citation1997). A previous study has shown that acetylsalicylic acid with ibuprofen and interferon-gamma can significantly reduce bacteria presence in the spleen, liver, and main organs of a patient having infection of Listeria monocytogenes (Hockertz and Heckenberger Citation1996). In addition, the combination of acetylsalicylate with lidocaine hydrochloride was shown to have antibacterial activity with Gram-negative and Gram-positive bacteria (Obsuka et al. Citation1994).
Our work shows the efficacy of utilizing acetylsalicylic acid and nicotinic acid to transport a dipeptide moiety and initiate significant antibacterial affects. Each of the parent compounds convey molecular properties that differentiate the two drugs and imparts useful pharmaceutical activity. In addition, previous studies have shown clearly that concomitant use of acetylsalicylic acid in the clinical treatment of bacterial infections is beneficial (Fouda Citation2001).
MATERIALS AND METHODS
Reagents and Instrumentation
All reagents were obtained from Aldrich Chemical Co. (Milwaukee WI USA). Infrared spectra were determined by a Mattson Galaxy 3000 Fourier transform-IR spectrometer in dimethylsulfoxide dried over molecular sieves. Molecular modeling and properties were determined by ChemSketch (Toronto, Ontario, Canada), CS Chem3D Pro (CambridgeSoft Corp., Cambridge MA, USA), SPARTAN (Wavefunction, Irvine, CA, USA) and Molinspiration (Slovensky Grob, Slovak Republic). Tissue culture experiments were accomplished at the Dept. of Veterinary & Biomedical Sciences, University of Nebraska, Lincoln, Nebraska, USA.
Synthesis of Dipeptide Drugs
We placed into separate reaction vessels 0.607 g (0.03367 mol) of acetyl-salicylic acid and 0.415 g (0.03367 mol) of nicotinic acid. Then, we placed 20 mL to 30 mL of acetonitrile (dried of molecular sieves) into each vessel and 1 mL of pyridine (proton sink). Next 1 mL of thionyl chloride was added to each vessel and refluxed for at least 1 hr under mild heat. We distilled out excess thionyl chloride, adding additional acetonitrile as needed. Then, 0.30 g of D-alanine was added to both mixtures and refluxed mildly for 60 to 90 min. We cooled the mixtures and added 1 to 2 mL of pyridine to each reaction solution. Then, we added to each vessel 1 mL of thionyl chloride, refluxed for at least 1 hr under mild heat. Again we distilled out excess thionyl chloride and added acetonitrile to maintain volume. Last we added an additional 1 mL of pyridine, then 0.30 g D-alanine and refluxed mildly for 60 to 90 min. The final volume was reduced by distillation, then precipitated out the final product by overnight incubation at −10°C and filtration at 4°C. Finally we washed the product with diethyl ether chilled to −10°C and stored desicated at −10°C.
Culture Method
The following mixtures of parent compounds, dipeptide drugs, and ampicillin were tested in culture with penicillin-resistant E. coli: Nicotinic acid and acetylsalicylic acid at a mole ratio of 1:1; derivatives (I) and (II) at a mole ratio of 1:1; and ampicillin. The mixtures above were first solubilized in liquid Luria-Bertani (LB) media and dispensed in known concentrations, followed by inoculation with penicillin-resistant E. coli. Number of colony-forming units were assayed and optical densities of cultures were measured at 600 nm after 5 hr at 37°C to evaluate antibacterial activity.
RESULTS AND DISCUSSION
Many important pathogens are found to be resistant to multiple antimicrobial classes, resulting in expensive treatment that is prone to failure (Deshpande, Fritsche, and Jones Citation2004). Efforts to respond to this growing threat has yielded new agents. In addition, the formation of novel derivatives of existing antimicrobials has been shown to have merit (Poole, Russell, and Lambert Citation2005). Ampicillin is a member of the penicillin family of antibiotics (classified as an aminopenicillin) that are able to penetrate Gram-negative bacteria and are considered inhibitors of cell wall synthesis. The structure of ampicillin is based upon the beta-lactam ring and these types are primarily bactericidal (Davis et al. Citation1990). Ampicillin interferes with cell wall formation by inhibition of the transpeptidation reaction due to the close structural analogy between D-alanine-D-alanine to the beta-lactam ring.
If the normal transpeptidation reaction within the bacterial cell wall occurs then new cross-linking occurs involving the initial-L-lysine-D-alanine-D-alanine side chain to form a new-L-lysine-D-alanine-glycine linkage. The aim of forming the D-alanine-D-alanine dipeptide derivatives shown in this work is to provide an alternative target in place of the-L-lysine-D-alanine-D-alanine side chain. If successful the bacterial cell wall cannot close normally and in consequence will form perturbations in the cell wall that may be lethal. This will manifest itself in terms of growth inhibition of the bacteria (E. coli in this work).
Acetylsalicylic acid and nicotinic acid are medications utilized as a NSAID or cholesterol management, respectively. Both parent compounds have an aromatic ring and carbonyl carbon suitable for activation and formation of an amide or ester group. The structural substituents of the parent compounds influence the molecular properties of their dipeptide derivatives and consequently their “druglikeness”. Although the parent compounds have structual features in common, the formation of dipeptide derivatives having a D-alanine-D-alanine substituent showed significant dissimilarites. The molecular structures of (I) and (II) are presented in with SMILES notation. The amino acid D-alanine has polar surface area of 63.32 Angstroms2 with significant hydrophilic nature illustrated by a 1-octanol/water partition coefficient of −2.692. The formation of a dipeptide D-alanine-D-alanine combination significantly alters the partition coefficient that becomes −3.26 (more hydrophilic) and increases the polar surface area to 92.42 angstroms2 (a 46% increase). Thus, a dipeptide moiety may significantly affect druglikeness properties of a derivatized agent.
FIG. 1 Molecular structures of dipeptide derivatives (I) and (II) are shown with SMILES notation. The D-alanine-D-alanine substituents are attached to the carbonyl carbon present in the parent compounds. Both (I) and (II) have aromatic rings within their structures.
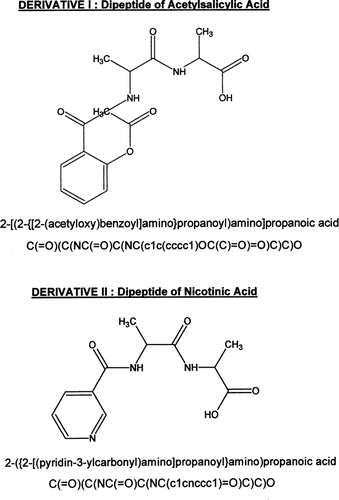
The affects on molecular properties and influence on parent compound structure after addition of D-alanine-D-alanine substituent can be readily seen in . Comparison of molecular properties of (I) and (II) to the parent structures acetylsalicylic acid and nicotinic acid, respectively, shows some striking alterations. The polar surface area of (I) and (II) are approximately double that of their respective parent compound. Derivatives (I) and (II) have substantially less water solubility than their repective parent compounds that decrease by more than 85% for acetylsalicylic acid and nicotinic acid. This is reflected by a more hydrophilic Log Kow for (I) and (II) (i.e., negative values for Log Kow). The Log Kow values for acetylsalicylic acid and nicotinic acid are more lipophilic at values of 1.13 and 0.69, respectively. Both (I) and (II), the parent drugs, in addition to ampicillin show zero violations of the Rule of 5. This indicates favorable propperties for drug absorption and less probability of problems in permeation (Lipinski et al. Citation1997).
TABLE 1 Molecular properties of compounds
Additional properties (descriptors) of molecular size, dipole, and penetration of the blood-brain barrier (BBB) membrane are presented in . The aromatic ring, and in the case of acetylsalicylic acid the acetyl group (─C(O)CH3), contributes to the dissimilarity of (I) from (II). The molecular dipole of I is 10.295 Debye compared with 5.083 Debye of (II). In general, a large dipole value does not enhance membrane permeation. The pyridine-like ring of (II) clearly affects the overall molecular dipole and reduces the value compared with the influence of the acetylsalicylic acid moiety. The acetyl group contained within (I) as expected would contribute to a larger value of molecular size, indicated by greater values for molar volume, parachor, and molar refractivity. Molar refractivity is a measure of both molecular volume and efficacy of polarization.
TABLE 2 Properties of dipeptide derivatives
Numerical values for Log BB (log (Cbrain/Cblood)) are determined by correlation with partition coefficient and molecular mass as a descriptor of bulkiness (Kaznessis et al. 2001): Log BB = −0.088 + 0.272(Log P) − 0.001116(molecular weight). The results of Log BB determination give BB values of 0.349 and 0.215 for (I) and (II), respectively. These values indicate only a small fraction of drug present at the BBB interface would be expected to successfully cross into the central nervous system.
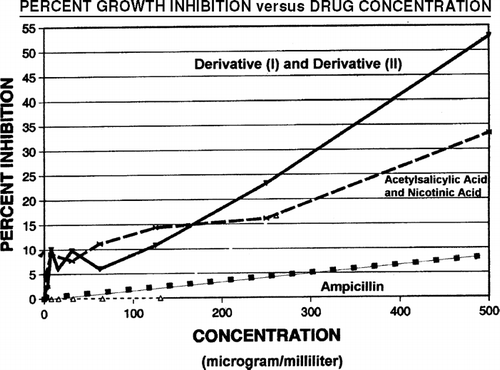
Significant growth inhibition of penicillin-resistant E. coli was induced by a mixture of (I) and (II) (mole ratio of 1:1) and by the parent compounds at concentrations of greater than 300 μg/mL. Results of in vitro growth inhibition evaluation are shown in . A mixture of (I) and (II) induced growth inhibition of 8%, 17.5%, 28%, and 42.5% at concentrations of 100 μg/mL, 200 μg/mL, 300 μg/mL, and 400 μg/mL, respectively. Linear regression analysis of the trend line results in the linear equation: y = 0.1050 × −1.800 (r = 0.9934 and R2 = 0.9868). Ampicillin showed considerably less growth inhibition (less than 10%) of this penicillin-resistant E. coli bacteria at all concentrations. Ampicillin showed less than 5% growth inhibition at all concentrations less than 300 μg/mL. Clearly a more beneficial results are obtained with a mixture of (I) and (II). In addition, a mixture of acetylsalicylic acid and nicotinic acid (mole ratio 1:1) induced growth inhibition of greater than 15% when at concentrations greater than 250 μg/mL.
FIG. 2 There is substantial inhibition of bacterial growth as indicated by the plot of Percent Inhibition versus Concentration in μg/mL. The mixture of nicotinic acid and acetylsalicylic acid expresses greater than 15% inhibition at concentrations above 200 μg/mL. The mixture of dipeptides (I) and (II) express greater than 15% inhibition at even lower concentrations of 160 μg/mL. Growth inhibition induced by (I) and (II) increases at a faster rate than other compounds to reach greater than 50% inhibition at 500 μg/mL. In the concentration ranges shown to be effective for (I) and (II), the growth inhibtion induced by ampicillin remains below 10%.
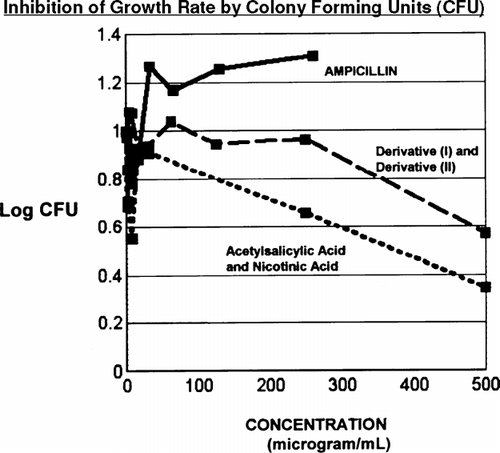
Reduction of colony-forming units (CFUs) also is indicative of growth inhibition. A pair, chain, or cluster of bacterial cells in close proximity to each other can multiply to produce a single colony. The term CFU is applied to consider the common origin for the cells of any colony. Whether colony counts are performed or not, bacterial colonies still arise from CFUs. Assessment of CFUs is presented in versus concentration in micrograms per milliliter. Throughout all concentrations of ampicillin, the number of CFUs increases, indicating no growth inhibition. However in both cases of mixtures of (I) with (II) and acetylsalicylic acid with nicotinic acid a substantial reduction of the number of CFUs occurs. Assay for (I) and (II) mixture shows large reduction of CFUs at concentrations greater than 240 μg/mL.
FIG. 3 Inhibition of colony-forming units also is indicative of bacterial growth inhibition. For ampicillin the number of CFUs continually increases throughout the studied region. However, for (I) and (II) the number of CFUs immediately decreases and continues to decrease throughout the concentration range. The parent nicotinic acid and acetylsalicylic acid also induce significant reduction of CFUs throughout the range of concentrations.
CONCLUSION
Two-D-alanine-D-alanine dipeptide derivatives were formed using acetylsalicylic acid and nicotinic acid as the parent compounds. The two derivatives were sufficiently stable and soluble in aqueous solution and physiological 37°C to inhibit the growth and formation of CFUs of a penicillin-resistant E. coli bacteria. Both derivatives showed zero violations of the Rule of 5. A mixture of both derivatives induced growth inhibition at levels of 8%, 17.5%, 28%, and 42.5% at concentrations of 100 μg/mL, 200 μg/mL, 300 μg/mL, and 400 μg/mL, respectively, whereas the antibiotic ampicillin showed less than 10% growth inhibition at concentrations up to 400 μg/mL. The dipeptide derivatives also demonstrated significant reduction of CFUs as opposed to ampicillin that actually showed increased number of CFUs.
REFERENCES
- Adwan K., Abu-Hasan N., Adwan G., Jarrar N., Abu-Shanab B., Al-Masri M. Molecular epidemiology of antibiotic-resistant Escherichia coli isolated from hospitalized patients with urinary tract infections in Northern Palestine. Pol. J. Microbiol. 2004; 53(1)23–26
- Al Sweih N., Jamal W., Rotimi V. Spectrum and antibiotic resistance of uropathogens isolated from hospital and community patients with urinary tract infections in two large hospitals in Kuwait. Med. Princ. Pract. 2005; 14(6)401–407
- Blomberg B., Olsen B., Hinderaker S., Langeland N., Gasheka P., Jureen R., Kyale G., Midtvedt T. Antimicrobial resistance in urinary bacterial isolates from pregnant women in rural Tanzania: Implications for public health. Scand. J. Infect. Dis. 2005; 37(4)262–268
- Davis B., Dulbecco R., Eisen H., Ginsberg H. Microbiology. J. B. Lippincott Company, New York 1990
- Deshpande L. M., Fritsche R., Jones R. Molecular epidemiology of selected multidrug-resistant bacteria: a global report from the SENTRY Antimicrobial Survellience Program. Diag. Microbiol. Infect. Dis. 2004; 49: 231–236
- Edge T., Hill S. Occurrence of antibiotic resistance in Escherichia coli from surface waters and fecal pollution sources near Hamilton, Ontario. Can. J. Microbiol. 2005; 51(6)501–505
- Estrada-Garcia T., Cerna J., Paheco-Gil L., Velasquez R., Ochoa T., Torres J., DuPont H. Drug-resistant diarrheogenic Escherichia coli, Mexico. Emerg. Infect. Dis. 2005; 11(8)1306–1308
- Kumar A., Herbert P., Schweizer P. Bacterial resistance to antibiotics. Active efflux and reduced uptake. Adv. Drug Deli. Rev. 2005; 57: 1486–1513
- Fouda S. I. Antibacterial activity of some nonsteroidal anti-inflammatory drugs. J. Microbiol. 2001; 53: 283–295
- Hockertz S., Heckenberger R. Treatment of an acute bacterial infection with a combination of acetylsalicylic acid/ibuprofen and interon gamma. Arzneimittelforschung 1996; 46(10)1012–1015
- Hong H., Chun J., Lee Y. Detection of extended-spectrum beta lactamase producing, multidrug-resistant environmental isolate of Escherichia coli that bind to human bladder cells. Microb. Drug Resist. 2004; 10(2)184–189
- Katler E., Weissmann G. Steroids, aspirin, and inflammation. Inflammation 1977; 2(4)295–307
- Lau S., Peng M., Chang F. Resistance rates to commonly used antimicrobials among pathogens of both bacteremic and non-bacteremic community-acquired urinary tract infection. J. Microbiol., Immunol. Infect. 2004; 37(3)185–191
- Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deli. Rev. 1997; 23: 1–25
- Marangos M., Nightingale C., Quintiliano R., Nicolau D. Influence of aspirin on the treatment of experimental Enterococcus faecalis and methicillin resistant Staphylococcus aureus endocarditis. J. Infect. Dis. Pharmacother. 1997; 2(3)47–53
- Nataro J., Kaper J. B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998; 11: 142–201
- Nguyen T., Van Le P., Le C., Weintraub A. Antibiotic resistance in diarrheagenic Escherichia coli and Shigella strains isolated from children in Hanoi, Vietnam. Antimicrob. Agents Chemother. 2005; 49(2)816–819
- Obsuka S., Ohta M., Masuda K., Arakawa Y., Kaneda T., Kato N. Lidocaine hydrochloride and acetylsalicylate kill bacteria by disrupting the bacterial membrane potential in different ways. Microbiol. Immunol. 1994; 38(6)429–434
- Poole K., Russell A., Lambert P. Mechanisms of antimicrobial resistance: opportunities for new targeted therapies. Adv. Drug Deli. Rev. 2005; 57: 1443–1445
- Reinthaler F., Posch J., Feieri G., Wust G., Haas D., Ruckenbauer G., Mascher F., Marth E. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 2003; 37(8)1685–1690