Abstract
Enhanced delivery of bifidobacteria and fecal changes were compared following multiple oral administrations of protected bifidobacteria-loaded alginate poly-l-lysine microparticles (bap-microparticles) and unprotected bifidobacteria cultures over a period of 1 month to healthy human volunteers as preliminary in vivo studies. When bap-microparticles were orally administered, enhanced delivery of bifidobacteria was achieved. The viability of the bifidobacteria was significantly increased ∼ 11.5–30 times (1.06–1.48 log cycles) during the ingestion period when compared with the bifidobacteria culture group (p < 0.05). However, other gut microflora such as bifidobacteria, enterobacteriaceae, lactic acid bacteria, and staphylococci in feces were not significantly different between the two groups. Encapsulated bifidobacteria resulted in more frequent defecation and decreased fecal viscosity.
Bifidobacteria, the normal inhabitants of the human and animal gut, have been shown to play an important beneficial role in human health (Rasic and Kurmann Citation1983; Holzapfal et al. Citation1998; Fukushima et al. Citation1997; Lian, Hsiao, and Chou Citation2003). Although the composition of the intestinal microflora of healthy adults is quite stable, factors such as stomach and intestinal disorders, antibiotic therapy, stress and other diseases can cause it to change quite significantly (Holzapfal et al. Citation1998; Chung et al. Citation1999). The consumption of milk or dairy products containing bifidobacteria can help to stabilize the intestinal flora in a manner that has both preventive and curative effects (Hoover Citation1993; Fukushima et al. Citation1997).
Although the usefulness of bifidobacteria-loaded products has long been recognized throughout the world, it has been impossible to maximize the benefit that could be derived from these bacteria, because of their low stability in acidic conditions. Ingested bifidobacteria must survive their transit through the gastric environment and reach the colon in sufficient quantities to allow colonization. However, it is well known that the low pH of the stomach environment is detrimental to their survival (Rao, Shiwnarain, and Maharaj Citation1989; Berrada et al. Citation1991; Chung et al. Citation1999; Lee et al. Citation1999; Cui et al. Citation2000a, Citation2000b; Lee and Heo Citation2000; Truelstrup Hansen et al. Citation2002; Lian et al. Citation2003). In a previous study, we observed large variations in the survival of the bifidobacteria present in various Korean dairy products when they were exposed to gastric fluid (Lee et al. Citation1999).
Although the selection of specific bifidobacteria that are tolerant to the pH of the human stomach seemed at one point to be the best way to overcome these problems (Clark, Cotton, and Martin Citation1993; Chung et al. Citation1999), microencapsulation methods using biocompatible alginate in either a matrix or coated microparticles have been used to improve the survival of bifidobacteria (Rao et al. Citation1989; Lee and Heo Citation2000; Truelstrup Hansen et al. Citation2002; Lian et al. Citation2003).
Sodium alginate can be commonly used as an immobilizing vehicle for bioactive substances. It has been found to form a gel when it contacts with calcium and multivalent cations, and this gel can be dried to produce larger alginate beads or microsized microparticles (Kwok, Groves, and Burgess Citation1991; Lee and Min Citation1995; Lee, Min, and Kim Citation1996; Lee and Heo Citation2000; Truelstrup Hansen et al. Citation2002). The dried alginate beads (or microparticles) are stable in low pH conditions, but they swell in weak basic solutions and ultimately disintegrate and erode.
In our previous study, small-sized bifidobacteria-loaded alginate microparticles were prepared using an atomization method (Cui et al. Citation2000a, Citation2001). The resulting microsized alginate microparticles were then complexed with cationic poly-l-lysine to improve the viability of bifidobacteria in low pH conditions. Cationic poly-l-lysine can allow the alginate membrane to be constructed more tightly (Kwok et al. Citation1991; Liu and Krishnan Citation1999). Although the effectiveness of microencapsulated bifidobacteria has been well recognized in terms of in vitro stability at low pH, there is no report to evaluates their in vivo roles in healthy human volunteers.
The purpose of our study was to compare the viability of fecal microflora (bifidobacteria, enterobacteriaceae, lactic acid, and staphylococci) following the multiple oral administration of either protected bap-microparticles or unprotected bifidobacteria cultures over a period of 1 month to healthy human volunteers. The fecal changes including the interval between bowel movements, and the viscosity and volume of the feces, also were examined.
MATERIALS AND METHODS
Sodium alginate was purchased from Junsei Chemical Co. (Tokyo, Japan). Poly-l-lysine (Mw 38,500), sodium azide, and L-cysteine HCl were purchased from Sigma (St. Louis, MO, USA). Freeze dried bifidobacterium bifidum culture (Bb-11), which is the normal inhabitant species of the human intestine, was purchased from Chr. Hansen's Lab. (Horsholm, Denmark). The manufacturer guarantees the purity of the bifidobacteria bifidum and that a minimum cell concentration of 5.0 × 1010 colony forming units (cfu g− 1) are obtained when they are enumerated on MRS agar culture at 37°C for 3 days. BL Agar was purchased from Tanabe Seiyaku Co. (Osaka, Japan). Lactobacilli MRS broth, EMB Agar, Baird-Parker Agar base, and Bacto Egg yolk Tellurite were purchased from Difco Laboratories (Detroit, MI, USA). The anaerobic GasPak jar, GasPak generator envelope, GasPak catalyst, and indicator were purchased from Becton Dickinson Microbiology Systems (BBL, Cockeysville, MD, USA). Bifidobacteria culture was used as supplied by the manufacturer. All other chemicals were of reagent grade and used without further purification.
Preparation of Protected Bap-Microparticles
The method of preparing the protected bap-microparticles against low pH conditions was described previously in detail (Cui et al. Citation2000a, Citation2001). Briefly, the components used for the preparation of the bap-microparticles included sodium alginate (1.5 g), bifidobacteria cultures (0.5 g), yeast extracts (0.5 g), glycerol (5.0 g), NaHSO3 (0.5 g), and Mg3(PO4)2 (1.0 g), which were suspended in distilled water (100 ml). Using a peristaltic pump, the solution was then sprayed into a bath containing 500 ml of 0.2 M CaCl2 solution using an air atomization device (Turbotak, Waterloo, Ontario) under aseptic conditions at a pressure of 0.75 bar and a delivery rate of 8 ml/min. The microgel droplets that were formed were cured for 15 min and filtered through two layers of filter paper (11 cm, circles, Whatman, Maidstone, UK) in a Buchner funnel and then washed twice with distilled water. Thereafter, 0.02% poly-l-lysine solution was used to cross-link the microparticles for 6 min. The resulting microparticles were then separated under vacuum filtration on a Buchner funnel.
The filtered microparticles were washed twice again with distilled water to remove excess CaCl2 solution and then frozen at −40°C for 2 hr. The final free flowing bap-microparticles were obtained after freeze-drying using a commercial freeze dryer (Ilshin, Seoul, South Korea) at 52°C under a pressure of 8 mmTorr for 14–16 hr. All solutions, including the sodium alginate and calcium chloride solutions, were autoclaved using a steam sterilizer at 121°C for 20 min before use. The poly-l-lysine solution was sterilized by manually passing it through a 0.22 μ m membrane filter.
In Vivo Study Protocol
The healthy young male subjects aged 27 ± 4.1 years (25–35), weight 64.1 ± 3.8 kg (59–69), and height 171.4 ± 4.6 cm (163–179) participated in this study. None had a history of gastrointestinal disease. They showed no signs of lactose intolerance and took no antibiotics during the study periods. Written informed consent was obtained from all the volunteers. The experimental protocol was approved by the Ethics Committee of the College of Pharmacy in Kangwon National University.
The healthy human volunteers were divided into 2 groups; 8 each. The protected bap-microparticles and unprotected bifidobacteria cultures without adding any excipients were packed in hard gelatin capsules. The gelatin capsules were stored in a refrigerator at 4°C before oral administration. One capsule, containing 5.6 × 109 cfu g− 1 of unprotected bifidobacteria culture or protected 5.0 × 109cfu g− 1 of bap-microparticles, was given twice a day 30 min in a fasting state before breakfast and dinner during ingestion period for each group.
The fecal samples were collected at 7 and 3 days before the experiment as a control, and 3, 7, and 14 days after the multiple oral administration of the protected bap-microparticles and unprotected bifidobacteria cultures. After 14 days, the subjects stopped taking the capsules. The fecal samples also were collected at 5 and 10 days after the subjects stopped taking the capsules.
The viability of the total bifidobacteria, lactic acid bacteria, staphylococci, and enterobacteriaceae in the feces as a function of the study period (i.e., the control, ingestion, and stopping periods) was compared between the two groups. The intervals between bowel movements (days) and the physical status of the feces, viz. their volume and viscosity, were kindly examined by the pathologist. The number of the volunteers were then counted based on the following arbitrary indices for the assessment of the volume of feces (reduced, normal, or increased) and viscosity of feces (soft, normal, or hard).
Microflora in the Feces
The bacteriological examination was completed within 4 hr after the collection of the feces from the human volunteers. An aliquot of the fecal samples was accurately weighed and suspended in phosphate buffer solution (pH 7.0) supplemented with L-cysteine HCl (0.05%). The solution was further diluted in decimal steps down to 1:108. Enumeration was carried out using the pour plate counting method (Romond et al. Citation1998; Lee et al. Citation1999). Dilutions were plated onto BS agar for the enumeration of bifidobacteria, MRS agar supplemented with sodium azide (0.02%) for the enumeration of lactobacilli, and EMB agar for the enumeration of the enterobacteriaceae, especially Escherichia coli. The MRS agar culture consisted of MRS broth (5.5 g), L-cysteine (0.05 g), and Bacto-Agar (1.5 g) in sterile water (100 ml). Staphylococcus was enumerated using Baird–Parker agar to which prewarmed 5% Bacto Egg yolk Tellurite was added aseptically. The plates used for the enumeration of the bifidobacteria were incubated in anaerobic conditions at 37°C for 72 hr. All the other plates were incubated in aerobic conditions at 37°C for 72 hr.
Statistical Analysis
The results are presented as means ± standard deviation. Student's t-test was used for the statistical analysis. On also, the categorical data for fecal volume and fecal viscosity were analyzed by the chi-square test for contingency tables (Bolton Citation1997). The differences were considered to be significant at p < 0.05.
RESULTS AND DISCUSSION
Changes of Fecal Microflora
These small-sized protected alginate poly-l-lysine microparticles were found to improve the viability of bifidobacteria, by protecting them from the effects of low pH conditions (Cui et al. Citation2000a). We observed that the number of surviving bacteria in commercial bifidobacteria cultures rapidly declined from 1011 cfu g− 1 to less than 103 cfu g− 1 when they were exposed to simulated gastric fluid for 2 hr. But the number of surviving bacteria in bap-microparticles remained at ∼ 108 cfu g− 1 when they were exposed to gastric fluid at pH 1.5 for 2 hr.
After freeze-drying, the average viability of the bifidobacteria remained at 9.3 × 109cfu g− 1. The encapsulation efficiency of the bifidobacteria in the alginate poly-l-lysine microparticles was ∼ 37.2%. The geometric mean size of the alginate poly-l-lysine microparticles ranged from 80 to 130 μ m, based on repeated measurements in which the result was highly reproducible. However, no attempt has yet been made to examine their in vivo roles in the human body.
The gut microbiota is a complex ecosystem that compromises over 1000 species from more than 190 genera. However, only four populations such as bifidobacteria, enterobacteriaceae, lactic acid bacteria, and staphylococci were assessed for preliminary in vivo studies in a small number of volunteers. The viability of the bifidobacteria in the fecal microflora after the oral administration of protected bap-microparticles in the human volunteers is shown in . Enhanced delivery of bifidobacteria was achieved during ingestion period of bap-microparticles. The number of bifidobacteria in the human feces showed higher levels (> 108 cfu g− 1, wet sample) compared with other micro-organisms.
FIG. 1 Viability of bifidobacteria in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers. * Significantly different from unprotected bifidobacteria cultures.
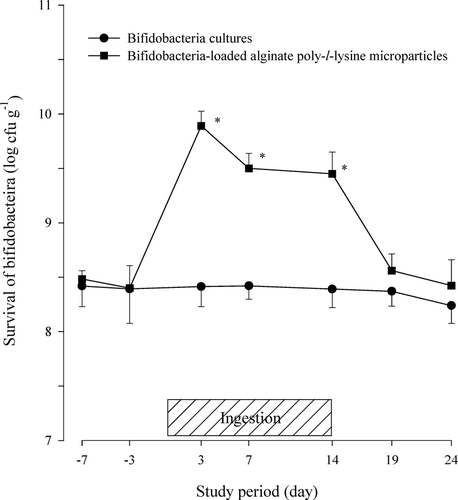
The viability of the bifidobacteria in the feces of the group taking bap-microparticles was significantly higher than that of the group taking the bifidobacteria culture (p < 0.05). The viability of the bifidobacteria was increased ∼11.5–30 times (1.06–1.48 log cycles) during the ingestion period. In contrast, the survival of the bifidobacteria was almost unchanged in the control group given only bifidobacteria cultures, because the bifidobacteria rapidly lost their viability when exposed to the low pH conditions of the stomach and the bile salts of the intestine as discussed in previous in vitro studies (Rasic and Kurmann Citation1983; Cui et al. Citation2000a; Truelstrup Hansen et al. Citation2002; Lian et al. Citation2003).
Unlike the unprotected bifidobacteria cultures, the protected alginate poly-l-lysine microparticles would be expected to improve the viability of the bifidobacteria in the feces, by shielding them against the low pH conditions (Cui et al. Citation2000a). However, following its increase during the ingestion period, the viability of the bifidobacteria in the feces returned to the predose levels within 10 days of the ingestion of the product being discontinued.
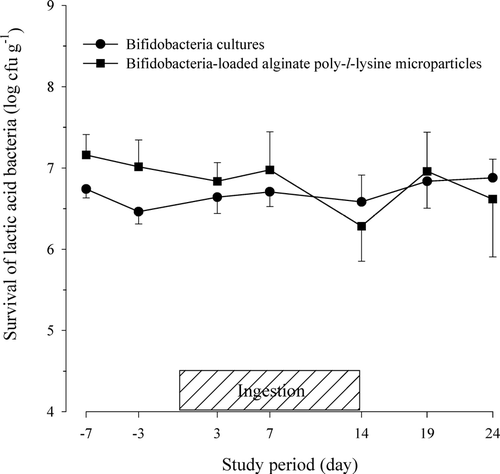
Contrarily, other gut microflora such as enterobacteriaceae, lactic acid bacteria, and staphylococci in feces were not significantly different between the two groups. The viability of the lactic acid bacteria in the fecal microflora after the oral administration of the bap-microparticles in the human volunteers is shown in . The viability of the lactic acid bacteria, as measured by the cell numbers, varied widely among the subjects and ranged from 106 to 108 cfu g− 1. The average viability of the lactic acid bacteria was slightly higher in the feces of the subjects who ingested the bap-microparticles than in the feces of those who ingested the bifidobacteria cultures, but the difference was not statistically significant. It has been observed that the ingestion of bifidobacteria may alter the population of intestinal pathogens, especially enterobacteriaceae and staphylococci.
FIG. 2 Viability of lactic acid bacteria in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers.
The viabilities of the enterobacteriaceae and staphylococci in the fecal microflora after the oral administration of the bap-microparticles in human volunteers are shown in and , respectively. The viability of the enterobacteriaceae ranged from 106 to 108 cfu g− 1 and showed wide variations among the subjects. When the bap-microparticles were given, the viability of enterobacteriaceae in the fecal samples gradually increased even after the oral ingestion was discontinued. In contrast, the oral ingestion of bifidobacteria cultures decreased the viability of the enterobacteriaceae in the fecal samples from the start of the experiment but a sharp increase at the end could not be explained. The progressive effect of bap-microparticles on enterobacteriaceae may not be due to the treatment itself but to a nonstabilized microbiota for the tested subjects as also can be seen for the control group.
FIG. 3 Viability of enterobacteriaceae in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers.
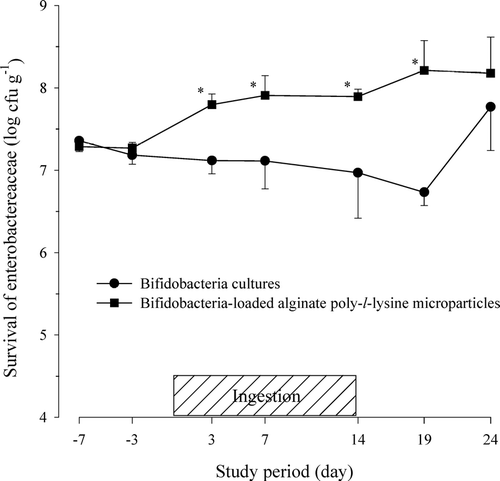
FIG. 4 Viability of staphylococci in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers.
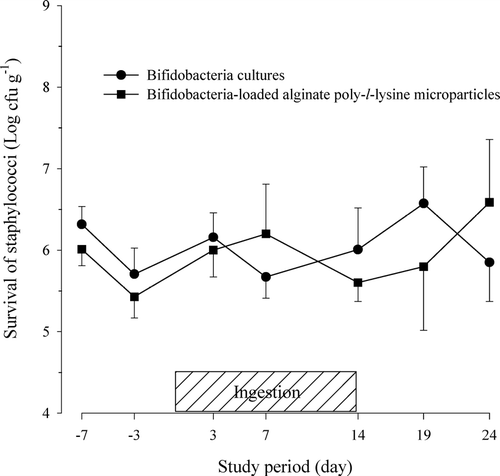
The previous results reported by other research groups concerning the ingestion of unprotected bifidobacteria also have been divergent (Rasic and Kurmann Citation1983; Romond et al. Citation1998; Fukushima et al. Citation1997). Further studies are needed to determine why the viability of enterobacteriaceae in the fecal samples in this study showed contradictory results compared with those reported in previous works. The viability of staphylococci in the fecal samples ranged from 105 to 107cfu g− 1 and the difference between the two groups also was quite large.
Although the viability of bifidobacteria might be increased in a small group, this increase does not necessarily lead to a reduction of viability in putrefactive bacteria. The viability of other micro-organisms such as lactic acid bacteria, enterobacteriaceae, and staphylococci also showed wide variations among the subjects. The viability of lactic acid bacteria and staphylococci was not significantly different between the two groups. However, bifidobacteria was the predominant micro-organism in the feces, followed by enterobacteriaceae (106-108 cfu g− 1), lactic acid bacteria (106-108 cfu g− 1), and staphylococci (105–107cfu g− 1). The viability of bifidobacteria in the feces was relatively stable, whereas the viability of the other microflora varied during the study period.
Observation of Fecal Status
Mild constipation or diarrhea may be partially corrected through the consumption of unprotected bifidobacteria preparations, possibly due to the changes of the microflora ecosystem in the intestine (Rasic and Kurmann Citation1983; Tojo et al. Citation1987; Benno and Mitsuoka Citation1992). The colonic transit times in women also were significantly accelerated in the sigmoid colon after the consumption of milk containing bifidobacteria, due to increased colonic motility (Marteau et al. Citation2002). However, the effectiveness of the protected bap-microparticles on in vivo fecal changes was not reported in human subjects. Intervals between bowel movements (days) after the oral administration of unprotected bifidobacteria cultures (group A) and protected bap-microparticles (group B) to healthy human volunteers are compared in . The intervals between the bowel movements were unchanged during the period of oral ingestion of the bifidobacteria cultures. In contrast, the intervals between the bowel movements had a tendency to increase during the period of ingestion of the bap-microparticles, except in one subject. In other words, the frequencies of the bowel movements were significantly reduced (p < 0.05). The intervals between bowel movements returned to the predose level once the oral ingestion of the bifidobacteria preparations was discontinued.
TABLE 1 Intervals between bowel movements (days) after the oral administration of unprotected bifidobacteria cultures (group A) and protected bifidobacteria-loaded alginate poly-l-lysine microparticles (group B) to healthy human volunteers
Number of volunteers by scoring indices of fecal volume and viscosity after the oral administration of unprotected bifidobacteria cultures (group A) and protected bap microparticles (group B) are given in and , respectively. The volume and viscosity of the feces during the study period were not significantly different between unprotected bifidobacteria cultures and protected bap-microparticles by the chi-square test (p > 0.05). However, if we noted that the fecal volume had a tendency to be increased during the ingestion periods of the protected bap microparticles in group B (p < 0.05). The viscosity of the feces also was significantly decreased by the oral administration of the protected bap-microparticles in group B. The feces contained more water during the ingestion period. It was evident that the multiple oral ingestions of the protected bap-microparticles could be more effective to modify the physiology of the intestinal ecosystem in human volunteers.
TABLE 2 Number of volunteers by scoring indices of fecal volume after the oral administration of unprotected bifidobacteria cultures (group A) and protected bifidobacteria-loaded alginate poly-l-lysine microparticles (group B)
TABLE 3 Number of volunteers by scoring indices of fecal viscosity after the oral administration of unprotected bifidobacteria cultures (group A) and protected bifidobacteria-loaded alginate poly-l-lysine microparticles (group B)
CONCLUSION
The protected bap-microparticles significantly increased the viability of the bifidobacteria in human volunteers, as compared with the unprotected bifidobacteria cultures used as a control, by improving the resistance of the bifidobacteria to gastric acid via microencapsulation. The viability of other fecal microflora such as lactic acid bacteria, enterobacteriaceae, and staphylococci showed wide variations among the subjects. Encapsulated bifidobacteria resulted in more frequent defecation and decreased feces viscosity. The bap-microparticles could be used to deliver bifidobacteria without losing their viability in the low pH conditions in human volunteers.
This work was partially supported by a grant from the Ministry of Science and Technology-National Research Laboratory (NRL) program (M1030000-06J0000-31910), Republic of Korea. The authors also thank Dr. Myung Ja Choi for her invaluable editorial assistance in preparing this manuscript.
REFERENCES
- Benno Y., Mitsuoka T. Impact of Bifidobacterium longum on human fecal microflora. Microbiol. Immunol. 1992; 36: 683–694
- Berrada N., Lemeland J., Laroche G., Thouvenot P., Piaia M. Bifidobacterium from fermented milk: survival during gastric transit. J. Dairy Sci. 1991; 74: 409–413
- Bolton S. Pharmaceutical Statistics. Practical and Clinical Applications, 3rd ed. Marcel Dekker, Inc, New York 1997
- Chung H. S., Kim Y. B., Chun S. L., Ji G. E. Screening and selection of acid and bile resistant bifidobacteria. Int. J. Food Microbiol. 1999; 47: 25–32
- Clark P. A., Cotton L. N., Martin J. H. Selection of Bifidobacteria for use as dietary adjuncts in cultured dairy foods: II–Tolerance to simulated pH of human stomachs. Cultured Dairy Prod. J. 1993; 11: 11–14
- Cui J.-H., Goh J.-S., Park S.-Y, Kim P. -H., Lee B.-J. Preparation and physical characterization of alginate microparticles using air atomization method. Drug Dev. Ind. Pharm. 2001; 27: 309–319
- Cui J.-H., Goh J.-S., Kim P.-H., Choi S.-H., Lee B.-J. Survival and stability of bifidobacteria-loaded alginate poly-l-lysine microparticles. Int. J. Pharm. 2000a; 210: 51–59
- Cui J.-H., Shim J.-M., Lee J.-S., Lee B.-J. Gastric acid resistance of lactobacilli and bifidobacteria in commercial drink and liquid yogurts. Kor. J. Microbiol. 2000b; 36: 161–165
- Fukushima Y., Li S. –T., Hara H., Terada A., Mitsuoka T. Effect of follow-up formula containing bifidobacteria (NAN BF) on fecal floral and fecal metabolites in healthy children. Biosci. Microflora 1997; 16: 65–72
- Holzapfal W. H., Haberer P., Snel J., Schillinger U., Veld H, Jos J. H. Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998; 41: 85–101
- Hoover D. G. Bifidobacteria: activity and potential benefits. Food Tech. 1993; 47: 120–124
- Kwok K., Groves M. J., Burgess D. J. Production of 5–15 μm alginate-poly-l-ysine microcapsules by an air-atomization technique. Pharm. Res. 1991; 8: 341–344
- Lee B.-J., Cui J.-H., Park O.-S., Goh J.-S., Ahn T.-S., Park S.-Y. Stability and gastric acid resistance of lactobacilli and bifidobacteria in commercial yogurts. Kor. J. Microbiol. 1999; 35: 89–93
- Lee B.-J., Min G. H. Preparation and release characteristics of polymer-reinforced and coated alginate beads. Arch. Pharm. Res. 1995; 18: 183–188
- Lee B.-J., Min G. H., Kim T. W. Preparation and in vitro release of melatonin-loaded multivalent cationic alginate beads. Arch. Pharm. Res. 1996; 19: 280–285
- Lee K.-Y., Heo T.-R. Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Appl. Environ. Microbiol. 2000; 66: 869–873
- Lian W.-C., Hsiao H.-C., Chou C.-C. Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int. J. Food Microbiol. 2003; 86: 293–301
- Liu P., Krishnan T. R. Alginate-pectin-poly-l-lysine particulate as a potential controlled release formulation. J. Pharm. Pharmacol. 1999; 51: 141–149
- Marteau P., Cuillerier E., Meance S., Gerhardt M. F., Myara A., Bouvier M., Bouley C., Tondu F., Bommelaer G., Grimaud J. C. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Alimentary Pharmacol. Ther. 2002; 16: 587–593
- Picot A., Lacroix C. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Int. Dairy J. 2004; 14: 505–515
- Rao A. V., Shiwnarain N., Maharaj I. Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can. Institute Food Sci. Tech. 1989; 22: 345–349
- Rasic L. J., Kurmann J. A. Bifidobacteria and Their Role. Birkhauser Verlag, BernSwitzerland 1983
- Romond M. B., Ais A., Guillemot F., Bounouader R., Cortot A., Romond C. Cell-free whey from milk fermented with Bifidobacterium breve C50 used to modify the colonic microflora of healthy subjects. J. Dairy Sci. 1998; 81: 1229–1235
- Tojo M., Oikawa T., Morikawa T., Yamashita N., Iwata S., Satoh Y., Hanada J., Tanaka R. The effect of Bifidobacterium breve administration on Campylobacter enteritis. Acta Paediatrica. (Jpn). 1987; 29: 160–167
- Truelstrup Hansen L., Allan-Wojtas P. M., Jin Y.-L., Paulson A. T. Survival of Ca-alginate microencapsulated Bifidobacterium spp. In milk and simulated gastrointestinal conditions. Food Microbiol. 2002; 19: 35–45