Abstract
Erythrocytes as the most readily available and abundant cells within the body have been studied extensively for their potential application as drug delivery carries. In this study, human erythrocytes were loaded by bovine serum albumin (BSA) as a model antigen/protein using hypotonic preswelling method for targeted delivery of this antigen-to antigen-presenting cells. The average loaded amount, efficiency of entrapment, and cell recovery upon loading procedure were 1979.25 ± 9.4 μg, 30.06 ± 0.20%, and 87.53 ± 0.66%, respectively. The total BSA recovery upon loading procedure was 97.20 ± 4.90%. The apparent mechanism of entrapment was simple concentration-based gradient in/out the cells with some minor limiting factors against protein entry into the cells. We have shown that the intra- and intersubject variations of the method were interestingly low (i.e., less than 5% in all cases).
Cellular carriers, including erythrocytes, leukocytes, platelets, islets, hepatocytes, and fibroblasts, all have been suggested as potential delivery systems for drugs and other bioactive agents (Banker and Rhodes Citation2002, 560; Rossi et al. Citation2005). They can be used to provide slow release of entrapped drugs in the circulatory system, to deliver drugs to a specific site within the body, as cellular transplants to provide missing enzymes and hormones (in enzyme or hormone replacement therapy), or as endogenous bioreactors to synthesize and secret molecules capable of affecting the metabolism and function of other cells (Banker and Rhodes Citation2002, 560; Highley and De Bruijn Citation1996; Jain, Jain, and Dixit Citation1997). Erythrocytes, as the most abundant and readily available cells in the body, have gained the highest degree of interest among the aforementioned cells, owing to a series of advantages. These cells are nonimmunogenic, biodegradable, and biocompatible and may have nearly normal life-span in circulation, among other advantages (Gutierrez Millan et al. Citation2004a, Citation2004b; Hamidi and Tajerzadeh Citation2003; Magnani et al. Citation2002). One of the main advantage of erythrocytes is that they act as a true drug targeting system that achieve selective drug distribution to different organs and tissues, specially the phagocytic cells of the mononuclear phagocyte system (MPS). The selective accumulation of therapeutic agents in phagocytic cells, such as macrophages, by the use of carrier erythrocytes has been reported for antibiotics (Jaitely et al. Citation1996), antileshmanials (Summers Citation1983), antimalarials (Talwar and Jain Citation1992), antineoplasm agents (Al-Achi and Boroujerdi Citation1990), anti-HIV peptides (Rossi et al. Citation1998), and enzymes such as asparaginase, uricase, urokinase, and arginase (Gutierrez Millan et al. Citation2004b).
In recent years, a major goal of researchers in vaccine design and formulation has been the development of sustained-release or pulsed-release delivery systems capable of eliminating the requirement for a multiple dosing schedule inherent to the administration of conventional vaccines (Storni et al. Citation2005; Coombes et al. Citation1996). This would result in the decreased number of vaccination periods and therefore reduced problems with patient noncompliance and population coverage (Powell Citation1996; Zhao and Leong Citation1996). Aside from this, a new generation of vaccines are poorly immunogenic and need potent adjuvants to induce an effective protective immunity (Zhou et al. Citation2003; O'Hagan, Singh, and Gupta Citation1998). Therefore, development of more efficient and safe adjuvant/vaccine delivery systems to obtain high immune responses is of primary importnace. Most of the vaccine delivery systems, especially those based on polymeric systems, have high immunogenic potency but are toxic and immunogenic such that they cause nonspecific stimulation of host immune systems (Giudice, Podda, and Rappuoli, et.al. Citation2002; Babiuk et al. Citation2000; Gupta and Siber Citation1995).
Erythrocytes, as the autologous cells of the bost body, can be regarded as a safe adjuvant/vaccine delivery system. Beside the general advantages of erythrocytes as an ideal drug delivery system, they have two remarkable characteristics for use as a vaccine delivery system: They can be used for controlled release of vaccines with an aim to reduce the number of doses needed for primary immunization or to develop single dose vaccines; they may serve as a vehicle to target antigens to antigen-presenting cells (APC); for instance macrophages and dendritic cells, for improved and potentiated immunity.
Bovine serum albumin (BSA) is one of the most widely used model antigens during vaccine delivery studies (Hoshi et al. Citation1998; Sprott, Tolson, and Patel Citation1997; Ramaldes et al. Citation1996) owing to its ease of assay, well-known immunogenicity pattern, well-known physicochemical properties, low cost, and general availability (Hoshi et al. Citation1998). In our study was encapsulated in human erythrocytes using the hypotonic preswelling method. The encapsulation method was validated and characterized in terms of the antigen-loading parameters of the prepared vehicles.
MATERIALS AND METHODS
BSA (Sigma, St. Louis, MO, USA; Art. No. A2153) was purchased locally. Heparin sodium was purchased from Iranian Pharmaceutical Development and Investment Company (Rasht, Iran). Other chemicals and solvents were from chemical lab or HPLC purity grades, as needed, and were purchased locally.
Preparation of Human Erythrocytes
Blood samples were withdrawn by venipuncture from healthy volunteers aged 25 to 30 years using 19-gauqe hypodermic needles connected to disposable polypropylene syringes. After centrifuging at 600 g for 10 min, the plasma and buffy coat were separated by aspiration, and the remaining packed erythrocytes were washed three times with phosphate-buffered saline (PBS; 150 mM NaCl and 5 mM K2HPO4; pH = 7.4).
Encapsulation of BSA
A hypotonic preswelling method described by Tajerzadeh and Hamidi (Citation2000) was used for loading the human erythrocytes by BSA. For this purpose, 1 ml of washed packed erythrocytes was transferred gently to a polypropylene test tube, 4 ml of a hypotonic PBS with osmolarity of 0.67 that of the eutonic solution was added, and the resulting cell suspension was mixed gently by 10-times inversion. The swollen cells were separated by centrifugation at 600 g for 10 min and the supernatant was discarded. A 200-μl aliquot of a hemolysate, prepared by diluting another portion of erythrocytes with distilled water (1:1), was then added gently to the remaining swollen cells. We assumed that this hemolysate layer plays an important role as an osmotic shock barrier and also as a reservoir of cell constituents for underlying cells and thus prevents them from substantial loss of cellular components near the lysis point. Then, 250 μl of an aqueous solution of BSA (8 mg/ml) was gently added to the cell suspension, and the resulting mixture was inverted gently several times and centrifuged at 600 g for 5 min. Addition of protein solution, mixing, and centrifuging were repeated three more times to achieve the lysis point of the cells.
This point was detectable by a sudden increase in transparency of the cell suspension and the disappearance of a distinct boundary between cells and supernatant on centrifuging. Then, the erythrocytes were resealed by the rapid addition of 100 μl of hypertonic PBS with an osmolarity of ten times the eutonic solution, followed by gentle mixing of the suspension by several inversions. Finally, the resulting mixture was incubated at 37°C for 30 min to reanneal the resealed cells. The carrier erythrocytes obtained by this manner were washed three times using 10 ml aliquots of PBS to wash out the unentrapped BSA and the released hemoglobin and other cell constituents during the loading process.
BSA Assay
A reversed-phase HPLC method was developed and used throughout this study for BSA assay. The method consisted of a gradient system of 0.1% trifluoroacetic acid (TFA) in water (A) and 0.08% TFA in acetonitrial (B) with initial A/B ratio of 70/30 changed linearly to the final ratio of 35/65 (A/B) within 20 min. The reversal to the initial condition then occurred within 2 min, and finally the system was re-equilibrated over 8 min (total run time of 30 min). The flow rate was 1 ml/min all over the gradient steps. The analyte separation was carried out using a wide-pore Symmetry 300® C4 protein analysis column (50 × 4.6 mm; particle size 5 μm; pore size 300 Å; Waters, MA, USA) operated at 40°C and equipped by the corresponding guard column (Waters).
The solvent delivery system used was a double-reciprocating pump (mode 600, Waters, MA, USA) and a UV-detector (model 746, Waters, MA, USA), with a wavelength of 280 nm was used for analyte detection with the outputs processed and record by a compatible integrator (model 486, Waters, MA, USA). Sample injection was made by a loop injector (Rheodyne®, Cotati, CA, USA) equipped with a 50 μl loop.
To determine the amount of loaded BSA, 0.1 ml of final washed erythrocytes was diluted with 0.1 ml of distilled water to completely lyse the cells. Then, the suspension was centrifuged at 10000 g for 20 min and the supernatant was filtered through a 0.45 μm syringe filter (Teknokroma, Spain). Finally, 50 μl of the filtrate was injected to the chromatograph.
Loading Parameters
To evaluate the effect of any changes in encapsulation method variables on the loading efficiency, three indices were defined as loading parameters:
Loaded amount, the amount of BSA encapsulated in 0.1 ml of the final packed erythrocytes.
Efficiency of entrapment, the percentage ratio of the loaded amount of BSA to the amount added during the entire loading process.
Cell recovery, the percentage ratio of the hematocrit value of the final loaded cells to that of the initial packed cells, measured on equal volumes of two suspensions.
Methodological Tests
Incubation of Intact Erythrocytes with Isotonic BSA Solution
To investigate the possible uptake and/or degradation of BSA by intact human erythrocytes, 0.5 ml of washed packed erythrocytes was incubated at 37°C with 0.5 ml of BSA solutions in PBS with a concentration of 8 mg/ml (the same as used in loading procedure). At 15, 30, and 60 min, equal volumes of the cell suspension were harvested and centrifuged at 1000 g for 10 min, and the concentrations of remaining BSA in the supernatant were determined, as described, by direct injection to the chromatograph. In addition, the BSA concentration in the cellular fraction also was determined at each time point after lysis, as described above.
Concentration and Volume of Added BSA Solution
The encapsulation procedure was performed using aqueous solutions of BSA with concentrations of 1, 2, 4, 6, 8, 10, and 15 mg/ml. The loading parameters were determined in each case.
To verify the observed point of lysis and also to optimize the volume of the protein solution used during the loading procedure, the process was performed on four separate erythrocyte samples such that to each of the cell suspensions, 2, 3, 4, and 5 successive 250 μl aliquots of BSA solution were added. After completion of the procedure, the loading parameters were determined in each case.
Mechanism of Entrapment
To investigate the possible mechanism of BSA entrapment by erythrocytes, an encapsulation procedure was performed and the concentrations of protein in each of three final washing solutions as well as in final packed cells were determined by HPLC. Then, the total amount of washed out (unentrapped) protein was calculated by considering the total volume of discarded solutions. Also, the total amount of entrapped protein was determined using the loaded amount multiplied by the cell recovery of the method. Finally, taking the volume fraction of cells in whole suspension at the point of lysis, the mechanistic behavior of erythrocytes against the protein molecules was exploited.
Process Validation Tests
To validate the encapsulation process, the following three tests were carried out:
Intrasubject variations. Three blood samples were collected from a healthy volunteer and the loading procedure was carried out on each sample separately. The loading parameters for each sample as well as the corresponding coefficients of variations (CV %) were determined.
Intersubject variations. Blood samples were collected from 6 healthy volunteers (3 male and 3 female subjects), and the loading procedure was carried out in each case. The loading parameters for each of the subjects as well as the corresponding coefficients of variations were determined.
Recovery. The measured entrapped, unentrapped, and total amount of BSA recovered after completion of the encapsulation procedure were compared with those calculated by considering the total added amount and the volume fraction of cells and supernatant at the point of lysis.
Statistical Analysis
The experiments were carried out in triplicate (n = 3) and the differences between the results were judged using T-test or ANOVA parametric tests at a significance level of 0.05.
RESULTS AND DISCUSSION
BSA Uptake by Erythrocytes
The results of incubation of intact human erythrocytes with BSA isotonic solutions are shown in . As can be seen, no detectable amount of protein was taken up by intact erythrocytes. Therefore, we considered that the erythrocyte membrane had no significant active role in the encapsulation of BSA in human erythrocytes. On the other hand, a small amount of BSA was degraded by intact erythrocytes. Therefore, caution should be taken when calculating the protein loading or interpreting the results of loading experiments with respect to this slight degradation, whenever applicable.
TABLE 1 Concentration of BSA determined in supernatant and cell lysate upon incubation of intact erythrocytes with isotonic BSA solutionFootnotea(n = 3)
Concentration of BSA Solution
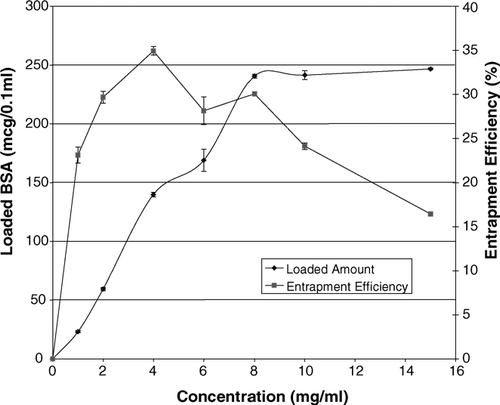
The effect of BSA concentration on the loaded amount and efficiency of entrapment is shown in . According to these data, it is clear that the loaded amount of protein is related directly to the concentration of BSA solution used, throughout the concentration range of 1–8 mg/ml and approaches a plateau beyond this range. The efficiency of entrapment increases slightly up to 4 mg/ml, beyond which a declining trend is evident. While the use of concentrations higher than 4 mg/ml resulted in some lower efficiency of entrapment, the concentration of 8 mg/ml was selected to be used during the process. We chose 8 mg/ml mainly because of the suitable (not optimal) entrapment efficiency with optimal absolute amount of loaded protein in unit volume of packed carrier cells, a parameter that is critical for dose adjustment during in vivo studies on this delivery system.
Volume of Added BSA Solution
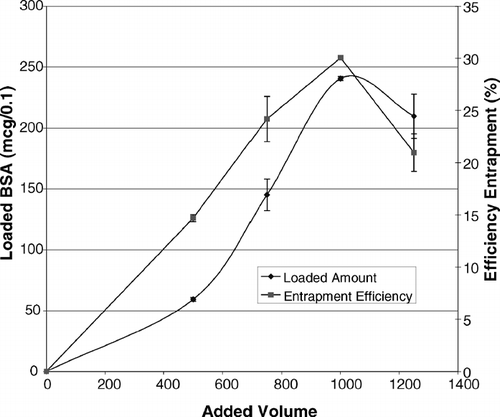
The effect of the volume of protein solution added during the encapsulation process on the loading parameters is shown in . An interesting consistency was found between these findings and the macroscopically detected point of lysis, at which a sudden increase in loaded amount and efficiency of entrapment occurs exactly when the transparency of erythrocyte suspension increases remarkably. Thus, the macroscopic evidence for the point of lysis can be used reliably for the detection of the lysis point. Furthermore, since both the loaded amount and the efficiency of entrapment were shown to be optimum with 1000 μl of protein solution, which is high enough to ensure the achievement of the lysis point, this volume was selected as the optimum volume to be added during the process.
Mechanism of Entrapment
As shown in , for an experimental run, ∼5.8 mg of the total 8 mg BSA added during the encapsulation procedure was discarded as three washing solutions. At the same time, the total amount of BSA remaining in the erythrocytes was ∼1.9 mg per total packed cells recovered after the loading process. The total volume of the reaction mixture was 2.5 ml at the point of resealing. This volume consist of 1.2 ml for swollen cells, 0.2 ml for hemolysate, 1 ml for total protein solution added in four steps, and 0.1 ml for hypertonic resealing solution. From this volume, 0.82 ml belongs to the carrier cells. Accordingly, we expected that if the distribution of protein between the intracellular and extracellular fractions would be governed only by a simple concentration gradient-based diffusions, from the total amount of 8 mg of added protein during the process, about 2.62 mg would be entrapped in the erythrocytes, and the rest (i.e., 5.38 mg) would be discarded as unentrapped protein.
TABLE 2 Entrapped and unentrapped amounts of BSA at the end of the encapsulation process (n = 3)
In fact, as shown in , we can say that the partitioning of protein at the lysis point, mainly (∼75%) depends on the volume fraction of cells in the suspension, considering the fact that the main part (i.e., more than 65%) of the 25% difference between the expected and measured protein mass inside erythrocytes, has been found as extra protein mass in washing solution. We concluded that there has been a limiting factor for complete protein entry to erythrocyte in samples at lysis point (e.g., large size, high polarity, limited diffusion time, and adsorption of BSA to erythrocyte surface). However, the results of the incubation test of intact erythrocytes with BSA showed that the erythrocyte membrane had no significant active role in the uptake of BSA, the protein only passed via the pores made in the membrane, upon hemolysis, inward and outward the erythrocyte.
TABLE 3 Recovery of BSA as entrapped, unentrapped, and total protein after the encapsulation process in human erythrocytes (n = 3)
Loading Parameters
The average loading parameters of BSA in human erythrocytes are shown in . The loaded amount of BSA is comparable to those values reported in the literature for a variety of proteins (Gutierrez Millan et al. 2004). This can ensure sufficient entry of BSA into the body on reinjection of fairly low volumes of the packed cells. A cell recovery of ∼87.53%, being practically appreciable, is comparable to recovery results of other drugs and proteins reported by others (Magnani et al. Citation2002; Rossi et al. Citation2005).
TABLE 4 Loading parameters of encapsulation method of BSA in human intact erythrocytes (n = 3)
Process Validation Tests
shows that the intrasubject variations of the loading procedure are fairly low. Also, as shown in , the intersubject variations of the loading procedure are low too, even when samples taken from subjects of different sexes were included in the study. Considering the biological nature of the carriers used in this study, these markedly low variations of loading parameters are highly interesting and promising with respect to the popular use of this carrier system.
TABLE 5 Intrasubject variations of loading parameters of BSA in human intact erythrocytes (n = 3)
TABLE 6 Intersubject variations of loading parameters of BSA in intact human erythrocytes of three male and female volunteers
The recovery of the encapsulation process is shown in with respect to entrapped, unentrapped, and total protein. These data may provide a reasonable basis for investigation of the mechanism of entrapment, as discussed earlier, and indicate a remarkable degree of accuracy and validity for the whole process, particularly considering the high total protein recovery (i.e., 97.2%).
CONCLUSION
The hypotonic preswelling method for encapsulation of drugs/proteins in human erythrocytes was evaluated using BSA as a model antigen. The effect of different variables such as volume, concentration, and method of addition of protein solution were studied. A knowledge of the optimal conditions of the loading process to yield the best results allows the design of a suitable encapsulation procedure for a particular antigen and/or protein with some minor modification, if necessary, and this study can serve as a model for such evaluations. In addition, the mechanism of entrpment of BSA in intact human erythrocytes was exploited and we showed that the probable mechanism is the entry of protein into the cells via the pores made on the cell membrane at the point of hemolysis. Then, on closure of pores by hypertonic resealing, the protein is trapped in the cells.
Development of this delivery system for BSA may not only be of remarkable importance in vaccine delivery, but also provide some valuable information on the possibility of controlled release of proteins using this delivery system. Finally, validation tests on the loading method indicated a remarkable degree of accuracy, precision, and reproducibility for the loading method in preparation of carrier erythrocytes for antigen delivery studies using BSA as a model.
REFERENCES
- Al-Achi A., Boroujerdi M. Pharmacokinetics and tissue uptake of doxorubicin associated with erythrocyte-membrane: Erythrocytes-ghosts vs. erythrocytes-vesicles. Drug Devel. Ind. Pharm. 1990; 16: 2199–2219
- Babiuk S., Baca-Estrada M., Babuik L. A., Ewen C., Foldvari M. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J. Control. Rel. 2000; 66: 199–214
- Banker G. S., Rhodes C. T. Modern Pharmaceutics. Marcel Dekker Inc, New York 2002
- Coombes A. G. A., Lavelle E. C., Jenkins P. G., Davis S. S. Single dose, polymeric, microparticle-based vaccines: the influence of formulation conditions on the magnitude and duration of the immune response to a protein antigen. Vaccine 1996; 14: 1429–1438
- Giudice G. D., Podda A., Rappuoli R. What are the limits of adjuvanticity?. Vaccine 2002; 20: S38–S41
- Gupta R. K., Siber G. R. Adjuvants for human vaccines-current status, problems and future prospects. Vaccine 1995; 13: 1263–1276
- Gutierrez Millan C., Zarzuelo Castaneda A., Sayalero Marinero M. L., Lanao J. M. Factors associated with the performance of carrier erythrocytes obtained by hypotonic dialysis. Blood Cells Mol. Dis. 2004a; 33: 132–140
- Gutierrez Millan C., Sayalero Marinero M. L., Zarzuelo Castaneda A., Lanao J. M. Drug, enzyme and peptide delivery using erythrocytes as carriers. J. Control. Rel. 2004b; 95: 27–49
- Hamidi M., Tajerzadeh H. Carrier erythrocytes: an overview. Drug Del. 2003; 10: 9–20
- Highley M. S., De Bruijn E. A. Erythrocytes and the transport of drugs and endogenous compounds. Pharm. Res. 1996; 13: 186–195
- Hoshi S., Saito N., Kusanagi K., Ihara T., Ueda S. Adjuvant effects of fluoride on oral immunization of chickens. Vet. Immunol. Immunopathol. 1998; 63: 253–263
- Jain S., Jain S. K., Dixit V. K. Magnetically guided rat erythrocytes bearing isoniazid: preparation, characterization, and evaluation. Drug Devel. Ind. Pharm. 1997; 23: 999–1006
- Jaitely V., Kanaujia P., Venkatesan N., Jain S., Vyas S. P. Resealed erythrocytes: drug carrier potentials and biomedical applications. Ind. Drugs. 1996; 33: 589–549
- Magnani M., Rossi L., Fraternale A., Bianchi M., Antonelli A., Crinelli R., Chiarantini L. Erythrocyte-mediated delivery of drugs, peptides and modified oligonucleotides. Gene Ther. 2002; 9: 749–751
- O'Hagan D. T., Singh M., Gupta R. K. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv. Drug Deliv. Rev. 1998; 32: 225–246
- Powell M. F. Drug delivery issues in vaccine development. Pharm. Res. 1996; 13: 1777–1785
- Ramaldes G. A., Deverre J.-R., Grognet J.-M., Puisieux F., Fattal E. Use of an enzyme immunoassay for the evaluation of entrapment efficiency and in vitro stability in intestinal fluids of liposomal bovine serum albumin. Int. J. Pharm. 1996; 143: 1–11
- Rossi L., Brandi G., Schiavano G. F., Balestra E., Millo E., Scarfi S., Damonte G., Gasparini A., Magnani M., Perno C. F., Benatti V., De Flora A. Macrophage protection against human immunodeficiency virus or herpes simplex virus by red blood cell-mediated delivery of a heterodinucleotide of azidothymidine and acyclovir. AIDS Res. Hum. Retroviruses. 1998; 14: 435–444
- Rossi L., Serafini S., Pierige F., Antonelli A., Cerasi A., Fraternale A., Chiarantini L., Magnani M. Erythrocyte-based drug delivery. Expert Opin. Drug Del. 2005; 2: 311–322
- Sprott G. D., Tolson D. L., Patel G. B. Archaeosomes as novel antigen delivery systems. FEMS Microbiol. Lett. 1997; 154: 17–22
- Storni T., Kundig T. M., Senti G., Johansen P. Immunity in response to particulate antigen-delivery systems. Adv. Drug Del. Rev. 2005; 57: 333–355
- Summers M. P. Recent advances in drug delivery. Pharm. J. 1983; 230: 643–645
- Tajerzadeh H., Hamidi M. Evaluation of hypotonic preswelling method for encapsulation of enalaprilat in intact human erythrocytes. Drug Dev. Ind. Pharm. 2000; 26: 1247–1257
- Talwar N., Jain N. K. Erythrocytes as carrier of primaquin preparation: characterization and evaluation. J. Control. Rel. 1992; 20: 133–142
- Zhao Z., Leong K. W. Controlled delivery of antigens and adjuvants in vaccine development. J. Pharm. Sci. 1996; 85: 1261–1270
- Zhou S., Liao X., Li X., Deng X., Li H. Poly-D, L-lactide-co-poly(ethylene glycol) microspheres as potential vaccine delivery systems. J. Control. Rel. 2003; 86: 195–205