Abstract
The primary objective of this study was to compare the lubrication properties of micronized poloxamer 188 (Lμ trol micro 68®) and micronized poloxamer 407 (Lμ trol micro 127®) with certain conventional lubricants such as magnesium stearate and stearic acid. The secondary objective was to use these micronized poloxamers as water-soluble tablet lubricants in preparation of effervecsent tablets. The results showed that these micronized poloxamers have superior lubrication properties compared with stearic acid, with no negative effect on tablet hardness, friability, disintegration, or dissolution. Moreover, lubricant mixing time had no significant effect on tablet properties when poloxamers were used as lubricants. Effervescent tablets also were produced successfully using micronized poloxamers as lubricants. The micronized poloxamers had a better lubrication effect in compariason with that of water-soluble lubricant l-leucine.
Lubricants represent a very important class of excipients in manufacturing of tablets. They are used in tablet formulation to ease the ejection of tablets from the die and to prevent sticking of tablet components to punches as well as to prevent excessive wear on punches and die (Garnet et al. Citation1989). An ideal lubricant should reduce the tablet ejection force effectively, when used in small quantities, and with no adverse effects on a tablet formulation. It should be unaffected by changes in process variables (Miller and York Citation1988). Numerous reviews have indicated that no universal lubricant is available at present.
Parameters associated with lubricants also may have a distinct effect on the tablet formulation; these include the concentration and type of lubricant, mixing time, speed of mixing, type of mixer, and lubrication method. Of all lubricants, magnesium stearate, although good and economical, has a few deleterious effects on tablet formulation, such as reduction in tablet strength that prolongs tablet disintegration time and reduces percentage dissolution (Lerk et al. Citation1982; Strickland, Higuchi, and Busse Citation1960; Hwang and Parrott Citation1993). A lubricant generally increases the relaxation on tablet by reducing interparticulate bonding that reduces tablet strength. Over time, magnesium stearate and several other hydrophobic lubricants form a hydrophobic film around the particle during mixing to give molecular coverage (Bolhuis et al. Citation1975). The hydrophobic surface thus retards water penetration into tablet and results in prolongation of disintegration time and (Lerk et al. Citation1982; Granderton Citation1969). Disintegration time also depends on the type, mixing time, type of mixer, and mixer load as well as the swelling capacity of disintegrants in a formulation (Lerk et al. Citation1982; Russo Citation1984; Van der Watt and de Villiers Citation1997).
Shah and Mlodozeniec (Citation1977) have described lubricant mixing as a two-step mechanism. During the mixing process, lubricant particles are first adsorbed onto the surface and then distributed uniformly across the granule surface. Kikuta and Kitamori (Citation1994) have observed that with an increase in lubricant mixing time, the tablet hardness decreases at a first-order rate and then continues to decrease with another very slow first-order rate process. The effect of lubricant mixing on dissolution is attributed to the hydrophobicity of the lubricant as well as its film forming nature. Interestingly, a lower concentration of magnesium stearate does not affect dissolution rate of crystalline lactose but decreases dissolution rate of tablets containing Avicel PH101 and Aerosil 200 (Delattre et al. Citation1976; Khan, Musikabhimma, and Rubinstein 1983).
Lubricants are the most important components of the effervescent tablet formulation aiding in the production of effervescent tablets on high-speed equipment (Mohrle Citation1989; Saleh et al. Citation1984; Schmidt and Christin. Citation1990). Many materials are effective as lubricants at certain concentrations but may quench tablet disintegration (Mohrle Citation1989). For effervescent tablets, it is desirable to get a clear solution after the table disintegrates in water. For that, lubricants should be water soluble, which limits the selection of lubricant, as most of the lubricants are water-insoluble and give cloudy appearances. Excipients with both lipophilic and hydrophilic properties can provide good internal lubrication to an effervescent formulation and give clear solution of the disintegrated tablets (Mohrle Citation1989; Roscheisen and Schmidt Citation1995a; Saleh et al. Citation1984). Several researchers have even suggested extrinsic lubrication and punch coating as a useful mean for lubricating effervescent formulations (Sendall et al. Citation1983; Loeffler and Ebey Citation1989, Schmidt and Cheistion Citation1990).
Materials such as l-leucine (Shinozaki et al. Citation1971; CitationRöscheisen and Schmidt 1995b), glycine, sodium benzoate, sodium propionate, and adipic acid have been tried as water-soluble lubricants. In industry, 1-leucine is used as a lubricant in effervescent tablets. The only drawback regarding 1-leucine is the cost and requirement of higher concentration. From a structural point of view poloxamer may be a good candidate as a water-soluble lubricant, due to the presence of POE and POP in the polymer. Micronized poloxamers may prove to be good candidates as lubricants for effervescent tablet formulation due to their particle size and water-solubility nature.
One of the objectives of this study was a comparison of poloxamers with conventional hydrophobic lubricants magnesium stearate and stearic acid, as well as with a water-soluble lubricant l-leucine. Caffeine was used as a model drug for the first set of comparative experiments. The effect of lubricant type, concentration, and tablet compression force on the tablet lubrication effect was measured using ejection force of the final tablet formulation. Effects of these parameters on tablet properties such as hardness, disintegration time, friability, and dissolution were measured. Compression force was kept constant while evaluating the mixing time effect. Two different mixing times were used with two different concentrations of lubricants. Direct compressible grade acetaminophen was used as model drug for this set of experiments. Minitab, statistical software, was used for the statistical analysis to determine the effect of this parameter on the lubrication effect. Last, effervescent tablets were manufactured using micronized poloxamer 188 (MP 188) and micronized poloxamer (MP 407) as tablet lubricants using two different formulations namely aspirin and acetaminophen.
MATERIALS AND METHOD
Caffeine, anhydrous powder, acetaminophen DC, and aspirin (BASF, NJ, USA) were used as active components. Kollidon VA 64® (BASF, NJ, USA), lactose monohydrate USP (Mutchler, NJ, USA), and HPMC E5 LV USP (Dow Chemical, MI, USA) were used as fillers or binders. Avicel PH 101(FMC, PA, USA), MCC, NF (viyarur Type 101) (JRS, NY, USA), sodium croscarmellose (Mutchler, NJ, USA), and Kollidon CL® (PVP XL) (BASF, NJ, USA) were used as external binder and disintegrants, respectively. Magnesium stearate and stearic acid (Mallinckrodt, NJ, USA), Lμtrol 68 micro® (MP 188), Lμ trol 127 micro® (MP 407) (BASF), and spraydried l-leucine (Calibiochem, CA, USA) were used as lubricants.
A ROTO-P® single pot high shear granulator (Romaco, NJ, USA), a Korsch® XL 100 rotary tablet press (Korsch MA, USA), an Alexanderwerk® modular processing system (Alexanderwerk, PA, USA), a Hanson® dissolution tester (Hanson Research, CA, USA), an HPLC 2690 model (Waters Corporation, MA, USA), and a Shimadzu® UV 160 spectrophotometer (Shimadzu, MD, USA) were used. The tap density and bulk density were measured by a tap density instrument (Varian, NC, USA). Tablet hardness was measured using a hardness tester (Key, NJ, USA). Disintegration testing was conducted using Pharma Test PTZ AUTO 1® apparatus and a Vanderkamp® friabilitor was used for friability test.
EXPERIMENTAL PROCEDURES
Hydrophobic Lubricants
Caffeine was used as a model drug for comparison of micronized poloxamer 188 (MP 188) and micronized poloxamer 407 (MP 407) with magnesium stearate and stearic acid as tablet lubricants. The wet granulation method was used for preparation of granules using 5% HPMC, E5 LV USP as a granulating fluid. Granules were dried in a forced air oven (45°C) for 24 hr to achieve the constant moisture content. The dried granules were milled using Alexanderwerk® modular processing system and blended with the other excipients for 5 min using a bin blender. Lubricant was added prior to the compression and mixed for 2 min using a bin blender. Formulation was selected based on pervious experience.
General factorial design was used for evaluation purpose. Ejection force was used as response variable. The significant variable at a 95% confidence level were determined. Two different concentrations of lubricant were used: 1% and 1.5%. All the batches were compressed at three different compression forces (3–4, 7–8, and 11–12 kN) to evaluate the effect of compression force on lubrication effect. Tablets were compressed and ejection force was recorded. Tablets were collected from all the batches and evaluated for weight variation, thickness, tablet hardness, friability, and disintegration time and dissolution profile.
Water Soluble Lubricant
The purpose of this study was to compare MP 188 and MP 407 with a water-soluble tablet lubricant such as 1-leucine using a conventional tablet formulation. Caffeine was used as model drug for this study. Granules were prepared as described previously. A 33 factorial design was employed. The three factors were lubricant type, lubricant concentration (%), and compression force (kN). Three different concentrations, 1%, 1.5%, and 2% of each lubricant, and three different compression forces 3–4, 7–8, and 11–12 kN were used. The significant variables at a 95% confidence level were determined (α = 0.05). Granulation and tabletting were performed as described.
Direct Compression Acetaminophen Formulation
Direct compressible grade acetaminophen was used as a model drug for this study. Avicel PH 101 was used as a compression aid; PVP-VA copolymer 60/40 (Kollidon VA 64®) (BASF) was used as a dry binder and PVP XL (Kollidon CL®) (BASF) was used as a disintegrant. Drug was mixed with other mentioned excipients for 5 min; lubricant was added just prior to tablet compression and mixed for 2 or 10 min according to the experimental design. A full factorial experimental design was used to evaluate the effect of lubricant mixing time, lubricant type, and concentration on tablet properties. Tablet hardness was used as a response variable. Three different lubricants magnesium stearate micronized poloxamer 188 and micronized poloxamer 407, with two different concentrations, 0.5% and 1.5%, and two different mixing time 2 min and 10 min were used in the experimental design. All the batches were compressed at constant compression force. The compression and ejection forces were recorded using PMA 3 (Pharmaceutical Measurement Analysis) software. One hundred tablets were collected for all the batches and evaluated for thickness, tablet hardness, friability, and disintegration time and dissolution test.
Preparation of Acetaminophen, Asprin Effervescent Tablet Formulation
For each batch of acetaminophen and aspirin formulation, all the preweighted ingredients were mixed and blended for 15 min using a V-shaped blender. The acetaminophen formulation experiments were carried out as per randomized complete block design (RCBD); a simple comparative design was used for the aspirin formulation (Montgomery Citation2001). ANOVA was used for analyzing the results. For acetaminophen formulation, 2% and 4% of lubricants and for aspirin, 4% of lubricants, were added prior to tablet compression and blended for 5 min. The tablets were compressed using a Korsch XL 100 rotary tablet press. Humidity was maintained below 25% RH throughout the experiment. Tablets were collected and packed in doubled layer polyethylene bags to prevent humidity exposure. Hardness, friability, and disintegration test were conducted as soon as the tablets were compressed.
Evaluation Methods
Bulk and Tap Densities
The bulk and tap densities were determined using a 100-ml graduated cylinder. A preweighted sample was introduced into the measuring cylinder and the density was calculated by dividing the mass by volume occupied by sample. The same cylinder was then placed in an optimal control tap density instrument and tapped for 100 taps. Percentage compressibility value (Carr's index value) was calculated using bulk and tap density values for each blends. The formula for Carr's index is as below.
where V is the volume occupied by a sample of the powder after being subjected to a standardized tapping procedure and Vo is the volume before tapping.
Tablet Nardness and Friability
Tablet hardness was measured using a Key® hardness tester. Ten tablets from each batch were selected randomly and hardness was measured in kP (Kilo Pascal) unit.
Twenty tablets were selected randomly for friability test using a Roche® friability tester. The tablets were weighted and placed in the plastic friability chamber. Closed chamber were rotated on for 4 min at 25 RPM, dropping the tablets a distance of 6 IN. with each revolution. After completed dedusting, tablets were weighed again and the percentage weight loss was determined along with a visual examination of the tablets for capping and chipping effect.
Disintegration Time
For conventional tablets, tablet disintegration tests were performed using a PTZ AUTO 1® (Pharma Test) apparatus. This instrument has a built-in thermostatically controlled heater that warms up water to 37°C and a pump that circulates water within a Plexiglas bath. The instrument has a rack assembly with 6 glass tubes that has the USP specifications. For effervescent tablets, the disintegration test was conducted using a 200-ml beaker, containing 150 ml of distilled water. Tests were conducted at room temperature by placing a tablet in water and the time for the tablet to disintegrate into small granules was recorded.
Dissolution Test
A USP paddle apparatus was used for the caffeine dissolution study. The dissolution studies were carried out in 900 ml of water. The media temperature was 37°C. The paddle speed was 100 rpm; 5 ml media aliquots were withdrawn at 0,10,20,30,45, and 60 min and replaced with 5 ml of media. Analysis of caffeine was performed by an HPLC method, on a model 2690 Waters® instrument, and using a mixed water/methanol/glacial acetic acid (69:28:3) as mobile phase, and a C18, 4.6 × 100-mm, 5 μm column. A UV/visible detector at 275 nm and a flow rate of 2 mL was used.
Dissolution tests for acetaminophen tablets were conducted according to the USP 25. Medium: pH 5.8 phosphate buffer, 900 mL; apparatus: USP type 2, 50 rpm; time: 30 min were used. The media temperature was kept at 37°C Then, 5 ml aliquots were withdrawn at 10, 20, 30, 45, and 60 min and replaced with 5 mL fresh media. Drug content was analyzed by Shimadzu UV spectrophotometer at 243 nm. For dissolution study of each lubricant and concentration, a compression force of 7–8 kN was used, as tablets compressed at 7–8 kN were neither soft nor overcompressed.
RESULTS AND DISCUSSION
Evaluation of Lubricants Using Hydrophobic Lubricants
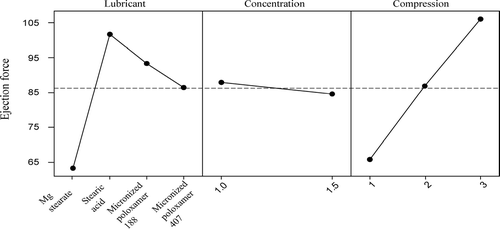
shows effect of compression force on various tablet parameters for two concentration levels. It is evident from this table that an increase in compression force results in a reduction of tablet thickness, an increase of tablet disintegration time, an increase in tablet hardness, and an increase in tablet ejection force. These results are quite similar to the findings of other researchers (Bolhuis et al. Citation1975; Bowden Citation1958). reveals the results of an ANOVA analysis for caffeine formulation. We can see from this table that lubricants, lubricant concentration, and tablet compression forces all have a significant effect (p value < 0.05) on tablet ejection force. Even the 2-way and 3-way interactions among these factors are significant. Since lubricant type is the factor of interest, further analysis using Tukey simultaneous test (all pair-wise comparison among the lubricant type) has shown () that all four types of lubricants have different effects from each other (p value < 0.05).
TABLE 1 Effect of lubricant type, lubricant concentration, and tablet compression force on tablet ejection force, tablet hardness, thickness, friability, and disintegration time
TABLE 2 ANOVA Comparison with insoluble lubricants (General linear model: ejection force versus lubricant, concentration, compression force)
TABLE 3 P-value obtained from Tukey pair-wise comparison test, comparison with insoluble lubricants, response variable ejection force, and all pair wise comparisons among levels of lubricant
shows that all the caffeine formulations have high-quality compressibility value (Carr's index value) below 24, indicating a good compressibility and fair flow property of the blends (Lachman, Lieberman, and Kanig Citation1986).
TABLE 4 Caffeine formulation bulk density, compressibility value for blends made with different lubricants and concentrations.
is a typical main effect plot, indicating the effect of lubricant type, concentration, and compression force on tablet ejection force. Magnesium stearate has the lowest ejection force followed by MP 407, MP 188, and stearic acid. Moreover, with an increase in lubricant concentration a reduction in the ejection force can be observed. Higher percentage of lubricant in formulation may reduce the friction between the tablet and sidewall of die as well as the friction reduction of punches and tablets. As a result, an increase in concentration of lubricant may decrease the ejection force (Miller and York Citation1988; Bowden Citation1958).
From , it may be concluded that an increase in concentration of magnesium stearate has a negative effect on tablet hardness. Bolhuis et al. (Citation1975) have reported a similar observation in their report. With micronized poloxamers, there is no such negative effect on tablet hardness. However, with increasing concentrations of lubricant a nagging effect on disintegration time of tablets was seen in all cases. Tablets made with magnesium stearate demonstrate a marked increase in disintegration time known to be due to its hydrophobic nature. The increase in disintegration time for tablets with micronized poloxamers is probably due to the binding effect of these poloxamers. All the batches of tablets had weight uniformity consistence with the US Pharmacopoeia (2002) requirements. The dissolution tests were conducted according to the USP method. All the batches passed the USP dissolution test.
Evaluation of Lubricants using Water-Soluble Lubricants
The statistical analysis data for the water-soluble lubricants are is shown in . We can confirm that lubricant type, lubricant concentration, and compression force all have a significant effect on ejection force (p value < 0.05). From Tukey pair-wise comparison test, it may be concluded that all the three lubricants (1-leucine, MP 188, and MP 407) have significantly different lubrication activity from each other. Interaction plot is an important tool for conclusions, as it has the ability to give discriminative analysis of results for each factor at different levels. The interaction plot () shows that MP 407 gives better lubrication effect than 1-leucine and MP 188 at all factorial levels. Higher number of ethylene oxide units may be responsible for the better lubrication effect of MP 407. The MP 188 gives better lubrication in comparison to the 1-leucine, particularly, at higher concentrations. At 1% concentration, MP 188 and 1-leucine performed equally in terms of lubricant effectiveness. However, MP 188 and MP 407 gave a better tablet hardness than spray-dried 1-leucine at all lubrication concentrations, which may be attributed to their strong binding nature. But, unlike other lubricants, spray-dried 1-leucine has no negative effect on tablet hardness with an increase in concentration. Spray-dried 1-leucine may have a high amount of air entrapped in the particles which in turn may be responsible for lower hardness (Roscheisen and Schmidt Citation1996b).
FIGURE 2 Interaction among different factors and their effect on ejection force for water-soluble lubricant comparison study.
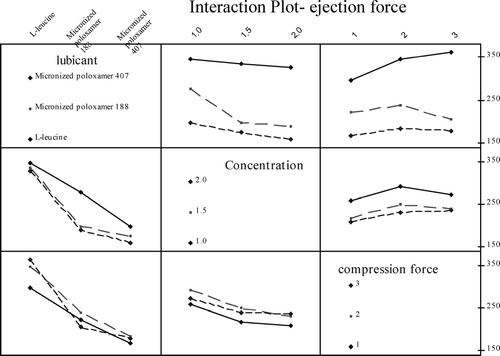
TABLE 5 P-values obtained from ANOVA for water-soluble lubricant comparison experiments general linear model: ejection force versus lubricant, concentration, compression force
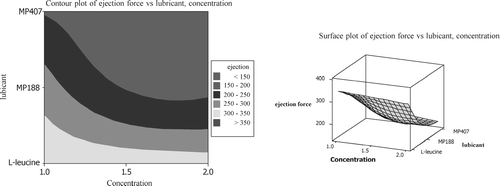
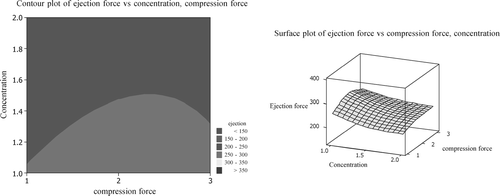
Contour plots and wire-frame plots ( and ) can be used to assist in the selection of lubricant and optimum lubricant concentration for a particular formulation. As per the surface plot, for caffeine formulation, MP 407 at 2% concentration may give better lubrication effect as the surface height is lowest for this combination of lubricant type and concentration. Lower surface height indicates better lubrication effect. The selection of compression force should be based on required tablet hardness. These water-soluble lubricants have no negative effect on disintegration time of conventional caffeine tablets. All the batches have passed USP dissolution test.
Lubricant Mixing Time
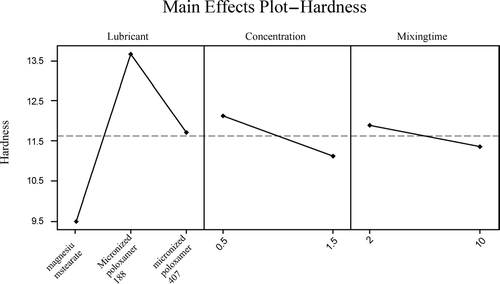
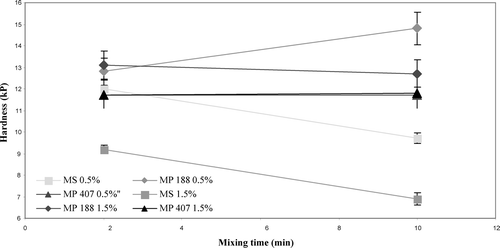
The direct compression formulation was used to assess the effect of lubricant mixing time as such formulations are more sensitive to lubricants and their overmixing effect (Shangraw Citation1989). The full factorial design was used to evaluate the effect of lubricant mixing time on tablet properties using direct compressible acetaminophen formulation with two different concentrations of lubricant: 0.5% and 1.5%. ANOVA and mail effect plot () have shown that lubricant type, concentration, and lubricant mixing time have a significant effect on tablet hardness (p value < 0.05). Magnesium stearate may have deleterious effects on tablet hardness as the lubricant mixing time is increased; the tablet hardness is decreased significantly for both lubricant concentration levels (). Even interaction plot () has shown that hardness of the tablets, made up with magnesium stearate, decreased from 12 to 9.7 kP for 0.5% and 9.9 kP to 6.7 kP for 1.5% lubricant level as the lubricant mixing time was increased, whereas there was no effect on hardness of tablets manufactured using micronized poloxamers. This finding is consistent with other published studies (Bolhuis et al. Citation1975; Khan et al. 1983).
TABLE 6 Effect of lubricant mixing time on tablet properties
These results were supported by Tukey pain-wise comparison test. From first two comparisons ( and ) are concluded that magnesium stearate has a significant effect on tablet hardness (p value < 0.05) at both 0.5 and 1.5% concentrations, whereas with MP 188 at 1.5% concentration and MP 407 at both concentration mixing time, they appeared to have no significant effects (p value > 0.05) on tablet hardness (). Mixing time appears to have no negative effect on tablet disintegration and dissolution, which contradicts other published reports (Shah and Mlodozeniec Citation1977; Murthy and Samyn Citation1977). This may be attributed to the excellent disintegration property of PVP XL (Lopez-Salys and Robles Citation2001; Kibbe, Wade, and Weller 1994; CitationCaramella et al. 1887). Our results indicate that poloxamers have no negative effect on tablet properties. Moreover, lubricant mixing time may not be a critical parameter when poloxamers are used as a lubricant.
TABLE 7 Tukey simultaneous test, response variable hardness (All pair: wise comparisons among levels of lubricant*mixing time*concentration)
Acetaminophen and Aspirin Effervescent Tablet Formulation
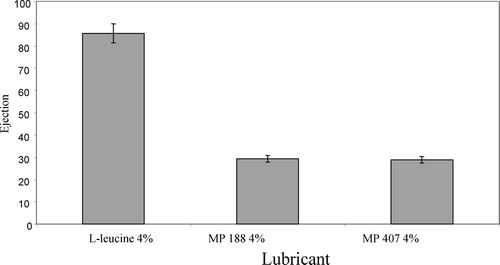
For acetaminophen formulation, lubricant type and lubricant concentration were used as input variables. Compression force was kept constant to simplify the design and reduce the number of experiments. Additional tests such as tablet hardness, disintegration time, and friability test were conducted to assess the effect of lubricant type and lubricant concentration on tablet properties. From , it may be concluded that tablets with MP 407 exert lowest ejection force among the three studied lubricants. Similar results were observed with both 2% and 4% of lubricant concentrations. It is evident from these results that in increasing the lubricant concentration, all three (l-leucine, MP 188, and MP 407) gave better lubrication efficacy. The observed results are in agreement with the observations of Röscheisen and Schmidt (Citation1995a) in which they showed that with increase in lubricant concentration, there was an improvement in lubricant effectiveness for effervescent formulation. Statistical results have shown that randomized complete block design was effective to carry out the experiments successfully, and ANOVA showed that both lubricants as well as the lubricant concentration have significant effects on the tablet ejection force.
FIGURE 8 Effect of lubricant type and lubricant concentration on tablet ejection force (acetaminophen effervescent tablet formulation).
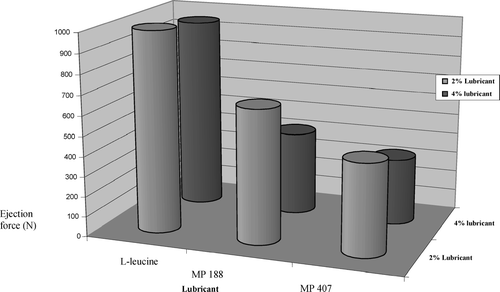
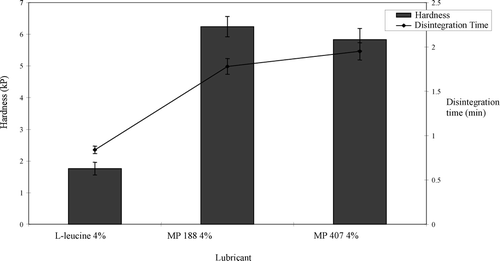
At 2%, spray-dried 1-leucine was not effective as tablet lubricant, since tablet compression was not possible due to higher ejection force at this concentration. With Korsch XL 100 tablet press, one cannot compress tablets if the tablet ejection force is higher than 1000 N as the tablet press has an internal safety feature to prevent the machine from being damaged due to excessive friction. However, tablets were compressed successfully with 2% of MP 188 or MP 407. At 4% lubrication level, it was possible to compress tablets using l-leucine, though the ejection force was very high and was near the 1000 N limit (). However, these tablets had low hardness of 1.75 kP and were not able to pass the friability test as tablets were being fragmented and powdered (). These results are in accordance with CitationRoscheisen and Schmidt, 1995b, who reported that spray-dried 1-leucine has a high amount of air entrapped in the leucine particles causing production of soft tablets. A second report by Rotthauser, Kraus, and Schmidt (Citation1998) also indicates that due to antibinding properties of l-leucine, there is a negative influence on tablet hardness.
FIGURE 9 Effect of lubricant type and lubricant concentration on tablet hardness (acetaminophen effervescent tablet formulation).
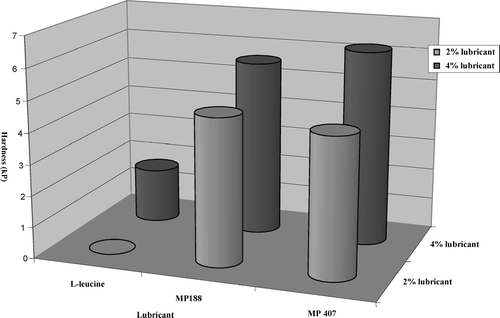
FIGURE 10 Effect of lubricant type and lubricant concentration on tablet disintegration time (acetaminophen effervescent tablet formulation).
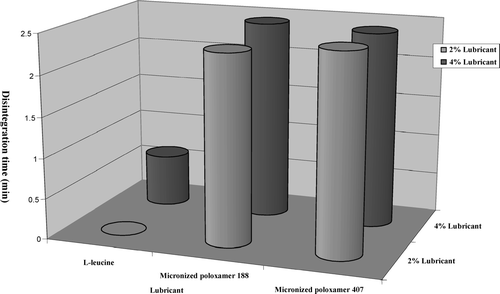
However, tablet hardness may be improved by increasing the concentration of MP 407 and MP 188 (). From the results obtained, it may be concluded that MP 188 and MP 407 have no negative effects on tablet hardness, and it may be attributed to binding properties of poloxamers. These observations are consistent with the findings of previous studies. Tablets containing MP 188 and MP 407 took longer time to disintegrate compared to tablets with l-leucine (). This phenomenon can be attributed to binding effect of poloxamers. Poloxamer with higher molecular weight has the tendency to gel; this phenomenon also may play a role in such behavior.
The aspirin effervescent formulation was evaluated using paired comparison design (Montgomery Citation2001). Lubricant concentration was kept constant at 4% and only one factor, lubricant type, was compared. Results of this formulation study seems to be similar to the acetaminophen formulation. Results were analyzed using paired t-test Hypothesis was set to test the difference between paired observations. At 4% concentration, MP 407 and MP 188 gave better lubrication than 1-leucine, which is also supported by . represents the effect of lubricants on tablet hardness and tablet disintegration time. Tablets compressed with 4% 1-leucine have hardness of 1.76 kP and failed to pass friability test as the tablets were fragmented and powdered, although all the batches were compressed at the same compression force, tablets compressed with micronized poloxamers gave better hardness and passed the friability test. These tablets have higher disintegration time as hardness and disintegration time go hand-in-hand.
CONCLUSION
Factorial design can be used successfully or evaluation of the new lubricant materials. All the factors, such as lubricant type, lubricant concentration, tablet compression force, and overall their interactions appear to have significant effects on tablet ejection force. Magnesium stearate has the best lubrication efficacy among the excipients evaluated in the preliminary study, followed by micronized poloxamer 407, micronized poloxamer 188, and stearic acid. However, magnesium stearate decreases tablet hardness with increase in lubricant concentration and/or lubricant mixing time, whereas micronized poloxamers have no negative effect on tablet hardness. Further, with new lubricants unlike other lubricants, the mixing time may not be a critical operational factor for their applications.
Study of water-soluble lubricants indicates that micronized poloxamer 188 and micronized poloxamer 407 have better lubrication properties than l-leucine. Also, they may improve the hardness of tablets. However, since they increase the disintegration time of effervescent tablets, this may be a drawback and needs additional optimized work to produce tablets with faster disintegration time. Micronized poloxamers can be used as water-soluble lubricants where water-solubility of all excipients is the priority. Further in-depth analysis of gelling and binding behavior of poloxamers is necessary to understand the behavior of these polymers in solid dosage forms.
REFERENCES
- Bolhuis G. K., Lerk C. F., Zijlstra H. T., De Boer A. H. Film formation by magnesium stearate during mixing and its effect on tabletting. Pharm, Weekbl. 1975; 110: 317–325
- Bowden F. P. A review of the friction of solids. Wear 1958; 4: 333–346
- Caramella C., Colombo P., Conte U., La Manna A. Tablet disintegration update: the dynamic approach. Drug Dev. Ind. Pharm. 1987; 13(12)2111–2145
- Delattre L., Gillard J., Jaminet F., Roland M. Etude Comparative D'agent lubricants dans les excipients pour compression directe. J. Pharma. Belg. 1976; 31: 497–508
- Garnet P., Baley G., McCurdy G., Banker G. Tablet formulation and design. Pharmaceutical Dosage Forms: Tablets, H. A. Lieberman, L. Lachman, J. B. Schwartz. Marcel Dekker, New York 1989; 1
- Granderton D. The effect of distribution of magnesium stearate on the penetration of a tablet by water. J. Pharma. Pharmaco. 1969; (21 suppl): 95–185
- Hwang R., Parrott E. Effect of a lubricant on wear rate of tablets. Drug Dev. Ind. Pharmacy 1993; 19(12)1379–1391
- Khan K. A., Musikabhimma P., Rubinstein M. H. The effect of mixing time of magnesium stearate in the tabletting properties of dried microcrystalline cellulose. Pharm, Acta Helv. 1984; 58: 109–111
- Kibb A. H., Wade A., Weller P. J. Handbook of Pharmaceutical Excipients3rd. ed., , et al. American Pharmaceutical Association, Washington, DC 2000
- Kikuta J., Kitamori N. Effect of mixing time on the lubricating properties of magnesium stearate and the final characteristics of the compressed tablets. Drug Dev. Ind. Pharmacy 1994; 20(3)343–355
- Kikuta J., Kitamori N. Friction properties of tablet lubricants. Drug Dev Ind. Pharmacy 1985; 11(4)845–854
- Lachman L., Liebermann H. A., Kanig J. L. The Theory and Practice of Industrial Pharmacy3rd. ed., , et al. Lea & Febiger, Philadelphia 1986
- Lerk C. F., Bolhuis G. K., Smallenbroek A. J., Zuurman K. Interaction of tablet disintegrants and magnesium stearate during mixing II effect on dissolution rate. Pharma, Acta Helva 1982; 57: 282–286
- Loeffler G., Ebey G. Pharmaceutical tablet compression tooling. Pharmaceutical Dosage Forms: Tablets, H. A. Lieberman, L. Lachman, J. B. Schwartz. Marcel Dekker, New York 1989; 2
- Lo'pez-Soly's J., Villafuerte-Robles L. Effect of disintegrants with different hygroscopicity on dissolution of Norfloxacin: Pharmatose DCL 11 tablets. Int. J. Pharma. 2001; 216: 127–135
- Miller T. A., York P. Pharmaceutical tablet lubrication. Int. J. Pharma. 1988; 41: 1–19
- Mohrle R. Effervescent tablets. Tablet formulation and design. Pharmaceutical Dosage Forms: Tablets, H. A. Lieberman, L. Lachman, J. B. Schwartz. Marcel Dekker, New York 1989; 1
- Montgomery D. C. Design and Analysis of Experiments, , et al. John Wiley & Sons, New York 2001
- Murthy K., Samyn J. Effect of shear mixing on in vitro drug release of capsule formulations containing lubricants. J. Pham. Sci. 1977; 66: 1215–1219
- Röscheisen G., Schmidt P. C. The combination of factorial design and simplex method in the optimization of lubricants for effervescent tablets. Eur. J. Pharm. Biopharm. 1995a; 41: 302–308
- Röscheisen G., Schmidt P. C. Preparation and optimization of 1-leucine as lubricant for effervescent tablet formulations. Pharma. Acta Helva. 1996b; 70: 133–139
- Rotthauser B., Kraus G., Schmidt P. C. Optimization of an effervescent tablet formulation containing spray dried l-leucine and polyethylene glycol 6000 as lubricants using a central composite design. Eur. J. Pharm. Biopharm. 1998; 46: 85–94
- Russo E. Typical scale-up. Pharma. Tech. 1984; 8: 46–56
- Saleh S. I., Aboutaleb A., Kassem A. A., Stamm A. Evaluation of some water soluble lubricants for direct compression. Lab. Pharma. Probl Tech. 1984; 32: 588–591
- Schmidt P. C., Christin I. Effervescent tablets—a nearly forgotten drug form. Die Pharmazie 1990; 45(2)89–101
- Sendall F., Staniforth J. N., Rees J. E., Leatham M. J. Effervescent tablets. Pharma. J. 1983; 230: 289–294
- Shah A. C., Mlodozeniec A. R. Mechanism of surface lubrication: influence of duration of lubricant-excipients mixing on processing characteristics of powders and properties of compressed tablets. J. Pharma. Sci. 1977; 66: 1377–1381
- Shangraw R. F. Compressed tablets by direct compression. In Pharmaceutical Dosage Forms: Tablets, H. A. Lieberman, L. Lachman, J. B. Schwartz. Marcel Dekker, New York 1989; 1
- Shinozaki I. Lubricating effect of 1-leucine and l-isolucine. Yakuzaigaku 1971; 31: 232–234
- Strickland W. A., Higuchi T., Busse L. W. The physics of tablet compression 10—mechanism of action and evaluation of tablet lubricants. J. Pharma. Am. Assoc. Sci. Edn. 1960; 49: 35–40
- United States Pharamacopoeia 25/National Formulary 20, , et al. United States Pharmacopeial Convention, Rockville, MD 2002
- Van Der Watt J. G., De Villiers M. M. The effect of V-mixer scale-up on the mixing of magnesium stearate with direct compression microcrystalline cellulose. Eur. J. Pharm. Biopharm. 1997; 43: 91–94, direct compression microcrystalline cellulose. Euro. J. Pharm. Biopharm 43:91–94