Abstract
Hard gelatin capsule was coated by a cellulose acetate as a semipermeable membrane with or without castor oil and filled with propranolol hydrochloride and sorbitol as an osmotic agent. After sealing the capsule with white bees wax plug, the onset of release and dissolution rate of the drug were studied. Water penetration into the capsule from the dissolution medium increases simultaneously the osmotic and hydrostatic pressures of its content. When the hydrostatic pressure is high enough to overweigh the gravity and frictional forces of the plug, the expulsion of the plug occurs and drug release starts. The effects of thicknesses of the membrane and plug as well as the concentrations of cellulose acetate and castor oil on the onset of drug release were presented by a polynomial model. We found that the effect of plug thickness on the onset of release is more important when the membrane is thicker. The results showed that the presence of caster oil in coating formulation (cellulose acetate 10% or 15%) increased the onset of release (to values). The onset of release varied from 0.6 to 10.5 hr among which the onset times of 4.2, 4.8, 5.9, 5.5, 7.5, 5.0, 7.8 and 10.5 hr could be of use for either chronotherapeutic purposes in protection of patients against heart attacks and strokes during early morning hours or reducing daily frequency of dosage.
Propranolol, a beta blocker, is used in the management of several cardiovascular diseases including hypertension, angina pectoris, myocardial infarction, various cardiac arrhythmias, and hypertrophic cardiomyopathy. The biological half-life of propranolol is relatively short (3–6 hr) and for this reason depending on the kind of disease and patient conditions it is administered twice to four times daily (Stavropoulou, Papadkostaki, and Sanopoulou Citation2005). To reduce the frequency of daily dosage, decrease drug side effects, increase efficacy, and enhance patient compliance several attempts have been made to sustain and/or extend propranolol release. The different delivery systems include pellets (Bodea and Leucuta Citation1998), matrices prepared with various materials (Huang et al. Citation2004a, Citation2004b; Sadeghi, Afrasiabi-Garekani, and Goli Citation2004; Nokhodchi et al. Citation2002; Takka, Rajbhandari, and Sakr Citation2001; Ueda et al. Citation1989), osmotic pump (Mohammadi Samani et al. Citation2000), microspheres (Varshosaz, and Keihanfar Citation2001), and beads (Dabbagh et al. Citation1999; Rekhi and Jambhekar Citation1996). In fact propranolol beads and spheroids are commercially available (Rekhi and Jambhekar Citation1996; Prisant and Elliott Citation2003).
One of the main disadvantages associated with most sustained-release delivery systems is delayed attainment of pharmacodynamic effect upon initiation of therapy (Prisant and Elliott Citation2003). To solve the latter problem as well as reduce dosage frequency, it would be desirable to administer a conventional dosage form simultaneously with a system that can deliver the drug after a predetermined onset of release. Several approaches are employed to delay onset of drug release. Some systems contain a drug reservoir surrounded by a barrier that erodes, dissolves, or ruptures (Gosthoskar, Joshi, and Joshi Citation2004). With eroding or dissolving system a potential problem is the retardation and therefore not immediate drug release after the loss of the barrier function or a premature release seen in particular with highly water soluble drug (Sangalli et al. Citation2001).
Capsular-shaped systems are more independent of the nature of the content, and systems that consist of an insoluble capsule body with swellable (Wilding et al. Citation1998), or erodible plugs (Krogel and Bodmeier Citation1998) have been reported. Another capsular system exploits the osmosis principle to control the onset of drug release. Such a system has been the subject of some patents (Amidon and Leesman Citation1993, Citation1995, Citation1997) one of which with the active ingredient of methylphenidate has been commercialized and used in attention deficit hyperactivity disorder, (Gosthoskar et al. Citation2004). The system consists of a hard gelatin capsule coated with a semipermeable membrane housing an insoluble plug and osmotically active agent along with the drug formulation.
The aim of the present study is to design and evaluate an osmotic capsular system of a widely used drug, propranolol hydrochloride, employing osmosis principle. Since the factors influencing the onset of drug release from the system can be controlled, it has at least the following advantages. First, simultaneous administration of such an osmotic system of drug with its conventional dosage form reduces the four times (qid) and twice daily (bid) regimens to bid and once a day (od) regimens, respectively. This reduction in dosage frequency can improve patient compliance. Second, whereas there are sharp increases in heart attacks in the middle of the night and during early morning hours (Neutel and Rotenburg Citation2005; Sica, Frishman, and Manowitz Citation2003), such a situation demands considerations of diurnal progress of the disease rather than maintaining constant plasma drug level. A drug delivery system administered at bedtime, but releasing drug well after the time of administration (during morning hours), would be ideal in this case.
We designed propranolol hydrochloride delayed-release osmotic capsule and evaluated factors affecting its onset of release including thicknesses of semipermeable membrane as well as plug, concentrations of semipermeable membrane forming polymer, and a hydrophobic plasticizer. An empirical quantitative relationship was established between onset of drug release and the mentioned independent variables. Also the extent of influence of the independent variables on the onset of drug release was determined.
MATERIALS AND METHODS
Colorless transparent hard gelatin capsule size 0 (Capsuline®, Pompano Beach, FL, USA), propranolol hydrochloride (Daru Pakhsh, Tehran, Iran); and cellulose acetate (Acros Chemical, NJ, USA), were purchased.Sorbitol, castor oil, acetone, and white bees wax were purchased from Merck (Darmstadt, Germany).
Coating Polymer Solution
Cellulose acetate (CA), as a semipermeable membrane-forming polymer with or without castor oil as a plasticizer, as well as hydrophobic agent (for controlling water penetration rate to semipermeable membrane) were dissolved in acetone by stirring at room temperature to obtain the desired concentrations. The composition of semipermeable membrane-forming solutions is seen in .
TABLE 1 Composition of semipermeable membrane forming solution, number of coatings, and thicknesses of semipermeable membrane and plug
Coating of Capsule Bodies
Transparent and colorless size 0 hard gelatin capsules were coated by dip coating method at room temperature. The coated capsules were then dried for 15 min at different temperatures ranging from ∼25°C to 50°C to find an optimum temperature for obtaining smooth coat without any shrinkage. Smooth coating was formed when the temperature was 25°C. Thickness of the semi permeable film applied to the capsule could be varied by either altering the number of coats applied or by varying the concentration of the film material. Number of coats was between 2 and 6, and each coating process was carried out after drying the previous coat. Detail of numbers of coating and thicknesses is provided in .
Semipermeable Membrane Thickness
Empty capsule body was coated by the dip coating process under the condition used in this study. Then the thickness of the dried coat after peeling was measured with a digital micrometer (Mitutoyo, Kawasaki, Japan) the sensitivity of which was 0.001 mm. The thicknesses of semipermeable membrane are shown in .
Filling capsule body and Determining Propranolol Hydrochloride Content
The coated capsules were hand filled with propranolol hydrochloride powder as an active ingredient (40 mg) and sorbitol as an osmotic agent (240 mg). Five capsules were randomly chosen from each formulation and the propranolol hydrochloride contents were determined by a spectrophotometer (Shimadzu-mini-1240, Japan) at 288 nm after dissolving and appropriate dilution with distilled water using the relevant Beer's plot. The results of assay showed that the drug contents of the capsules were between 98.7–103.3% of theoretical value.
Preparation and Placement of Plug
To form the wax layer, 27 g and 17 g of white bees wax were melted in glass Petri dish with diameter of 9.5 cm and were cooled at ∼35°C. Then, the Petri dishes were carefully inversed on the open end of the capsule body and the capsule was pushed to the layer of the wax for the formation of plug.
Plug Thickness
In the present study, to investigate the effect of plug thickness on the onset of release, plugs with two different thicknesses were aimed for 2.5 mm and 3.5 mm. After the preparation of the capsules, the plug was removed from the capsule and the actual thickness of the plug was measured using digital micrometer (Mitutoyo, Kawasaki, Japan) the sensitivity of which was 0.001 mm. The plug thickness of 3 plugs was measured for each formulation; the mean and standard deviation for each formulation are listed in . ANOVA test showed no significant difference (p > 0.05) between plug thicknesses of formulations having small plug thickness (F1–F3, F7–F9, F13–F15 and F19–F21) with the mean and standard deviation being 2.457 ± 0.140 mm. Similarly, formulations with large plug thickness (F4–F6, F10–F12, F16–F18 and F22–F24), showed no significant difference in the plug thickness with mean and standard deviation being 3.655 ± 0.211 mm.
Onset of Drug Release and In Vitro Drug Release
Onset of drug release and/or lag time in drug release was measured when the plug was displaced from open end of the capsule body in the dissolution medium using a chronometer. The release tests of the prepared formulations were performed in quadruplicate using a USP 27 standard dissolution apparatus apparatus II (Erweka DT 6R, Heusenstamm, Germany). The dissolution medium was 1000 ml distillated water. The temperature was 37°C and stirring rate was 50 rpm. Samples of 4 ml were taken every 10 min from the dissolution medium and equal volume of fresh 37°C distilled water was added to the dissolution vessel. Propranolol hydrochloride concentration was assayed spectrophotometrically as mentioned above.
Statistical Analysis
The statistical analyses were performed via ANOVA and Tukey's multiple range tests to detect significant differences among mean values of variables using SPSS version 11.5 package. The same package also was used for multiple linear regression.
RESULTS AND DISCUSSION
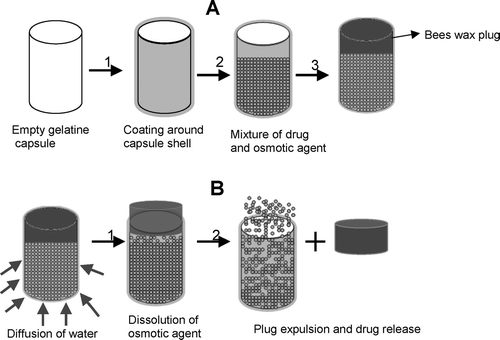
illustrates the key steps involved in the preparation and release of propranolol hydrochloride osmotic capsule with controlled onset of release. These are semipermeable coating, filling of ingredients, placement of plug, dissolution of the osmotic agent due to water diffusion from the sink, simultaneous build up of osmotic and hydrostatic pressures inside the capsule, plug expulsion because of hydrostatic pressure, and start of drug release.
FIGURE 1 Key steps involved in formulation and release of propranolol hydrochloride osmotic capsular system. A(1) Semipermeable membrane coating; (2) drug and osmotic agent filling; (3) plug placement. B(1) Diffusion of water from the sink, dissolution of the osmotic agent, simultaneous build up of osmotic, and hydrostatic pressures inside the capsule; (2) plug expulsion and start of drug release.
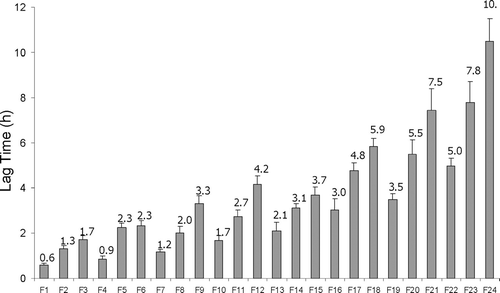
The mean values of onset of drug release (lag time in drug release), tos for all 24 formulations are shown in . Depending on the formulations, the mean to values varied between 0.6 and 10.5 hr. Formulations F12, F17, F18, F20–F24 with to values of 4.2, 4.8, 5.9, 5.5, 7.5, 5.0, 7.8, and 10.5 hr can have important clinical applications. For example if F12 (to = 4.2 hr), F18 (to = 5.9 hr), and F24 (to = 10.5 hr) are administered simultaneously with their corresponding conventional dosage forms, the six, four and two times daily dosage frequencies are reduced to three, twice, and once a day regimens. The reduction of dosage frequency improves patient compliance that in turn can affect the outcome of drug therapy.
FIGURE 2 Histograms representing times of release onset (release lag time) for different formulations. Each time is average of four experiments and the vertical bars indicate standard deviations.
There is increasing evidence that hypertension crisis accompanying increased incidence of myocardial infarction and stroke occur during the morning hours. These situations make a chronotherapeutically administered beta blocker, e.g. propranolol hydrochloride, of vital importance for protection of the patient against the mentioned diseases (Neutel and Rotenberg Citation2005; Sica et al. Citation2003). In such situations the mentioned formulations can be either administered alone or co-administered with a conventional dosage form at the bedtime in the evening to protect the patient against heart attack and stroke because these formulations can release the drug in the critical morning hours.
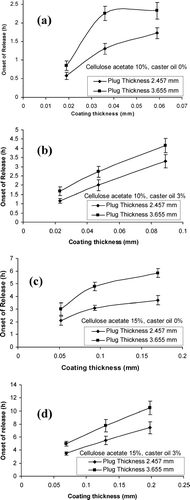
To discuss the effect of membrane thickness on the onset of release, the to data were plotted against membrane thickness for different formulations as shown in . The figure shows that the thickness of plug and the membrane both have significant effect on the onset of drug release; the higher the thickness of membrane and the plug, the longer the to. Comparison of the graphs in shows that the presence of caster oil in coating formulation (cellulose acetate 10% or 15%) increased the to values (compare with and with ).
FIGURE 3 The effect of bees wax plug thickness on the release lag time for different concentrations of cellulose acetate and caster oil.
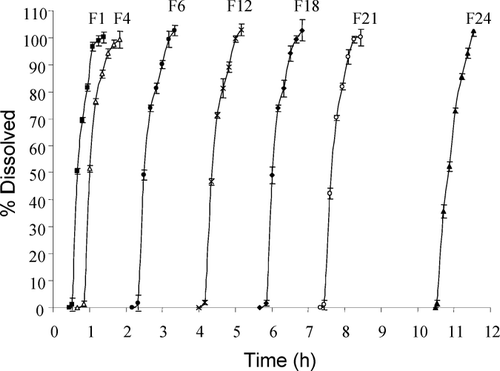
shows the mean dissolution profiles of propranolol hydrochloride osmotic capsules. For the sake of clarity data points before the expulsion of the plug have not been drawn in the figure and only the release profiles of 7 formulations have been shown in . Since the ingredients of all formulations (drug plus sorbitol) are the same and the dissolution rates of the drug are nearly equal as the time required for 100% dissolution is approximately 1 hr for all of them.
FIGURE 4 Dissolution curves of propranolol hydrochloride for formulations F1, F4, F6, F12, F18, F21, and F24 osmotic capsules. Each point is mean of four tests and error bars represent standard deviations.
When the capsular osmotic system is placed in the dissolution apparatus, water molecules penetrate into the capsule and dissolve the osmotic agent and a concentrated solution of the agent is formed inside the capsule. As a result, the chemical potential of water molecules in the solution inside the capsule is much less than outside the capsule. In other words a chemical potential gradient is created between the two sides. This chemical potential gradient increases the osmotic pressure and causes influx of water into the capsule accompanied by hydrostatic pressure build up inside it. When the hydraulic (hydrostatic) pressure inside the capsule is high enough to overcome the gravity and frictional forces of the plug, the expulsion of the plug occurs and the drug release starts. The events are schematically illustrated in . It is obvious that at the moment of the plug expulsion both hydraulic and osmotic pressures are equal. The osmotic pressure, π, at 37°C (T = 310 K) inside the capsule would be roughly 55 atm (1 atm = 101325 Pa) and 58 atm for the capsules sealed with the plug thicknesses of 0.2457 (F1) and 0.3655 (F18) cm, respectively, as calculated by the van't Hoff equation (Sinko Citation2006):
where n is the molarity of sorbitol in solution occupied the available volume inside the capsule and R = 0.0821 L.atm/K.mole. For the capsule with plug thickness 0.3655 cm, the available volume for sorbitol solution is 0.68 − [3.14 × (0.6/2)2× 0.3655] = 0.58 cm3, where 0.68 is the volume of capsule size 0 in cm3and 0.6 is the diameter of the capsule in cm. The molarity of sorbitol (MW = 182 g/mole) solution in this volume would be 240/ (182 × 0.58) = 2.27. Thus the osmotic pressure for this case according to Eq.1 is π = 2.27 × 0.0821 × 310 = 58 atm. A similar calculation in the case of 0.2457 cm plug thickness would result in 55 atm. The contribution of propranolol hydrochloride to the osmotic pressure is ignored because its amount (40 mg vs 240 mg) and water solubility (1g/20 cm3 vs 1g/0.45 cm3) are much less than that of sorbitol (USP 2005; Lund Citation1994).
To the best of our knowledge the mathematics of osmotic capsular delayed-release systems has not been elucidated in literature. However, intuitively, the onset of drug release, to, is directly related to thicknesses of semipermeable membrane, mt, and plug, pt, to the concentrations of the hydrophobic plasticizer, CO, as well as semipermeable membrane polymer, CA, provided that effective surface area of the capsule is kept constant with the dissolution media. Since the same capsule size has been used in this work, the latter variable is constant. The following polynomial empirical quantitative relation is obtained between the individual to values of the seven formulations whose dissolution profiles are seen (see ) and the mentioned independent variables after performing multiple linear regression analysis:
The goodness of fit of experimental data to equation 2 is evident from high squared correlation coefficient, r2, and low probability level, p The figures inside parenthesis represent the standard errors of coefficients and n is the number of individual data. The accuracy and prediction ability of this equation has been verified by calculating overall percent error (OPE):
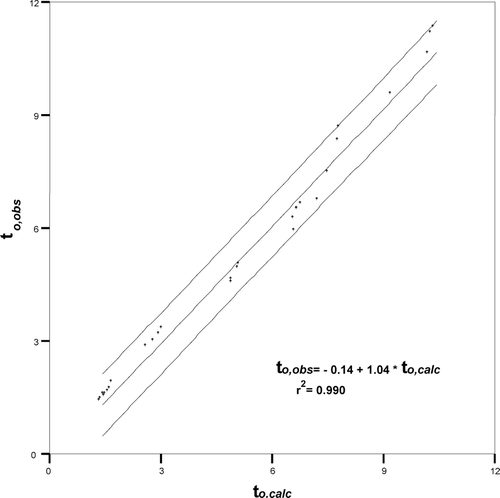
to,calc is the theoretically calculated value of to for each formulation after inserting its independent variables in equation 2, and to,obs is the experimental value of to. OPE value is 6.7 indicating the accuracy of the proposed empirical model (equation 2). In addition to OPE, the suitability of the model is evident from a good linear relationship between to,obs and to,calc (). Such a model can be of benefit in predicting to for any numerical variable(s) in the equation. The positive signs of the coefficients of the independent variables indicate a direct relationship between onset of drug release and the variables. The higher the values of the variables, the longer is the onset of drug release.
FIGURE 5 Linear plot of the experimental value of to, to,obs versus theoretically calculated value of to, to,calc by Equation 2. The side lines are 95% prediction interval plots.
To assess the contribution of the four independent variables appearing in equation 2 on the value of to, the standardized coefficients of the variables also were calculated using SPSS statistical package. These were 0.782, 0.177, 0.141, and 0.075 for log mt, CO, log pt, and log CA, respectively. Thus, the effect of semipermeable membrane thickness on the onset of release is the greatest followed by concentration of caster oil in membrane forming solution, plug thickness, and concentration of cellulose acetate in membrane.
A more detailed inspection of the results ( and ) reveals that the effect of plug thickness on the onset of release is more important when the membrane is thicker. For example, comparison of F1 and F4 with equally small mt value of 0.019 mm having plug thickness of 2.338 and 3.457 mm indicates that the corresponding to values (0.6 and 0.9 hr for F1 and F4, respectively) are not significantly affected by the plug thicknesses. On the other hand, formulations with thicker membranes (F2 and F5), a substantial difference is observed between the t0 of the thin and the thick plug formulation (compare t0 values of F2 and F5 formulations with membrane thickness of 0.036, F15 and F18 formulations with membrane thickness of 0.17 mm, and F21 and F24 formulations with membrane thickness of 0.19 mm). These observations imply that when the membrane is thin rapid influx of water into the capsule outweighs the effect of difference in pt on the release onset.
The presence of hydrophobic castor oil in the membrane as well as thicker membrane can be accounted for longer to of F12 in comparison to that of F6. The higher concentrations of CA in the semipermeable membrane as well as thicker membrane are the reasons for longer to of F18 with respect to F12. We should mention that in these two latter cases, in spite of absence of castor oil in the membrane of F18, the prominent role of log mt, the corresponding standardized coefficient (SC) of 0.872 together with that of log CA which is 0.075 can overweigh the effect of castor oil with SC of 0.177. Comparing F18 and F21, although F18 has thicker plug than F21, the presence of caster oil and thicker membrane in F21 caused longer to for F21 (7.5 hr) than F18 (5.9 hr). This clearly is the result of an opposing effect of SC values of log mt and caster oil (CO) against SC of log pt. In F21 and F24 in which all independent variables are constant with the exception of the plug thickness, the thicker plug in F24 is the main reason for its longer to.
CONCLUSION
The study presented preparation and characterization of propranolol osmotic capsules exhibiting chronopharmacological behavior. The osmotic capsules offer a solution for controlled delivery of propranolol, eliminating the necessity of nighttime dosing. It we showed that by changing the plasticizer concentration, membrane, and the plug thicknesses, the onset of release could be altered for drug delivery, purposes. Pulsatile drug release over a period of 4.2–10.5 hr, consistent with the requirements for chronopharmaceutical drug delivery, was achieved from gelatin capsules coated with cellulose acetate, in which propranolol and sorbitol powder were sealed by means of a suitable bees wax plug. Furthermore, effect of the formulation factors was investigated and discussed quantitatively.
REFERENCES
- Amidon G. L., Leesman G. D. Pulsatile drug delivery system. U.S. Patent 5229131, 1993
- Amidon G. L., Leesman G. D. Multi-stage drug delivery system. U.S. Patent 5387421, 1995
- Amidon G. L., Leesman G. D. Method for making a multi-stage drug delivery system. U.S. Patent 5674530, 1997
- Bodea A., Leucuta S. E. Optimization of propranolol hydrochloride sustained-release pellets using box-behnken design and desirability function. Drug Dev. Ind. Pharm. 1998; 24: 145–155
- Dabbagh M. A., Ford J. L., Rubinstein M. H., Hogan J. E., Rajabi Siahboomi A. R. Release of propranolol hydrochloride from matrix tablets containing sodium carboxymethylcellulose and hydroxypropylmethylcellulose. Pharm. Dev. Technol. 1999; 4: 313–324
- Gosthoskar A. V., Joshi A. M., Joshi N. H. Pulsatile drug delivery systems: a review. Drug Del. Tech. 2004; 4: 1–8
- Huang Y. B., Tsai Y. H., Yang W. C., Chang J. S., Wu P. C. Optimization of sustained-release propranolol dosage form using factorial design and response surface methodology. Biol. Pharm. Bull. 2004a; 27: 1626–1629
- Huang Y. B., Tsai Y. H., Yang W. C., Chang J. S., Wu P. C., Takayama K. Once-daily propranolol extended-release tablet dosage form: formulation design and in vitro/in vivo investigation. Eur. J. Pharm. Biopharm. 2004b; 58: 607–614
- Krogel R., Bodmeier R. Pulsatile drug release from an insoluble capsule body controlled by an erodible plug. Pharm. Res. 1998; 15: 474–481
- Lund W. The Pharmaceutical Codex, , et al. The Pharmaceutical Press, London 1994; 1025–1029
- Mohammadi Samani S, Adrangui M., Siahi-Shadbad M. R., Nokhodchi A. An approach to controlled-release dosage form of propranolol hydrochloride. Drug Dev. Ind. Pharm. 2000; 26: 91–94
- Neutel J. M., Rotenberg K. Comparison of a chronotherapeutically administered beta blocker vs. a traditionally administered beta blocker in patients with hypertension. J. Clin. Hypertens. 2005; 7: 395–400
- Nokhodchi A., Norouzi-Sani S., Saeedi M. The effect of various surfactants on the release rate of propranolol hydrochloride from hydroxypropylmethylcellulose (HPMC)-Eudragit matrices. Eur. J. Pharm. Biopharm. 2002; 54: 349–356
- Prisant L. M., Elliott W. J. Drug delivery systems for treatment of systemic hypertension. Clin. Pharmacok. 2003; 42: 931–940
- Rekhi G. S., Jambhekar S. S. Bioavailability and in-vitro/in-vivo correlation for propranolol hydrochloride extended-release bead products prepared using aqueous polymeric dispersions. J. Pharm. Pharmacol. 1996; 48: 1276–1284
- Sadeghi F., Afrasiabi-Garekani H., Goli F. Tableting of Eudragit RS and propranolol hydrochloride solid dispersion: effect of particle size, compaction force, and plasticizer addition on drug release. Drug Dev. Ind. Pharm. 2004; 30: 759–766
- Sangalli M. E., Maroni A., Zerona L., Busetti C., Giordano F., Gazzaniga A. In vitro and in vivo evaluation of an oral system for time and/or site-specific drug delivery. J. Control. Rel. 2001; 73: 103–110
- Sica D., Frishman W. H., Manowitz N. Pharmacokinetics of propranolol after single and multiple dosing with sustained release propranolol or propranolol CR (innopran XL), a new chronotherapeutic formulation. Heart Dis. 2003; 5: 176–181
- Sinko P. Nonelectrolytes. Martin's Physical & Pharmacy Science15th ed., P. Sinko. Lippincott Williams and Wilkins, Philadelphia 2006; 119–141
- United States Pharmacopoeia, , et al. United States Pharmacopoeial Convention, Rockville, MD 2005
- Stavropoulou A., Papadkostaki K. G., Sanopoulou M. Experimental and theoretical study of the release kinetics of propranolol hydrochloride from PVA matrices. J. Control. Rel. 2005; 101: 313–315
- Takka S., Rajbhandari S., Sakr A. Effect of anionic polymers on the release of propranolol hydrochloride from matrix tablets. Eur. J. Pharm. Biopharm. 2001; 52: 75–82
- Ueda Y., Hata T., Yamaguchi H., Ueda S., Kotani M. Time controlled explosion system and process for preparation for the same. U.S. Patent 4871549, 1989
- Varshosaz J., Keihanfar M. Development and evaluation of sustained-release propranolol wax microspheres. J. Microencapsul. 2001; 18: 277–284
- Wilding I. R., Davis S. S., Bakhshaee M. M., Stevens H. N. E., Sparrow R. A., Brenna J. Gastrointestinal and systemic absorption of captopril from capsuled-release formulation. Pharm. Res. 1998; 9: 654–657