Abstract
Various ocular diseases like glaucoma, conjunctivitis, and dry eye syndrome require frequent drug administration. Poor ocular bioavailability of drugs (< 1%) from conventional eye drops is due mainly to the precorneal loss factors that include rapid tear turnover, nonproductive absorption, transient residence time in the cul-de-sac, and the relative impermeability of the drugs to corneal epithelial membrane. These problems may be overcome by the use of in situ gel-forming systems that are instilled as drops into the eye and undergo a sol-gel transition in the cul-de-sac. Our present work describes the formulation and evaluation of an ocular delivery system of timolol maleate based on the concept of both temperature and pH-triggered in situ gelation. Pluronic F-127 (a thermosensitive polymer) in combination with chitosan (pH-sensitive polymer also acts as permeation enhancer) was used as gelling agent. The developed formulation was characterized for various in vitro parameters e.g., clarity, gelation temperature and pH, isotonicity, sterility, rheological behavior, drug release profile, transcorneal permeation profile, and ocular irritation. Developed formulation was clear, isotonic solution, that converted into gel at temperatures above 35°C and pH 6.9–7.0. A significant higher drug transport across corneal membrane and increased ocular retention time was observed using the developed formulation. The developed system is a viable alternative to conventional eye drops for the treatment of glaucoma and various other ocular diseases.
Conventional, liquid ophthalmic formulations show low bioavailability because of constant lachrymal secretion and rapid nasolachrymal drainage. Normal drainage of an instilled dose commences immediately on instillation and is essentially completed within 5 min. Typically; ophthalmic bioavailability of only 1–10% are achieved due to the short precorneal residence times of ophthalmic solutions. Consequently, there is a need for frequent instillation of concentrated solutions to achieve the desired therapeutic affect. Moreover, systemic absorption of the drug drained through the nasolachrymal duct may result in some undesirable side effects (Maurice Citation1987; Schoenwald Citation1990; Middleton et al. Citation1990).
To overcome these problems various ophthalmic vehicles, such as suspensions, ointments, inserts, and aqueous gels, have been investigated to extend the ocular residence time of medications for topical application to the eye (Swarbrick and Boylan Citation1995; Ranade and Hollinger Citation1996). These ocular drug delivery systems offer some improvement over conventional liquid dosage forms but because of blurred vision (e.g., ointments) or lack of patient compliance (e.g., inserts), they have not been universally accepted. As a result, good ocular bioavailability following topical delivery of a drug to the eye remains a challenge yet to be resolved satisfactorily (Felt and Baeyens Citation1999; Chiou and Watanabe Citation1986).
From the point of view of patient acceptability, a liquid dosage form that can sustain drug release and remain in contact with the cornea of the eye for extended periods of time is ideal. If the precorneal residence time of a drug could be improved only modestly, say to 1 or 2 hr, then improved local bioavailability, reduced dose concentrations, less total drug, improved patient acceptability, and reduced dosing frequency may be achieved. This relatively modest but important improvement can be achieved by delivery systems based on the concept of in situ gel formation. Such delivery systems consist of phase transition systems that are instilled in a liquid form and shift to the gel or solid phase once in the cul-de-sac of the eye. The principal advantage of this formulation is the possibility of administering accurate and reproducible quantities, in contrast to already-gelled formulations, and more over promoting precorneal retention (Gurny, Ibrahim, and Buri Citation1993; Cohen et al. Citation1997).
Glaucoma is a disease complex characterized mainly by an increase in the intraocular tension that if sufficiently high and persistent leads to irreversible blindness. Timolol eye drops are used in the management of glaucoma, which causes side effects on the Gastrointestinal tract, heart, mental state, and respiratory function due to systemic absorption. In situ hydrogel forming system of this drug can provide localized effect with reduced contraindications, improved patient compliance and better therapeutic index.
Our present work describes the formulation and evaluation of an ocular delivery system based on the concept of both temperature and pH-triggered in situ gelation. Pluronic F-127 (a thermosensitive polymer) in combination with chitosan (pH-sensitive polymer also acts as permeation enhancer) was used as gelling agent. Timolol maleate, the drug frequently used for glaucoma therapy, was used as model drug to check the efficacy of the formulation.
MATERIALS AND METHODS
Timolol maleate was received as gift sample from M/s, Ven Petrochem & Pharma (India) Pvt. Ltd. (Mumbai, India). Pluronic F-127 was purchased from Sigma Chemicals (St. Louis, mo, USA). Chitosan (practical grade, 75-85% deacetylated, molecular weight 150 kDa) was obtained as kind gift from M/s, Panacea Biotec Ltd., (India). All other chemicals and solvents used were purchased from local suppliers and of analytical grade unless mentioned.
Drug-Polymer Interaction Studies
The solutions of Pluronic F-127, chitosan, and timolol maleate were prepared individually and in combinations and were autoclaved. The ultraviolet spectra were taken before and after autoclaving using double beam ultraviolet-visible spectrophotometer. Both spectra were compared for any possible change in solution content due to interactions between different ingredients.
In situ Gelling System
Different combinations of placebo formulations were developed and evaluated for gelling capacity to identify the composition suitable for use as in situ gelling system (). Chitosan was dissolved in saline solution, pH adjusted to 5.5–6.0 by 1% v/v acetic acid. Pluronic F-127 also was dissolved in normal saline. The gelling capacity was determined by placing a drop of the system in a vial containing 2 ml of artificial tear fluid freshly prepared and equilibrated at 37°C and visually assessing the gel formation, noting the time of gelation and the time taken for the gel formed to dissolve. The composition of the artificial tear fluid used was sodium chloride 0.670g, sodium bicarbonate 0.200g, calcium chloride dehydrate 0.008g, and purified water q.s. 100 g.
TABLE 1 Combinations of chitosan/ Pluronic F-127 studied
Medicated Formulation
For antiglaucoma activity timolol is prescribed as 0.25% to 0.5% w/v solution. Hence a final drug concentration of 0.25% was used in formulation. Complete formula for the developed formulation is shown in . Drug solution was prepared in normal saline and mixed with previously optimized in situ gelling system containing chitosan and Pluronic in optimum ratio. Methyl paraben in a concentration 0.1%w/v was used as preservative. Osmolarity of formulation was determined by osmometer (Fiske Associate, USA) and required quantity of sodium chloride after calculation was added to make the solution isotonic. The developed formulation was then filled in 10-ml capacity amber-colored glass vials, a cap and dropper with the teat. The formulation in its final pack was subjected to terminal sterilization by autoclaving at 121°C and 15 psi for 20 min.
TABLE 2 Ingredients of the developed in situ gel formulation
Physicochemical Characterization
Clarity and Viscosity
The clarity of the formulations after and before gelling was determined by visual examination of the formulations under light alternatively against white and black backgrounds. Viscosity of formulation was determined before and after gelation by using Brookfield's viscometer (RVT model) in the small volume adaptor at 20 rpm. Gelation was induced in formulation by raising the pH to 7.4 by addition of 1M sodium hydroxide solution ().
TABLE 3 Physicochemical properties of the developed in situ gel formulation
Gelation pH and Temperature
Formulation was taken in a beaker and 1M NaOH was added dropwise with continuous stirring. pH was checked using pH meter (Equiptronics digital pH meter) and viscosity was determined. For determination of gelation temperature, the temperature of the formulation was gradually raised by heating the container in a water bath. The change in viscosity at each temperature was recorded. The pH and temperature at which sudden change in viscosity was observed were noted as gelation pH and gelation temperature, respectively ().
In Vitro Drug Release Profile
A 1 ml aliquot of the formulation was taken in the dialysis tube (Sigma Chemicals), which was suspended in beaker at 37 ± 0.5°C containing 100 ml artificial simulated tear fluid (pH 7.4) under continuous stirring. Aliquots of medium were withdrawn at different time intervals and equal volumes of fresh media were added to replace the withdrawn samples. Withdrawn samples were filtered, diluted appropriately, and estimated for the drug content by ultraviolet spectrophotometry 294 nm. Cumulative percent drug released was calculated.
In Vitro Transcorneal Permeation Study
Goat corneas were used to study the permeation of timolol maleate across the corneal membrane. Whole eyeballs of goat were procured from a slaughter house and transported to laboratory in cold condition in normal saline maintained at 4°C. The corneas were carefully removed along with a 5–6 mm of surrounding scleral tissue and washed with cold saline. The washed corneas were kept in cold freshly prepared solution of tear buffer of pH 7.4.
The study was carried out by using modified franz diffusion chamber (designed at ocular pharmacology lab, Dr. R.P. Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi). It consisted of four cells and each cell consisted of upper and lower chamber. The upper chamber served as a donor compartment in which 100 μ l of drug solution/formulation under study was placed. The upper and lower chambers were separated by goat cornea. The lower chamber served as a receiver compartment that was infused continuously with simulated tear fluid at the rate of 20 μ l/min. The whole system was maintained at 37 ± 0.5°C. The perfusate was collected at periodic time intervals for up to 4 hr in preweighed microcentrifuge tubes and subjected to the quantification of timolol maleate by using ultraviolet at 294 nm.
Ocular Irritation Test (HET-CAM test)
For the present study, modified HET-CAM test (the Henś Egg Test or Huhner-Embroynen-Test) as reported by Velpandian et al. Citation2006 was carried out. Briefly, fertilized hen's eggs weighing between 50–60 g were obtained from poultry farm. The eggs were candled to discard the defective ones. These eggs were incubated in a humidified incubator at a temperature of 37 ± 0.5°C for 3 days. The trays containing eggs were rotated manually in a gentle manner after every 12 hr. On day 3, egg albumin (3 ml) was removed by using sterile techniques from the pointed end of the egg. The hole was sealed by 70% alcohol sterilized parafilm (American Can Company, USA) with the help of heated spatula. The eggs were kept in the equatorial position for the development of chorioallantoic membrane (CAM) away from the shell. The eggs were candled on the 5th day of incubation, and everyday thereafter nonviable embryos were removed. On 10th day a window (2× 2 cm) was made on the equator of the eggs through which formulations (0.5 ml) were instilled.
A 0.9% NaCl solution was used as a control in the present study because it is reported to be practically nonirritant. The scores were recorded according to the scoring scheme as shown in .
TABLE 4 Scoring chart for HET-CAM test
Gamma Scintigraphy
In vivo precorneal drainage of the developed formulation was assessed by gamma scintigraphy. Albino rabbits of either sex weighing 2–3 kg were used for the study. Animals were procured from the animal house of INMAS (Delhi, India) and had free access to food and water. The study was carried out under the guidelines compiled by CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Culture, Government of India, and all the study protocols were approved by local institutional animal ethics committee. Utmost care was taken to ensure that animals were treated in the most humane and ethically acceptable manner.
Timolol maleate was dissolved in normal saline and radiolabeled with Tc-99m by direct labeling method using stannous chloride as reducing agent. The radiolabeled drug solution was then mixed with other formulation ingredients in such a way that the final solution contained 0.25% w/v timolol maleate and required concentration of polymers.
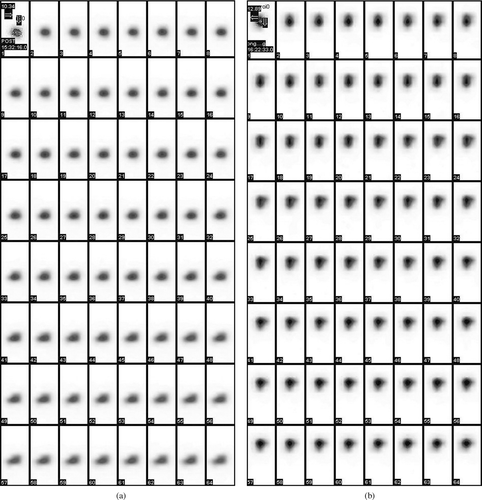
Gamma camera (Millenium VG, USA), autotuned to detect the 140 KeV radiation of Tc-99m, was used for scintigraphy study. Rabbits were anesthetized using ketamine HCl injection given intramuscularly in a dose of 15 mg/kg body weight. The rabbits were positioned under gamma camera and 25 μ l of the radiolabeled drug/formulation (equivalent to ∼100 μ ci) was instilled onto the left corneal surface of the rabbits. Recording was started 5 sec after instillation and continued for 20 min using 128 × 128 pixel matrix. Individual 68 frames (68 × 16 sec) were captured by dynamic imaging process. Region of interest (ROI) was selected on the one frame of the image and time-activity curve was plotted to calculate the rate of drainage from eye. A single whole body static image also was taken after 2 hr of instillation of drug/formulation. Each formulation was tested on 3 rabbits.
RESULTS AND DISCUSSION
Formulation and in Vitro Characterization
The interaction studies were carried out to check any possible physiochemical interaction among the formulation ingredients. Ultraviolet spectra of the ingredients before and after autoclaving were identical. No additional peak emerged or existent peak shifted that confirms two facts. First, the formulation ingredients were compatible to each other and no physicochemical reactions took place, and second the formulation can be terminally sterilized by autoclaving.
The present study used a combination of chitosan and Pluronic F-127 for the development of sustained ocular drug delivery system. Chitosan is reported to act as penetration enhancers that increase transcorneal permeation of the drug. Besides this, other properties of chitosan such as bioadhesiveness, viscous nature, and ability to convert into hydrogel at ocular pH (pH 7.4) make it the best suitable candidate for the development of this type of delivery systems (Lehr et al. Citation1992; Felt et al. Citation1998, Citation1999, Citation2000). Poloxamers, commercially available as pluronics (Desai and Blanchard Citation1998), also possess all the necessary characteristics such as good thermal gelling, nonirritation to eye, and tolerance that make them suitable for ocular administration. Poloxamers are composed of a central hydrophobic part (polyoxypropylene) surrounded by hydrophilic part (polyethylene oxide). Pluronic F-127, a very commonly used poloxomer for ophthalmic use converts into a colorless and transparent gel at temperature above 35°C. The mechanism involving the sol-to-gel transformation after an increase in temperature includes the gradual desolvation of the polymer, increased micellar aggregation, and the increased entanglement of the polymeric network (Vadnere et al. Citation1984).
Different combination of chitosan and Pluronic F-127 were prepared and evaluated for the gelling capacity and viscosity (). Gelling capacity and viscosity are the two main prerequisites of an in situ gelling system. The formulation should have an optimum viscosity that will allow easy instillation into the eye as a liquid (drops) that would undergo a rapid sol-to-gel transition (triggered by a rise in pH from 6.0 to 7.4). Additionally, the gel formed in situ should preserve its integrity without dissolving or eroding for a prolonged period of time. A concentration of 0.25% chitosan and 9.0% Pluronic F-127 was selected as it had satisfactory attributes of viscosity and gelling capacity ().
A medicated formulation was developed from selected combination of the two polymers. A 0.25% timolol was prescribed for glaucoma therapy; hence we also used the same concentration while preparing medicated in situ gel. Methylparaben at concentration 0.1% w/v was added as a preservative and NaCl was added in sufficient quantity to make formulation isotonic with eye. Final formulae of developed formulations are given in .
The developed formulations were further characterized for various physicochemical parameters (). Formulation was clear solution, isotonic to normal saline and formulation pH between 6.0–6.2. Gelation pH was found to be between 6.0–7.0 and gelation temperature was 35–37°C. Results clearly indicated that the formulation is converted into gel when the pH of the formulation is raised and resulted in sudden increases in the viscosity. Results confirm that the formulation was liquid at room temperature and at the pH formulated (pH 6.0–6.2) it underwent rapid transition into the gel phase at the pH of the tear fluid (pH 7.4) and physiological temperature (37°C). Terminal sterilization by autoclaving had no effect on the pH, gelling capacity, and viscosity of the formulation. The formulation that converted into stiff gel due to exposure to elevated temperature during autoclaving was converted into liquid again after cooling.
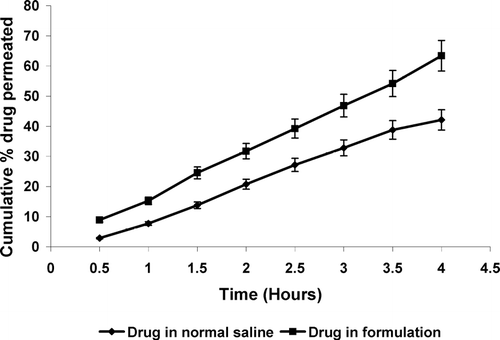
In vitro drug release profile of the formulation was determined in simulated tear fluid (pH 7.4) and the formulation displayed a slow release profile (). A cumulative drug release was 33.29 ± 1.6% after 2 hr, 79.12 ± 2.3% after 6 hr; and 98.03 ± 3.1% after 10 hr. In vitro transcorneal permeation studies also were conducted and a higher permeation across goat cornea was observed after 4 hr with developed in situ gelling system (63.41 ± 2.6%) as compared with plain drug solution (42.11 ± 2.1%) (). This can be attributed to well known transmucosal enhancer property of chitosan.
FIG. 1 In vitro release drug release profile from developed in situ gel system. Values are expressed as mean ± S.D. (n = 6)
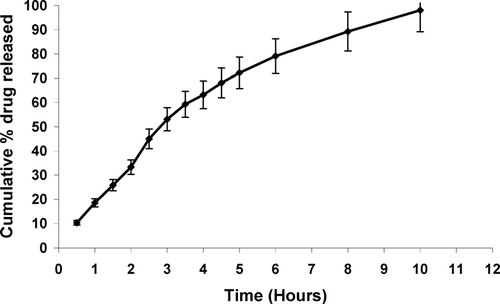
FIG. 2 In vitro drug transcorneal permeation profile from different formulations. Values are expressed as mean ± S.D. (n = 6)
For packing the formulations the amber colored glass vial, a cap and dropper with the teat were used and found to be an appropriate packaging system for current formulation. The packaging material was tested for resistance for autoclaving, leakage, and pourability. Packages passed all the tests and proved to be a good choice for packaging of present ocular formulation. Sterilization of the product was done by autoclaving at 121°C for 20 min at 15 psig and test for sterility was performed on autoclaved packaging according to IP 1996 standards. No microbial growth/microbial contamination was observed up to 14 days of incubation. Hence the formulation passed the sterility test.
Ocular Irritation Test
Ocular irritation of the developed formulations was checked by Hen's egg chorioallantoic membrane test that is a rapid, sensitive, and inexpensive test. Testing with incubated eggs is a borderline case between in vivo and in vitro systems and does not conflict with the ethical, and legal obligations. The chorioallantoic membrane of the chick embryo is a complete tissue including viens, artries and capillaries and is technically very easy to study. It responds to injury with a complete inflammatory process, a process similar to that induced in the conjuctival tissue of the rabbit eyes (Spielmann Citation1997). Developed formulation was tested by this test and results were compared with those obtained by using normal saline, which was used as control as this is supposed to be practically nonirritant. A means score of 0 was obtained for normal saline throughout the duration of the study (24 hr). Developed in situ gel formulation was nonirritant up to 1 hr (mean score 0) whereas the mean score was found to be 0.33 up to 24 hr (). It can be inferred from the results that the formulation is nonirritant to mild irritant and is well tolerated.
TABLE 5 Recorded scores in HET-CAM test
Gamma Scintigraphy
For scintigraphic studies drug was radiolabeled with radionuclide Tc-99m. It chosen because of its moderate half-life (6 hr). Further, it emits gamma rays, that have relatively low energy as compared with α and β rays, and leads to no serious health hazards for the teserrduers. Drug was instantaneously labeled with Tc-99m. Labelling efficiency was checked by instant thin layer chromatography (ITLC) using 100% acetone as mobile phase. The Rf value of free Tc is ∼ 0.9, so it reaches to the top of the ITLC strip while the complexed Tc (drug-Tc complex) cannot travel much due to difference in molecular weight and to being retained at the base of ITLC strip. Thus, from the difference in the top and bottom counts, labeling efficiency can be calculated. Various labeling parameters, e.g., SnCl2concentration and pH, were optimized and we observed that at 100 μ g SnCl2concentration and at pH 6.5 the maximum labeling efficiency (98.2%) was obtained. At these conditions minimum colloids (1.1%) were produced. In vitro stability of the labeled complex also was checked and the complex was found to be stable for up to 24 hr.
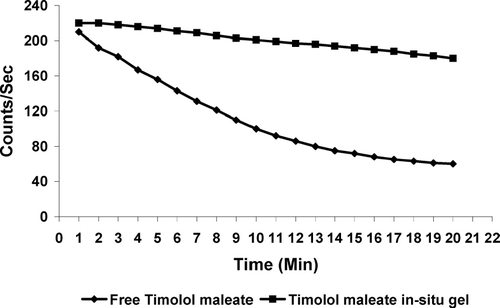
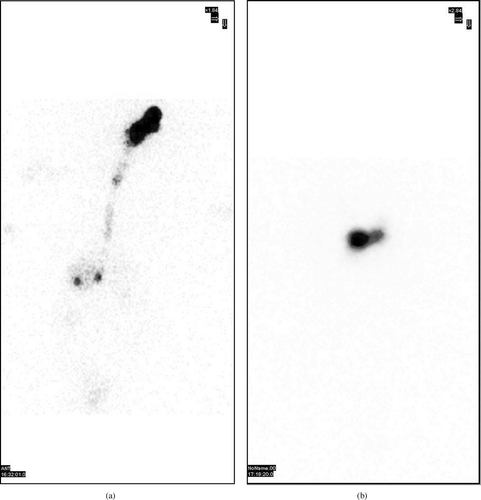
The observation of the acquired gamma camera images showed good spreading over the entire precorneal area for developed in situ gelling system () immediately after administration as compared with plain drug solution (). The curves of the remaining activity on the corneal surface as a function of time (time-activity curve) was plotted and is shown in . Plain drug solution cleared very rapidly from the corneal region and reached into systemic circulation via nasolachrymal drainage system, as significant activity was recorded in kidney and bladder after 2 hr of ocular administration (). Developed in situ gel formulation cleared at a slow rate (p < 0.05) and remained at corneal surface for longer time duration. No radioactivity was observed in systemic circulation (kidney and bladder) (). Chitosan is both viscous and bioadhesive. Further, the ocular retention was prolonged due to the gelation, that was induced within the formulation by chitosan and Pluronic as a function of both pH and temperature, respectively.
CONCLUSION
The developed chitosan/Pluronic F-127 based ophthalmic formulation of timolol maleate was nonirritant, enhanced transcorneal drug permeation, and prolonged the retention at corneal site. It is suitable for sustained topical drug delivery to eyes for rational drug therapy for glaucoma and other eye related disorders and can go up to the clinical level.
REFERENCES
- Chiou G. C. Y., Watanabe K. Drug delivery to the eyes. Methods of drug delivery: International Encyclopedia of Pharmacology and Therapeutics, G. M. Ihler. Pergamon Press, UK 1986; 203–210, section 120
- Cohen S., Lobel E., Trevgoda A., Peled Y. A novel in-situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J. Control. Rel. 1997; 44: 201–208
- Desai D., Blanchard J. In vitro evaluation of pluronic F-127 based controlled release ophthalmic delivery systems for pilocarpine. J. Pharm. Sci. 1998; 87(2)226–230
- Felt O., Baeyens V. Mucosal drug delivery: ocular. Encyclopedia of Controlled Drug Delivery, vol II. John Wiley and Sons. 1999; 605–626
- Felt O., Buri P., Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998; 24: 979–993
- Felt O., Carrel A., Baehni P., Buri P., Gurny R. Chitosan as tear substitute: a wetting agent endowed with antimicrobial efficacy. J. Ocul. Pharmacol. Ther. 2000; 16: 261–270
- Felt O., Furrer P., Mayer J. M., Plazonnet B., Buri P., Gurny R. Topical use of chitosan in ophthalmology: tolerance, assessment and evaluation of precorneal retention. Int. J. Pharm. 1999; 180: 185–193
- Gurny R., Ibrahim H., Buri P. The development and use of in situ formed gels triggered by pH. Biopharmaceutics of Ocular Drug Delivery, Y. Edman. CRC press. 1993; 81–90
- Lehr C. M., Bouwstra J. A., Schacht E. H., Junginger H. E. In vitro evaluation of mucoadhesive properties of chitosan and other natural polymers. Int. J. Pharm. 1992; 78: 43–48
- Maurice D. M. Kinetics of topically applied drugs. Ophthalmic Drug Delivery: Biopharmaceutical, Technological and Clinical Aspects, M. S. Saettone, P. Bucci, P. Speiser. Fidia Research Series, Liviana Press, Padova 1987; vol. 11: 19–26
- Middleton D. L., Leung S. S., Robinson J. R. Bioadhesive Drug Delivery Systems, V. Lenaerts, R. Gurny. CRC Press, Boca Raton, FL 1990; 179–202
- Ranade V. V., Hollinger M. A. Intranasal and ocular drug delivery. Drug delivery systems. CRC press, Boca Raton, FL 1996; 209–238
- Schoenwald R. D. Ocular drug delivery: pharmacokinetic considerations. Clin. Pharmacokinet. 1990; 18(4)255–269
- Spielmann H. Ocular irritation. In Vitro Methods in Pharmaceutical Research, J. V. Castle, M. J. Gomez. Academic Press, San dego, CA 1997; 265–287
- Swarbrick J., Boylan J. Ocular drug formulation and delivery. Encyclopedia of Pharmaceutical Technology. Marcel Dekker, Ney York 1995; 43–75
- Vadnere M., Amidon G., Lindenbaum S., Haslam J. L. Thermodynamic studies on the gel-sol transition of some pluronic polyols. Ophthalmology 1984; 28: 113–117
- Velpandian T., Bankoti R., Humayun S., Ravi A. K., Kumari S. S., Biswas N. R. Comparative evaluation of possible ocular photochemical toxicity of fluoroquinolones meant for ocular use in experimental models. Ind. J. Exp. Biol. 2006; 5: 87