Abstract
The objective of our study was to prepare and characterize basic fibroblast growth factor (bFGF)-loaded nanoparticles. Protein-loaded chitosan nanoparticles were obtained by ionotropic gelation process based on the interaction between chitosan and tripolyphosphate (TPP). The protein-loading capacity and encapsulation efficiency were 0.021% and 27.388%, respectively. The bFGF-loaded nanoparticles have a mean diameter of 424 nm, a narrow size distribution, spherical shape and positive surface charges. In vitro release showed that the extent of release was 68% at 24 hr. The protein integrity was investigated by SDS-PAGE analysis that confirmed protein integrity was not affected by the encapsulation procedure and release conditions.
Keywords :
Significant advances in biotechnology have resulted in the discovery of a large number of therapeutic and antigenic proteins (Vila et al. Citation2002). However, the in vivo application of biologically derived drugs remains limited due to problems in stability, short biological half-life, and the need to cross biological barriers and reach the target site (Babensee et al. Citation2000).
Basic fibroplast growth factor (bFGF) a protein with molecular mass of ∼18 kDa, is a potent mitogen that regulates angiogenesis during growth and development (Folkman and Shing Citation1992). It is a heparin-binding growth factor that stimulates the proliferation of a wide variety of cells, including mesenchymal, neuroectodermal, and endothelial cells (Li et al. Citation2006). bFGF is effective on protecting neurons from all kinds of insults such as glutamate exitotoxicity, ischemia, hypoglycemia, intracellular overloaded calcium ion, nitrogen, monoxide, and free radicals (Liu et al. Citation2006). However, bFGF has minimal pharmacological effects in the central nervous system in the absence of blood-brain barrier (BBB) disruption. BBB transport of bFGF occurs via an absorptive-mediated transcytosis mechanism that is relatively inefficient (Song et al. Citation2002).
The biodegradable polymeric delivery systems were used to overcome the shortcomings of bFGF therapy (i.e., susceptibility to enzymatic and thermal degradation, short duration of retention at wound sites, and the lack of ideal delivery vehicles) such as poly(lactic-co-glycolic acid) (PLGA) microspheres, gelatin nanoparticles and microspheres, and chitosan (CS) hydrogels (Andreopoulosa and Persauda Citation2006, Hiraoka et al. Citation2006, Cetin et al. Citation2005; Inoue et al. Citation2006; Obara et al. Citation2005).
Drug delivery to the central nervous system (CNS) is one of the most challenging fields of research and development for pharmaceutical and biotechnology products, which do not cross the BBB after systemic administration. The use of nanocarriers, such as liposomes and solid polymeric or lipid nanoparticles, may be advantageous over the current strategies. These nanocarriers cannot only mask the BBB limiting characteristics of the therapeutic drug molecule, but also may protect the drug from chemical/enzymatic degradation and also provide the opportunity for sustained release characteristics (Tiwari and Amiji Citation2006). The BBB is a diffusion barrier essential for the normal function of the central nervous system. The BBB endothelial cells differ from endothelial cells in the rest of the body by the absence of fenestrations, by more extensive tight junctions, by sparse pinocytic vesicular transport (Ballabh et al. Citation2004).
CS nanoparticles are obtained by the process of ionotropic gelation based on the interaction between the negative groups of the pentasodium tripolyphosphate and the positively charged amino groups of CS. This process has been used to prepare CS nanoparticles for the delivery of peptides and proteins. Based on this information, the aim of our present work was to develop bFGF-loaded CS nanoparticles for possible targeted delivery to the CNS.
MATERIALS AND METHODS
The polymer chitosan protasan Cl 113 (MW: < 150 kD, deacetylation degree: 75–90%) was purchased from FMC Biopolymers (Norway). TPP was supplied by Sigma Chemical Co. (USA). bFGF was obtained from Chemicon Int. (Temecula, CA, USA). Ultrapure water was obtained with MilliQ equipment (Waters, USA). hFGFb ELISA Kit was purchased from BioSource International (USA). Bio-Rad Mini Protean II Electrophoresis System was supplied by Bio-Rad (USA). All other chemicals and reagents were of analytical grade.
Preparation of CS Nanoparticles
CS nanoparticles were prepared according to the ionotropic gelatio process (Aktas et al. Citation2005; Calvo et al. Citation1997). Nanoparticles were obtained upon the addition of a TPP aqueous solution (0.4 mg/ml) to a CS solution (1.75 mg/ml) containing bFGF (2.5 μ g/ml) stirred at room temperature. The formation of nanoparticles was a result of the interaction between the negative groups of TPP and the positively charged amino groups of chitosan. Nanoparticles were collected by centrifugation at 10,000 rpm on a 10 μ l glycerol bed for 1 hr and supernatants were discarded.
Characterization of Nanoparticles
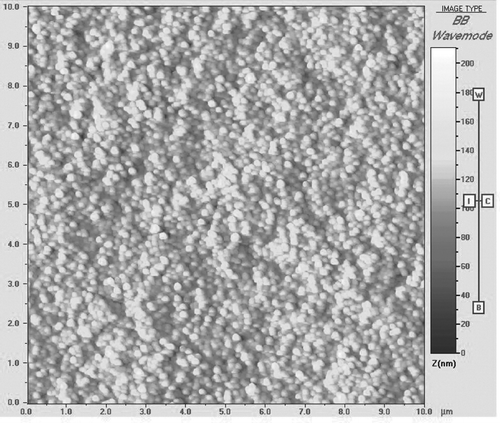
The morphological examination of the CS nanoparticles was performed using atomic force microscopy (AFM). Images of nanoparticles were taken with a Q-Scope™ 350 multiMode AFM (Quesant Instrument Corporation, Agoura Hills, CA, USA). The freshly prepared nanoparticles were centrifuged and washed three times with deionized water and later resuspended. A drop of nanoparticle suspension was placed on a glass slide, dried in air, and images were taken. AFM was performed in tapping mode using thin 230 μ m long, Si3N4 cantilevers part no. NSC16 with a nominal force constant from 25 to 60 N/m, oscillated resonant frequency ≈ 170 kHz (data from Quesant web site). During the scanning, the imaging setpoint was tried to fix at-0.39 V and scan rates were changed between 1-2 Hz.
The images have a 1024 × 1024 pixel resolution and their scan sizes were typically 10 μ m × 10 μ m and 5 μ m × 5 μ m. The microscope is enclosed in an acoustic/vibration isolation chamber that isolates microscope scanner from building vibrations, interior acoustical noise, and the thermal drift in the probes position caused by air drafts. Images are presented without filtering. The size (Z-average mean) and zeta potential of the nanoparticles were analyzed by photon correlation spectroscopy and laser doppler anemometry, respectively, in triplicate using a Zetasizer 3000HS (Malvern Instruments, UK).
Evaluation of bFGF Encapsulation
The peptide-loaded nanoparticles were separated from the aqueous suspension medium by ultracentrifugation at 10,000 rpm and 4°C for 1 hr. The amount of free peptide was measured in the clear supernatant by an ELISA kit. In brief, the bFGF assay was a solid-phase enzyme immunoassay that employed a multiple antibody sandwich principle. During the first incubation, the bFGF bound to the antibody-coated 96-wells microtiter plate. After washing, a biotinylated monoclonal antibody specific for bFGF was added. During the second incubation, this antibody bound to the immobilized bFGF captured during the first incubation. After removal of excess second antibody, streptavidin-peroxidase was added and incubated. Then, following the washing step, the substrate (tetramethylbenzidine) solution was added that was acted upon by the bound enzyme to produce color. The reaction was stopped by acidification, and bFGF amount was measured at 450 nm in the reader (VersaMax microplate reader, Molecular Devices, USA).
The protein-loading capacity (LC) of the nanoparticles and their association efficiency (AE) were calculated according to the following equations:
In Vitro Release Studies
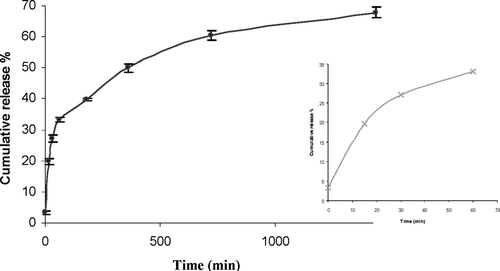
Protein release from CS nanoparticles was determined as follows: precipitated nanoparticles (1 mg) were resuspended in 1 ml of phosphate buffered saline solution (PBS) and incubated at 37°C under light agitation. At appropriate time intervals, individual samples were centrifugated and the amount of the peptide in the release medium was determined by using an ELISA kit.
Protein Integrity
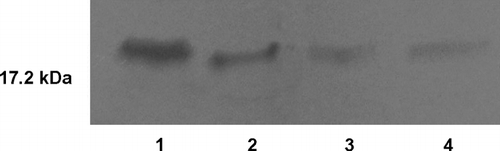
Integrity of the encapsulated protein after the release study was analyzed by SDS-PAGE. Released samples were removed and lyophilized for concentrating the protein. Then the protein samples were reconstituted in PBS. The bFGF samples and native bFGF were loaded onto 12% acrylamide gel and run using the Bio-Rad Mini Protean II electrophoresis system. The bands were visualized by silver staining (Blum et al. Citation1987).
RESULTS AND DISCUSSION
The CS nanoparticles were prepared by ionotropic gelation of cationic CS with TPP anions. This method involves the mixing of two aqueous solutions at room temperature. The mild conditions are suitable for preparation of protein-loaded nanoparticles (Amidi et al. Citation2006). The CS potentiated the FGF-2 activity and substantially prolonged its biological half-life perhaps by protecting it from inactivations by the interaction between FGF-2 and CS molecules (Masuoka et al. Citation2005).
AFM is used to observe the three-dimensional structures, which is capable of resolving surface details down to the atomic level and acquiring morphological images in high resolution. CS nanoparticles were formed spontaneously upon the incoporation of TPP and CS solution under magnetic stirring. AFM images revealed that the nanoparticles were in approximately spherical shape (). As seen in , the particle size of nanoparticles ranged from 301 to 424 nm. The image of the nanoparticles observed by AFM display lower values of diameter measured by particle size analyzer due to washing and drying of the sample suspension.
TABLE 1 Mean particle size and zeta potential of chitosan nanoparticles and bFGF-loaded chitosan nanoparticles
CS nanoparticles and bFGF-loaded have a positive zeta-potential (). Being a positive charged molecule, the bFGF addition resulted in increase in the zeta-potential values in comparison with the blank nanoparticles. CS nanoparticles displayed a low bFGF-association efficiency (27.388%) leading to final bFGF-loading values as low as 0.021%. These values are particularly low due to the positive charges of both bFGF (isoelectric point: 9.6) and CS at about pH 5.4 (). Because bFGF exerts its biological activity in the concentration range of 0.1 to 10.0 ng/mL, the bFGF loading is acceptable.
displays the release profiles of bFGF from the nanoparticles in sink conditions. In vitro release studies showed that around 30% of the loaded protein was immediately released into PBS. These results showed that some protein was localized on the surface of the nanoparticles and was loosely bound to surface. The highest extent of release (68% at 24 hr) was observed. These results may be explained again by a possible complex formation between CS and the entire amount of the protein loaded. The protein could be associated to the nanoparticles in three different states: at the nanoparticle surface, in the core as a reversible complex with CS, or in the core as an irreversible complex with CS. Size and repartition of the protein inside nanoparticles influence the release rate.
shows a representative SDS-PAGE gel of the bFGF samples. It we showed that released from the nanoparticles in vitro was predominantly intact. Our results showed that the integrity of the encapsulated bFGF was not affected by the entrapment procedure and release conditions. Thus, we assumed that the confomational stability is conserved in the nanoparticles.
CONCLUSION
Chitosan film, hydrogel, and fleeces containing bFGF were reported at the previous studies. In this study, we prepared bFGF-loaded CS nanoparticles that have not been reported yet. SDS-PAGE analysis confirmed that the structural integrity of bFGF was not altered following its entrapment in nanoparticles. This work can be considered for further studies on biological activity in cell line and in vivo it presents a promising step for possible targeting to the brain.
This project was supported by the Hacettepe University Research Fund (project no. 0202301008) and TUBITAK (project no. SBAG-2653).
REFERENCES
- Aktas Y., Andrieux K., Alonso M. J., Calvo P., Gursoy R. N., Couvreur P., Capan Y. Preparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitor. Int. J. Pharm. 2005; 298: 378–383
- Amidi M., Romeijn S. G., Borchard G., Junginger H. E., Hennink W. E., Jiskoot W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J. Controll. Rel. 2006; 111: 107–116
- Andreopoulosa F. M., Persauda I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials 2006; 27: 2468–2476
- Babensee J. E., Mcintire L. V., Mikos A. G. Growth factor delivery for tissue engineering. Pharm. Res. 2000; 17: 497–504
- Ballabh P., Braun A., Nedergaard M. The blood–brain barrier: an overview Structure, regulation, and clinical implications. Neurobiol. Dis. 2004; 16: 1–13
- Blum H., Beier H., Gross H. J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987; 8: 93–99
- Calvo P., Remuñán-López C., Vila-Jato J. L., Alonso M. J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997; 63: 125–132
- Cetin M., Capan Y., Vural I., Dogan A. L., Guc D., Hincal A. A., Wehrlé P., Dalkara T. Preparation and characterization of bFGF and BSA-loaded microspheres. J. Drug Del. Sci. Tech. 2005; 15: 371–375
- Folkman J., Shing Y. Angiogenesis. J. Biol. Chem. 1992; 267: 10931–10934
- Hiraoka Y., Yamashiro H., Yasuda K., Kimura Y., Inamoto T., Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006; 12: 1475–1487
- Inoue A., Takahashi K. A., Arai Y., Tonomura H., Sakao K., Saito M., Fujioka M., Fujiwara H., Tabata Y., Kubo T. The therapeutic effects of basic fibroblast growth factor contained in gelatin hydrogel microspheres on experimental osteoarthritis in the rabbit knee. Arthr. Rheum. 2006; 54: 264–270
- Li S.-H., Cai S.-X., Liu B., Ma K.-W., Wang Z.-P., Li X.-K. In vitro characteristics of poly(lactic-co-glycolic acid) microspheres incorporating gelatin particles loading basic fibroblast growth factor. Acta Pharmacologica Sinica 2006; 27: 754–759
- Liu Y., Lu J.-B., Ye Z.-R. Permeability of injured blood brain barrier for exogenous bFGF and protection mechanism of bFGF in rat brain ischemia. Neuropathology 2006; 26: 257–266
- Masuoka K., Ishihara M., Asazuma T., Hattori H., Matsui T., Takase B., Kanatani Y., Fujita M., Saito Y., Yura H., Fujikawa K., Nemoto K. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials 2005; 26: 3277–3284
- Obara K., Ishihara M., Fujita M., Kanatani Y., Hattori H., Matsui T., Takase B., Ozeki Y., Nakamura S., Ishizuka T., Tominaga S., Hiroi S., Kawai T., Maehara T. Acceleration of wound healing in healing-impaired db/db mice with a photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2. Wound Repair Regen. 2005; 13: 390–397
- Song B.-W., Vinters V. H., Wu D., Pardridge W. M. Enhanced neuroprotective effects of basic fibroblast growth factor in regional brain ischemia after conjugation to a blood-brain barrier delivery vector. J. Pharmacol. Exp. Ther. 2002; 301: 605–610
- Tiwari S. B., Amiji M. M.A. A review of nanocarrier-based CNS delivery systems. Curr. Drug Deliv. 2006; 3: 219–232
- Vila A., Sánches A., Tobio M., Calvo P., Alonso M. J. Design of biodegradable particles for protein delivery. J. Control. Rel. 2002; 78: 5–24