Abstract
Diclofenac sodium tablets consisting of core coated with two layers of swelling and rupturable coatings were prepared and evaluated as a pulsatile drug delivery system. Cores containing the drug were prepared by direct compression using microcrystalline cellulose and Ludipress as hydrophilic excipients with the ratio of 1:1. Cores were then coated sequentially with an inner swelling layer of different swellable materials; either Explotab, Croscarmellose sodium, or Starch RX 1500, and an outer rupturable layer of different levels of ethylcellulose. The effect of the nature of the swelling layer and the level of the rupturable coating on the lag time and the water uptake were investigated. Drug release rate studies were performed using USP paddle method. Results showed the dependence of the lag time and water uptake prior to tablet rupture on the nature of the swelling layer and the coating levels. Explotab showed a significant decrease in the lag time, followed by Croscarmellose sodium and finally by Starch RX 1500. Increasing the level of ethylcellulose coating retarded the diffusion of the release medium to the swelling layer and the rupture of the coat, thus prolonging the lag time.
Site-specific drug delivery systems are the main focuses of oral controlled-release solid dosage forms (Sinha et al.Citation2006). They are developed to obtain a slow delivery of drug at the beginning of the dissolution process followed by a fast release rate in definite parts of the stomach and intestine to increase local therapeutic effect (Li and Zhu Citation2002). Pulsatile drug delivery systems are characterized by two release phases. A first phase with no or little drug being released, followed by a second phase, during which the drug is released completely within a short period of time after a lag time. The release can be either time, or site-controlled (Karavas, Georgarakis, and Bikiaris Citation2006a).
Pulsatile delivery is desirable for drugs acting locally or having an absorption window in the gastrointestinal tract (Bussemer and Bodmeier Citation2001); drugs that develop biological tolerance, where the constant presence of the drug at the site of action diminishes the therapeutic effect (Bussemer, Otto, and Bodmeier Citation2001); drugs with an extensive first pass metabolism, e.g., beta-blockers (Lemmer Citation1999); or for drugs with special pharmacokinetic features designed according to the circadian rhythm of humans (Karavas, Georgarakis, and Bikiaris Citation2006b). Most pulsatile drug delivery systems are reservoir devices coated by a barrier polymeric coating (Krögel and Bodmeier Citation1999a, Citation1999b).
The coating prevents drug release from the core until the polymeric shell is completely dissolved, eroded (Karavas et al. Citation2006; Freichel and Lippold Citation2000) or ruptured (Bussemer, Dashevsky, and Bodmeier Citation2003) during/after a certain lag time, after which the drug is released rapidly from the inner reservoir core. The rupturing of the barriers are induced by an expanding core on water penetration through the barrier coating. The expansion can be caused by effervescent excipients (Krögel and Bodmeier Citation1999b) or swelling agents (Sangalli et al. Citation2001, Fan et al. Citation2001; Morita, Honda, and Takahashi Citation2000).
Diclofenac sodium is a nonsteroidal anti-inflammatory drug (NSAID) of wide use. Peptic ulcers associated with NSAID therapy represent a serious problem and sometimes limit the usefulness of these agents mainly in the chronic treatment of rheumatic diseases. Maggi et al. (Citation1993) designed press-coated tablets in which the inner coat contains diclofenac sodium and the outer shell has sucralfate to prevent NSAID-induced mucosal lesions and ulcer formation.
This study is a trial to develop pulsatile release tablets (PRTs) as a per oral, time–controlled, single-unit dosage form of diclofenac sodium to overcome or diminish its side effects. The proposed system consists of a directly compressed core tablet containing the drug and hydrophilic excipients namely, Ludipress and microcrystalline cellulose. The tablet core was then coated with two consecutive layers, an inner swelling layer and an outer rupturable coating layer. Superdisintegrants such as Croscarmellose sodium, Explotab and Starch RX 1500 were chosen as swellable materials. Ethylcellulose (EC) was used as the rupturable coating. The effect of the type of swelling layer as well as the level of the coating layer on the lag time, water uptake and drug release were investigated.
MATERIALS AND METHODS
Diclofenac sodium (Heumann Pharma GmbH, Germany). microcrystalline cellulose (Avicel PH 102, USA), Ludipress (BASF, FRG), talc (BDH, England), and colloidal silicon dioxide (Aerosil 200, Degussa, Germany) were brought. Croscarmellose sodium (Ac-Di-sol, FMC biopolymer, USA), Explotab (sodium starch glycolate, E. Mendell Co., USA), Starch RX 1500 (Colorcon, England), polyvinylpyrrolidone (PVP 90 F, BASF, Germany), ethylcellulose (EC, FMC Corp., USA), and triacetin (Alexandria Pharmaceutical Co., Egypt) were all purchased. All other reagents were of analytical grade.
Preparation of Core Tablets
The core tablets containing diclofenac sodium (100 mg/ tablet), Ludipress, and microcrystalline cellulose (in the ratio of 1:1 w/w) were prepared by direct compression. The tablet ingredients passed through 0.4 mm and retained on 0.315 mm mesh screen were mixed for 5 min in a Turbula mixer (Erweka, GmbH, Germany). A concentration of 0.5% w/w talc and 0.5% w/w aerosil 200 were added to the previous mixture and further blended for 10 min. The core tablets were compressed in a single punch tablet press (Korsch tablet press, type EKO, Erweka). The tablets were biconvex having an average diameter of about 9 mm, hardness about 10 N, thickness about 4 mm, and an average weight of 250 mg.
Coating Core Tablets
The core tablets were coated with two consecutive layers in a coating pan (Erweka). The inner swelling layer was Croscarmellose sodium, Explotab, or Starch RX 1500, whereas ethylcellulose was used as an outer rupturable coating layer. Each swelling substance was separately layered onto the core tablets using 4% w/v of an alcoholic solution of PVP as a binder. Croscarmellose sodium, Explotab, or Starch RX 1500 was then dispersed in PVP alcoholic solution at a ratio of 6:1, w/w and then stirred for 30 min using a shaft stirrer (VEB MLW Prufgerate, Germany) to obtain a homogenous dispersion prior to coating. The coating dispersion was then layered onto the core tablets in the coating pan to obtain swelling layer level of 21.5 mg/cm2.
The coating conditions were spraying rate of 10 ml/min. using an atomizing nozzle (Aeromatic AG, Muttenz-Switzerland); pan speed at 30 rpm, and inlet hot air at a temperature of 40°C. The coated tablets were further dried in the pan for 10 min with the hot air after the coating process was finished. The tablets were then placed in an oven (Despatch Oven Co., USA) at 40°C for 2 hr to remove the residual solvent. These tablets were then coated with 3.5% w/w ethanolic ethylcellulose solution, using triacetin as a plasticizer.
The plasticizer (5% w/w) was added to the polymer solution and stirred for 30 min prior to coating to obtain a homogenous solution. The plasticized polymer solution was sprayed onto the tablets in the coating pan at 40°C inlet air temperature and the same spraying rate to obtain coating levels of 1.4, 2.1, and 2.8 mg/cm2 of ethylcellulose. The tablets were placed in the oven at 40°C for at least 2 hr to remove the residual solvent. The coated tablets were stored in a closed desiccator prior to further evaluation.
Scanning Electron Microscopy (SEM)
The morphology of a cross-section of the pulsatile release tablet was observed under a SEM (Jeol JSM-5300, Japan). The dried sections of tablets were mounted onto the stages prior to coating with gold under vacuum. The pictures were taken at an excitation voltage of 25 KV and at 200 × magnification.
Rupture Test
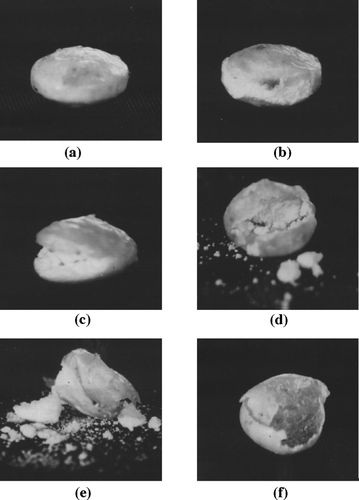
The rupture test of PRTs was determined visually using 750 ml of phosphate buffer pH 6.8, 37 ± 0.5°C, and at 50 rpm in the USP paddle dissolution apparatus (Pharma Test, type PTWS 3, USA). The rupture behavior of the tablets was monitored and photographed by a digital camera (FinePix F 401, Fuji Photo Film, Japan).
Water Uptake Study
The pulsatile release tablet was placed in a screw-caped flask containing 100 ml of phosphate buffer pH 6.8 at 37 ± °C. The flask was rotated 100 rpm in a horizontal shaker (VEB MLW Labortechnik). At predetermined time intervals, the tablet was removed from the dissolution medium and carefully blotted with tissue paper to remove surface water. The tablet was weighed with an analytical balance (Sartorius, GmbH, Germany), and then returned to the medium until the coating of the tablet ruptured. The percentage water uptake was calculated as follows:
where Wt is weight of wet tablet at time t and Wo is weight of dry tablet. The experiment was done in triplicate.
Swelling Index
The index was determined according to that reported in the literature (Wan and Lim Citation1992). Four tablets were separately weighed (m1) and each was placed into a beaker containing 6 ml of phosphate buffer, pH 6.8 at 37 ± 0.5°C. At specified time intervals, the tablets were removed and excess water on their surface was carefully absorbed using a tissue paper. Each swollen tablet was weighed (m2), and the swelling index was calculated by the formula:
The experiment was done in triplicate.
Release Rate Study
Dissolution study was conducted to determine the drug release from the PRTs using USP apparatus 2 paddles with the same conditions as in the rupture test. A 5-ml sample was withdrawn at predetermined time intervals and replaced by an equal volume of prewarmed phosphate buffer. The amount of diclofenac sodium released was assayed spectrophotometrically (Perkin Elmer, Lambda 3B, USA) at a wavelength of 276 nm. All dissolution experiments were done in triplicate. None of the excipients used affected the assay at that wavelength.
RESULTS AND DISCUSSION
SEM photographs of cross-sections of diclofenac sodium PRTs with three different swelling materials and ethylcellulose as a rupturable coating are shown in . In all the photos, the three parts of the system are clearly visible namely: the dense tablet core (Ludipress, microcrystalline cellulose, and drug); the more porous layer of the swelling ingredient; and the homogenous ethylcellulose coating as an outer rupturable coating layer. Ethylcellulose was the polymer of choice because of its water insolubility, adjustable water permeability, and its mechanical properties (Krögel and Bodmeier Citation1999b).
FIG. 1 SEM photomicrograph of a cross-section of a pulsatile release tablet with different swelling materials and a rupturable ethylcellulose coating. i = Dense tablet core layer, ii = Swelling layer, iii = Rupturable layer.
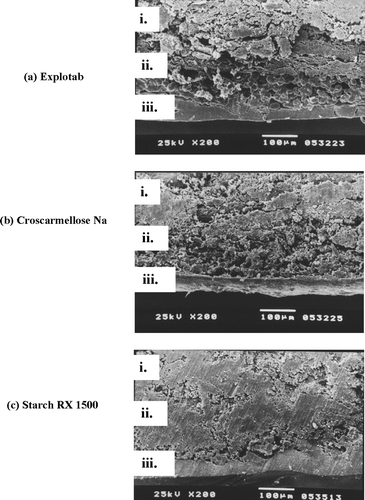
The swelling layer consisted of a superdisintegrant and PVP as a binder. The addition of PVP was necessary to facilitate the adherence of the swelling ingredient to the core surface, thus forming a continuous swelling layer. On solvent evaporation, the PVP formed a homogenous film with embedded superdisintegrant particles. The fibrous structure of the superdisintegrant was retained during the layering process, resulting in a porous structure of the swelling layer.
The type and the nature of the superdisintegrant determined the shape and the number of pores formed in the swelling layer. The Explotab-containing layer showed the highest frequency of large pores compared with those formed in the Croscarmellose sodium layer and Starch RX 1500 swelling layer that showed the least number of small pores respectively, (, , ). The swellable materials have high affinity for water and the subsequent volume expansion of the swelling agent caused the rupturing of ethylcellulose coating. The drug was then released rapidly within a short period after a certain lag time due to the strong rupturing of the coating.
The rupture test was performed to follow up the rupture sequence of diclofenac sodium PRTs (). The mechanism of membrane rupture consists of the following processes. Water is taken up by the tablet () through the outer semipermeable ethylcellulose membrane and hydrates the swelling layer causing stress against the membrane (). As soon as stress exceeds membrane strength, the membrane destruction is initiated (). The drug is released through the destructive membrane to the dissolution medium ( and ). Finally, the release of the drug was completed, leaving the evacuated coating membrane (). Ethylcellulose was reported to form a mechanically weak and semipermeable film that could rupture on exposure to the release medium and the resultant internal pressure developed within the tablet cores (Krögel and Bodmeier 1999).
The core composition could affect the swelling and rupturing behavior of the coated tablets (Krögel and Bodmeier 1999). Microcrystalline cellulose possesses a good disintegration property; therefore, it increases the disintegrating force of the core, resulting in a shorter lag time. Ludipress is highly soluble in water and has a high osmotic activity. According to this information and based on our preliminary results, the core tablet was prepared using microcrystalline cellulose and Ludipress with the ratio of 1:1 w/w and showed reproducible results.
When the coated tablet is exposed to an aqueous medium, water diffuses through the coating layers because of the activity gradient of water, hydrating the core. The solvation of the osmotic agent (Ludipress) creates a constant osmotic pressure difference between the core contents and the external environment. The hydration of the core causes the swelling of the disintegrant (microcrystalline cellulose), resulting in core disintegration. The drug is extruded through the exit ports via the hydrostatic pressure generated by the expansion of the water-swellable composition. After the drug has been delivered, the tablet shell remains intact ().
Besides the core composition, the amount and the type of swelling layer were other variables influencing the rupturing behavior. To study the swelling capacity and swelling pressure of each swelling agent needed to rupture the ethylcellulose coating layer, the amount of the swelling layer was kept constant at the level of 21.5 mg/cm2.
shows the swelling index of all formulations. Data from the table revealed that the swelling index of all formulations was higher at the lower level of ethylcellulose layer. As the coating level increased, the swelling index decreased. The PRTs lost their shape at 0.25, 0.5, and 1 hr for Explotab, Croscarmellose sodium, and Starch RX 1500 coated with 1.4 mg/cm2 of ethylcellulose layer, respectively. These results could be explained by the high water permeability of the thin coat. Because Explotab layer was highly porous, it absorbed water rapidly and the swelling pressure increased causing rapid rupturing of the coat. Croscarmellose sodium formed a less porous layer than Explotab. Thus it needed more time for absorption of water necessary for rupturing. Starch RX 1500 showed the longer rupture time due to its lower porosity compared with the other swelling agents ().
TABLE 1 Swelling Index of PRTs containing different swelling materials and coated with different ethylcellulose levels
Water uptake was investigated as a function of the ethylcellulose coating level and the type of swelling layer (, , ). The higher level of the coating layer reduced the water permeation rate and decreased the percentage water uptake of pulsatile release tablets made of the three different swellable materials.
FIG. 3 Effect of coating level on the percent water uptake of pulsatile release tablet containing Explotab.
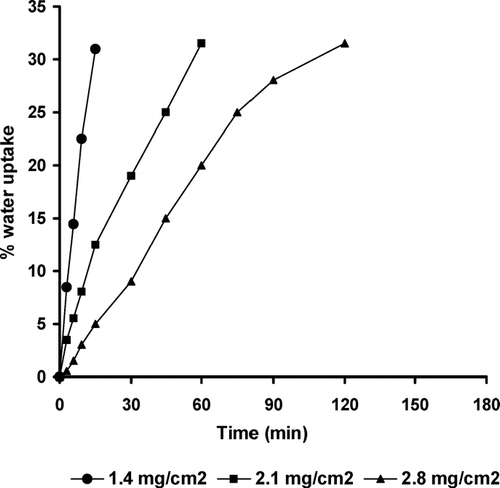
FIG. 4 Effect of coating level on the percent water uptake of pulsatile release tablet containing Croscarmellose sodium.
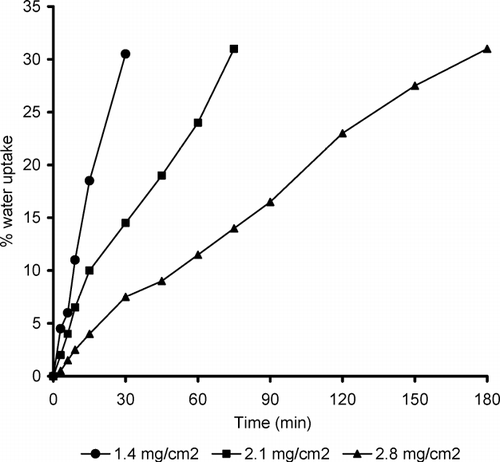
FIG. 5 Effect of coating level on the percent water uptake of pulsatile release tablet containing Starch RX 1500.
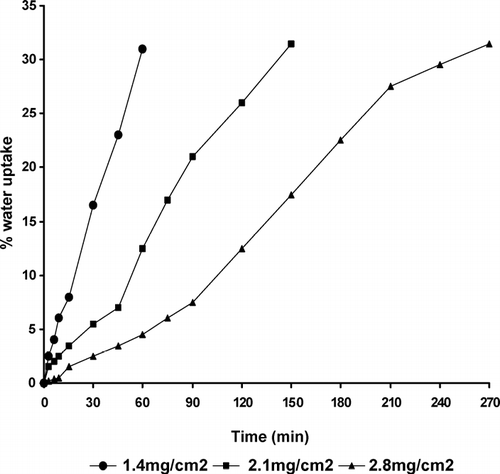
We observed that all curves showed an almost linear water uptake with time until a critical water level, at which the ethylcellulose coating ruptured. The critical water uptake level was slightly higher at higher level of ethylcellulose layer. This could be explained by the higher mechanical strength of the thicker coating, requiring a higher degree of swelling (water uptake) for rupturing (Sungthongjeen et al. Citation2004). The existence of an almost linear relation indicated that the volume of liquid penetrated into the porous body is proportional to time.
At the coating level at which the water permeation rate could be considered constant, the volume of liquid penetrated through the PRTs depends on the porosity of the swelling layer. Explotab-based PRTs showed the highest percentage of water uptake due to their highest degree of porosity, followed by Croscarmellose sodium and finally tablets containing Starch RX 1500 (, , ), respectively. We also noticed that the extent of water uptake at rupture was almost the same for the different coating levels. It ranged from ∼ 30 to 31.5% of weight gain based on the initial weight of PRT. This result was in agreement with the work done by Bussemer et al. (Citation2003).
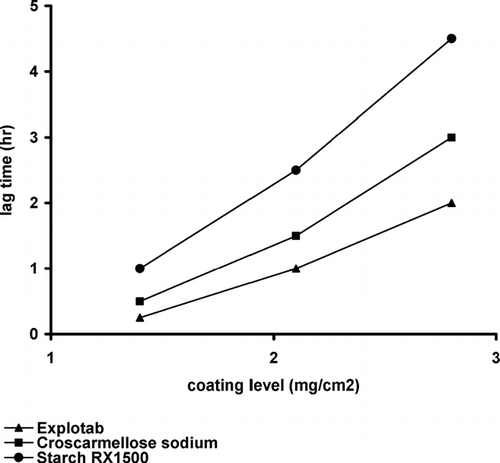
The lag time prior to tablet rupture is determined primarily by the water permeability and mechanical properties of the outer coating and the swelling behavior of the swellable materials (Bussemer et al. Citation2003). Lag time determination was carried out parallel to the drug release study. During the drug release study, the lag time was recorded for each formulation and plotted against the coating level (). Explotab shows the least values of the lag time along the three coating levels. At the coating level of 2.8 mg/cm2, the lag time was 2, 3, and 4.5 hr for Explotab, Croscarmellose sodium, and Starch RX 1500, respectively. This could be explained by an increase in the swelling pressure exerted by Explotab as it is considered an extragranular disintegrant forming a porous structure. This result was previously proved by SEM () and the swelling index. The swelling pressure of the three swellable materials could be arranged in the following order: Explotab > Croscarmellose sodium > Starch RX 1500.
Release studies were carried out to examine the pulsatile release characteristics of the prepared system. The release profiles (, , ) had a typical pulsatile shape, no drug release during the lag time, followed by a rapid and complete release phase. The lag time corresponds to the initial ingress of water into the tablet and hydration of the core. This is followed by nearly linear release of ∼ 100 % of initial drug load within a short period. With the PRT of this study, both the tablet core and the swelling layer influenced the rupturing process.
FIG. 7 Effect of coating level on the drug release from Explotab-containing pulsatile release tablets.
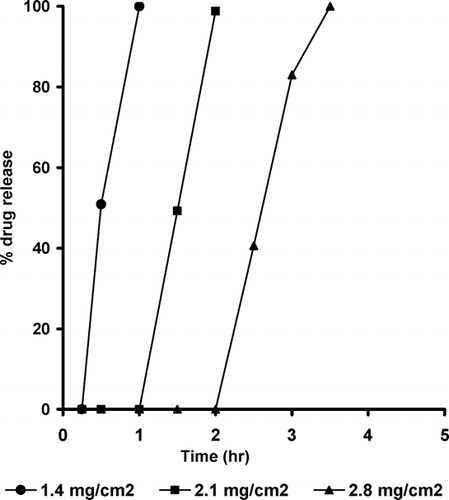
FIG. 8 Effect of coating level on the drug release from Croscarmellose sodium-containing pulsatile release tablets.
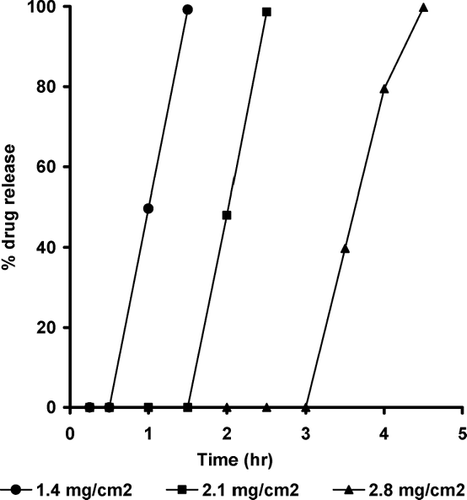
FIG. 9 Effect of coating level on the drug release from Starch RX1500-containing pulsatile release tablets.
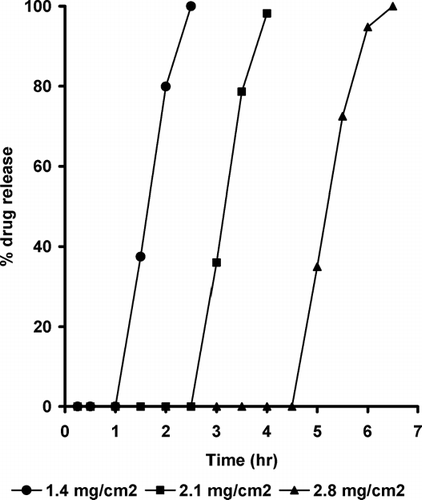
The drug release rate was directly related to the rate that water enters the tablet core, and the rate of water ingress depends on the osmotic pressure of the core and the permeability of the coating. Changing the coating level can alter the permeability of the coating; the thicker coating has lower permeability. The drug release profiles show that thicker coatings (2.8 mg/cm2) not only have slower release rates but also have longer lag times before the initiation of drug release. This can be attributed to the slower water penetration in tablets with a thicker coating, which results in a longer lag time required for sufficient water being present to fluidize the core so that it can be extruded (Thombre et al. Citation2004).
Among the components of the system, the swelling agent has the key role in drug release because the swelling force of the hydrated swelling agent destroys the outer membrane to create a lag time before release of the drug (Ueda et al. Citation1994). Explotab-based tablets showed the least lag time and higher drug release rates compared with Croscarmellose sodium- and Starch RX 1500-based tablets. At ethylcellulose coating level of 1.4 mg/cm2, the lag times were 0.25, 0.5, and 1 hr and the time for complete release of the initial loading dose were 1, 1.5, and 2.5 hr for Explotab, Croscarmellose sodium, and Starch RX 1500, respectively.
These results could be explained by the highest porosity and highest swelling index of Explotab-based tablets compared with the other swelling agents. Increasing the coating level from 1.4 to 2.8 mg/cm2 increased the lag time from 0.25 to 2 hr for Explotab based tablets. The increase in the lag time is due to the reduced water permeability and increased mechanical strength of the thicker coat. These results were in accordance with the reported results (Sungthongjeen et al. Citation2004; Efentakis, Koligliati, and Vlachou Citation2006). During the release study, the drug did not release prematurely by diffusion because it was shielded from the release medium by the outer polymer layer and the swelling layer. After complete rupture of the polymer coating, the tablet disintegrated and the drug released rapidly.
CONCLUSION
Diclofenac sodium as pulsatile release tablets with rapid release after a predetermined lag time were developed. The lag time could be adjusted and modified by varying the level of the coating layer and the type of the swelling materials.
REFERENCES
- Bussemer T., Bodmeier R. A review of pulsatile drug delivery. Am. Pharm. Rev. 2001; 4(4)18–24
- Bussemer T., Dashevsky A., Bodmeier R. A pulsatile drug delivery system based on rupturable coated hard gelatin capsules. J. Control. Rel. 2003; 93: 331–339
- Bussemer T., Otto L., Bodmeier R. Pulsatile-drug delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2001; 18(5)433–458
- Efentakis M., Koligliati S., Vlachou M. Design and evaluation of a dry coated drug delivery system with an impermeable cup, swellable top layer and pulsatile release. Int. J. Pharm. 2006; 311: 147–156
- Fan T. Y., Wei S. L., Yan W. W., Chen D. B., Li J. An investigation of pulsatile release tablets with ethylcellulose and Eudragit L as film coating materials and cross-linked polyvinylpyrrolidone in core tablets. J. Control. Rel. 2001; 77: 245–251
- Freichel O. L., Lippold B. C. A new oral erosion controlled drug delivery system with a late burst in the release profile. Eur. J. Pharm. Biopharm. 2000; 50: 345–351
- Karavas E., Georgarakis E., Bikiaris D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur. J. Pharm. Biopharm. 2006a; 64(1)115–126
- Karavas E., Georgarakis E., Bikiaris D. Felodipine nanodispersions as active core for predictable pulsatile chronotherapeutics using PVP/HPMC blends as coating layer. Int. J. Pharm. 2006b; 313(1-2)189–197
- Krögel I., Bodmeier R. Evaluation of an enzyme-containing capsular shaped pulsatile drug delivery system. Pharm. Res. 1999a; 16(9)1424–1429
- Krögel I., Bodmeier R. Floating or pulsatile drug delivery systems based on coating effervescent cores. Int. J. Pharm. 1999b; 187: 175–184
- Lemmer B. Chronopharmacokinetic: implications for drug treatment. J. Pharm. Pharmacol. 1999; 51: 887–890
- Li Y. H., Zhu J. B. Oral site-specific drug delivery system, World pharmacy-a branch of synthetic drug. Biochem. Drug Pharmac. 2002; 23(4)225–228
- Maggi L., Conte U., Giunchedi P., Colombo P. Press-coated tablets for the sequential pulsed administration of two different drugs. Int. J. Pharm. 1993; 99: 173–179
- Morita R., Honda R., Takahashi Y. Development of oral controlled release preparations, a PVA swelling controlled release system (SCRS); I. Design of SCRS and its release controlling factor. J. Control. Rel. 2000; 63: 297–304
- Sangalli M. E., Maroni A., Zema L., Busetti C., Giordano F., Gazzaniga A. In-vitro and in-vivo evaluation of an oral system for time and/or site-specific drug delivery. J. Control. Rel. 2001; 73: 103–110
- Sinha V. R., Bhinge J. R., Kumria R., Kumar M. Development of pulsatile systems for targeted drug delivery of celecoxib for prophylaxis of colorectal cancer. Drug Del. 2006; 13(3)221–225
- Sungthongjeen S., Puttipipatkhachorn S., Paeratakul O., Dashevsky A., Bodmeier R. Development of pulsatile release tablets with swelling and rupturable layers. J. Control. Rel. 2004; 95: 147–159
- Thombre A. G., Appel L. E., Chidlaw M. B., Daugherity P. D., Dumont F., Evans L. A. F., Sutton S. C. Osmotic drug delivery using swellable-core technology. J. Control. Rel. 2004; 94: 75–89
- Ueda S., Ibuki R., Kimura S., Murata S., Takahashi T., Tokunaga Y., Hata T. Development of a novel release system, time controlled explosion system (TES). III. Relation between lag time and membrane thickness. Chem. Pharm. Bull. 1994; 42(2)364–367
- Wan L. S., Lim L. Y. Drug release from heat-treated polyvinyl alcohol films. Drug Dev. Ind. Pharm. 1992; 18: 1895–1906