Abstract
The purpose of our studies was to assess in vitro nanoparticles cellular uptake and cellular budesonide levels after treatment of alveolar epithelial cell lines with wheat germ agglutinin (WGA)-conjugated budesonide nanoparticles and pharmacokinetic evaluation of drug after intratracheal instillation of nanoparticles in rats. Confocal microscopy was used to study the cellular uptake of nanoparticles, and the cellular and lung tissue drug levels were estimated by HPLC. Higher amount of fluorescence observed in the cells treated with WGA nanoparticles, higher and sustained cellular drug levels, and better bioavailability in lungs of WGA-conjugated nanoparticles indicate superiority of WGA-conjugated nanoparticles over unconjugated nanoparticles.
Local delivery to the lungs is desirable because it results in high local concentration and at the same time it minimizes systemic adverse effects. Use of sustained release delivery system would further reduce the dosing frequency, which might be particularly useful in chronic diseases like asthma. Colloidal drug delivery systems including liposomes and polymeric particles are useful in sustaining drug delivery to the lungs (Lai et al. Citation1993; Suarez et al. Citation2001; Konduri et al. Citation2003)
Among the polymers used for the preparation of nanoparticles, poly (D,L-lactic-co-glycolic) acid (PLGA) is highly preferred because it is biodegradable and biocompatible. The drug entrapped in PLGA matrix is released at a sustained rate through diffusion of the drug in the polymer matrix and by degradation of the polymer matrix (Anderson and Shive Citation1997). Polymeric drug carriers if attached to certain cytoadhesive ligands that bind to epithelial surfaces through specific receptor-mediated interactions enhance drug bioavailability by prolonged residence at the site of absorption owing to increased epithelial contact (Mo and Lim 2005).
Lectins, a family of natural nonenzymatic proteins/glyco- proteins, have cytoadhesive and cytoinvasive properties and specifically recognize and bind with carbohydrate residues on cell surface (Goldstein and Hayes Citation1978). Wheat germ agglutinin (WGA) from triticum binds specifically to N-acetyl-D-glucosamine residues located at the surface of alveolar epithelium(Abu-Dahab, Schafer, and Lehr Citation2001). Specific binding followed by internalization of WGA also has been demonstrated in intestinal and alveolar epithelium (Lehr Citation2000).
Budesonide is a potent corticosteroid with high glucocorticoid receptor affinity. BD has been shown to inhibit the expression and secretion of vascular endothelial growth factor (VEGF) in alveolar epithelial cells (A549) (Bandi and Kompella Citation2001). In the lung, VEGF is proposed to play a key role in the pathogenesis of inflammatory respiratory disorders. Because these inflammatory and angiogenic disorders are chronic, there is a need to sustain the inhibition of VEGF-mediated events in these disorders.
MATERIALS AND METHODS
PLGA, (lactide/glycolide ratio 50:50, inherent viscosity 0.45dl/g) was obtained as a gift sample from Boehringer Ingelheim (Germany). Budesonide (BD) was obtained as a gift sample from Cadila Healthcare (India). Polyvinyl alcohol (PVA, mw 125,000; hydrolyzed 87–89%) was purchased from S.D. Fine Chemicals (India). WGA and bichinconinic acid (BCA) protein assay kit were obtained from Banglore Genei (India). A549 cells were obtained from National Center for Cell Sciences (Pune, India). Dulbecco's Modified Eagle Medium (DMEM), sodium bicarbonate, streptomycin-penicillin solution, fetal bovine serum, fluorescein diacetate (FDA) and Hank's balanced salt solution (HBSS), propidium iodide, and ribonuclease-A all were purchased from Sigma Chemical (St. Louis, MO, USA). 1-ethyl-3(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC), N-hydroxy-succinimide (NHS), glycine, N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES), and sodium dodecyl sulphate (SDS) were purchased from National Chemicals (India). Methylene chloride and acetonitrile obtained from Loba Chemicals (India) were of HPLC grade.
Tissue culture flasks and 24-well plates were obtained from Tarsons Ltd (India). All the other reagents used for the present study were of analytical grade. The rats were procured from Cadila Healthcare (Ahmedabad, India). Animal experiments were approved by Social Justice and Empowerment Committee, Ministry of Government of India, New Delhi, India.
NP Formulation and WGA Conjugation
Budesonide-loaded PLGA nanoparticles were prepared by emulsion-solvent evaporation technique as described earlier (Naazneen, Citation2007). Briefly, a solution of BD and PLGA was poured into an aqueous PVA solution and the resulting mixture was stirred with the help of high speed homogenizer (Ultra-turaxx, T-25, Ultrapure Scientific, Mumbai) to get a primary O/W emulsion. The primary emulsion was passed through high pressure homogenizer (Emulsiflex, C5, Avestin, Canada) and the homogenized O/W emulsion was immediately added dropwise to an aqueous PVA solution. This was further stirred overnight with a magnetic stirrer (Remi Equipments, Mumbai) to evaporate the dichloromethane (DCM). Nanoparticles (NPs) were recovered by centrifugation for 30 min at 25000 rpm, washed and lyophilized for 24 hr.
WGA was conjugated onto the surface of BD-nanoparticles by two-step carbodiimide coupling method (Naazneen et al. Citation2007). Briefly, EDAC and NHS solution in 20 mM HEPES/NaOH buffer, pH 7.0, were added to a suspension of NPs in the same buffer. After 2 hr incubation at room temperature, excess EDAC and NHS were removed by centrifugation. The pellet was resuspended in WGA solution in 20 mM HEPES/NaOH buffer, pH 7.0 and incubated for 18 hr. Excess WGA was removed by centrifugation. To saturate the free coupling sites 1.0 ml of 20% glycine solution in 20 mM HEPES/NaOH buffer, pH 7.4 was added and incubated for 1 hr. Finally, the particles were washed and lyophilized for 24 hr. To estimate the amount of WGA conjugated to the surface of budesonide nanoparticles (BD-NPs), the amount of lectin in the supernatant and the washings was subtracted from the amount of lectin taken for conjugation.
NP Characterization
Unconjugated and WGA-conjugated NPs were characterized for particle size and zeta potential using the principle of laser light scattering with zeta sizer (Nano-ZS, Malvern Instruments, UK). The entrapment efficiency of BD in the nanoparticles was determined by extracting and quantifying the encapsulated BD(Naazneen et al. Citation2007). For in vitro drug release a suspension of NPs containing 500 μ g BD, in phosphate-buffered saline (PBS), pH 7.4 at 37°C, was placed in a dialysis bag and suspended in 15 ml PBS. Sampling was done at predetermined time intervals and volume was adjusted by replacing with fresh PBS. To determine the amount of BD in the samples, the samples were extracted with methylene chloride for 30 min. The methylene chloride was evaporated and the residue reconstituted with the mobile phase, centrifuged, and injected into the HPLC column. The release of BD from BD solution was used as a control.
Surface morphology was analyzed using EDAX (energy dispersion analysis by X-ray) scanning electron microscopy (ESEM). Aqueous nanoparticle suspensions were layered on the SEM stubs, and they were allowed to dry at room temperature. Samples were then observed with Phillips SEM 51S set at 10 kV.
Cell Culture
Alveolar epithelial cells (A549) were maintained on DMEM, supplemented with sodium bicarbonate, fetal bovine serum, and streptomycin-penicillin solution. All the incubations were performed at 37°C and 5% CO2.
Determination of Cellular Drug Levels
A549 cells (passage 82) were seeded in T 75 flasks in DMEM, supplemented with sodium bicarbonate and fetal bovine serum, at a density of 1.0 × 105 cells/flask and allowed to attach overnight. BD and NPs suspensions equivalent to 10− 5 M BD were added to the labeled wells. Untreated cells and blank NPs treated cells were used as controls. The medium was changed on day 2 and no further dose of the drug was added. At different time intervals, the media from the flasks was removed and the cells washed twice with PBS to remove uninternalized nanoparticles and free drug, and the cells were lysed by incubating them with distilled water for 30 min. A portion of the cell lysates was used to determine the total amount of cell protein using BCA-protein assay method. The total amount of drug (free and entrapped in NPs) was extracted from the cell lysates with DCM, the DCM evaporated and the residue reconstituted with the mobile phase, centrifuged, and injected into the HPLC column for determination of drug content.
In Vitro Antiproliferative Studies
Antiproliferative studies were conducted as described in our earlier study (Naazneen et al. Citation2007) using Fluorescein di-acetate as a stain to determine the number of viable cells.
Cell Cycle Analysis
To determine the mechanism of growth inhibition observed in the previous studies, the distribution of DNA in the cell cycle was studied by flow cytometry. A549 cells were seeded at a density of 1 × 106 cells per 2 ml in a 35-mm Petridish (PD) and allowed to attach overnight. BD or suspension of drug-loaded NPs and WGA-NPs equivalent to 10 − 5 M BD was added to the cells. Blank nanoparticles-treated cells and untreated cells were used as controls. The medium was changed after 24 hr, and no further dose of the drug was added. After 24 and 48 hr, cells were washed twice with PBS, trypsinized, and fixed with ethanol at 4°C until analyzed. Later the cells were stained with propidium iodide and the cellular DNA content was analyzed with a fluorescent activated cell sorter (FACS, flow cytometer) to create histograms of cell frequency versus propidium iodide fluorescence intensity.
Confocal Microscopy
Preparation of 6-Coumarin Loaded Nanoparticles
NPs containing fluorescent containing dye, 6-coumarin, were formulated using solvent evaporation technique. A solution of 6-coumarin and PLGA in chloroform was emulsified into aqueous PVA solution. This primary emulsion was passed through high pressure homogenizer (Emulsiflex, C5, Avestin, Canada) for 3 cycles at 15000 psi pressure. The homogenized O/W emulsion was immediately added drop wise to an aqueous PVA solution and the contents were stirred overnight with a magnetic stirrer (Remi Equipments, Mumbai) to evaporate the methylene chloride. Nanoparticles were recovered by centrifugation for 30 min at 25000 rpm, washed, and lyophilized for 24 hr.
Microscopic Studies
A549 cells were seeded on a coverslip placed in 35 mm PDs at a cell density of 0.1 × 106 cells per PD and allowed to attach overnight. The cells were treated with WGA conjugated and unconjugated PLGA nanoparticles loaded with 6-coumarin. At different time intervals, the media was removed, the monolayers were washed twice with PBS to remove uninternalized nanoparticles, and the cells were observed under a laser scanning confocal microscope LSM 10 (Zeiss, UK).
In Vivo Studies
Animals and Treatment
Wistar rats (weighing 200–250 g) were housed in polypropylene cages with free access to palletized chow and tap water. The animals were exposed to alternate cycles of 12 hr light and darkness. The temperature was maintained at approximately 26°C to 28°C. Three rats of either sex were used in each group, at every time interval. Animals were allowed free access to water and rat feed but were food-fasted overnight prior to each experiment.
Intratracheal Instillation
The intratracheal instillation of saline, drug solution, unconjugated and WGA-conjugated NPs were carried out by well adapted method (Brown and Schanker Citation1983). Intraperitoneal administration of ketamine (50 mg/kg) and diazepam 5 mg/kg, intramuscularly, was used to anesthetize rats. Anesthetized animals were placed in supine position on a 45° slanted support, and a small middle incision was made over the trachea. The trachea was exposed by blunt dissection of the sternohyoideus muscle. A small hole was made in trachea between the fifth and the sixth tracheal rings using as 20-gauge needle. The trachea was cannulated with a short (10- to 15-cm) length of PE50 tubing with the tip positioned approximately at the tracheal bifurcation. Dose equivalent to 150 μ g of drug in the form of solution or nanoparticles suspended in sterile saline was slowly instilled over a 1-min period using a 1-mL syringe attached to the PE50 tubing.
Following instillation, the tubing was withdrawn and a small drop of cyanoacrylate adhesive was placed over the hole to seal the opening. The skin was clothed with 3–0 Dexon sutures. Intraperitoneal ampicillin 10 mg/kg and diclofenac sodium were administered to the rats to combat infection and pain in the animals. The animal was removed from anesthesia and allowed to recover under a heating lamp. After recovery, animals were housed in individual plastic cages with access to food and water for the remainder of the study. At the end of each time point biological samples were collected and the animals are sacrificed.
Biological Sampling
Broncho alveolar lavage (BAL) was performed on anesthetized animals with PBS, prewarmed to 37°C. For performing the lavage the Hamilton syringe connected to the PE50 tubing was replaced with a 3-way stopcock attached with two 20 ml syringes. The tubing was reinserted through the cannula and advanced until tracheal bifurcation. Fluid (PBS) was slowly injected into the lung via one syringe and then BAL withdrawn by gentle aspiration via the other (Shek et al. 1990). This BAL yielded between 7 to 11 ml liquid, which was centrifuged at 4.38 × 103 × g for 5 min. The supernatant was extracted with DCM and assayed by HPLC method for BD. The lungs were excised and homogenized in 10 ml PBS. The lung homogenate was extracted with DCM and assayed for BD by HPLC method.
Budesonide HPLC Assay
The amount of BD in samples obtained from in vitro release and drug entrapment measurements were directly injected into the HPLC column after processing as described above. For drug analysis in lung tissue, the isolated lung tissues were homogenized in PBS buffer. To the lung homogenate, solution of celecoxib was added as an internal standard and mixed thoroughly. The drug was extracted from the homogenate with DCM. The DCM extract was evaporated to dryness and the dried residue was reconstituted with acetonitrile:water (70:30) mixture. This reconstituted mixture was vortexed for 1 min, centrifuged at 12,000g for 5 min, and the supernatant was injected into an HPLC system (Dionex Softron GmbH, Germany). The HPLC system was composed of a pump (P-680, Dionex), a simple 10-μ l loop injector (Reodyne 7125), and a UV-visible spectrophotometric detector (UVD 170U, Dionex).
For BAL, an appropriate concentration of internal standard was added and a similar extraction procedure was followed. The separation of drug was carried out on a 14 cm Kromasil C 18 150-4.6 HPLC column (Merck) having particle size of 5 μ m. The mobile phase for the assay consisted of acetonitrile and aqueous buffer mixture (70:30 v/v) mixture. The aqueous buffer was 0.1% acetic acid in water at pH 3. The run time of the assay was 15 min and the retention time of budesonide was 2.7 min. During the assay, budesonide was eluted isocratically at a mobile phase flow rate of 1 ml/min and monitored with a detector operating at 250 nm. Chromatographic runs were performed at room temperature and the data were analyzed using Chromeleon 6.5 software.
Statistical Analysis
All data are expressed as the mean ± SD or the mean ± SE of the mean, and comparison of the mean values was performed using either Student's t-test or ANOVA. Statistical significance was set at p < 0.05.
RESULTS
NP Formulation and WGA-Conjugation
NPs formulated by emulsion-solvent evaporation technique with a drug: polymer ratio of 1:2 and 2% aqueous PVA as an emulsifier were spherical with smooth surfaces. Amount of WGA conjugated onto the surface of NPs by carbodiimide coupling method was 12.2 ± 2.6 μ g/mg of nanoparticles.
Characterization of Unconjugated and WGA Conjugated NPs
The NPs were characterized for particle size, zeta potential, percent entrapment efficiency (% EE) and in-vitro drug release and results tabulated in . The ESEM microscopy pictures indicated spherical particles with smooth surfaces.
TABLE 1 Characterization of budesonide nanoparticles
Cellular Drug Levels
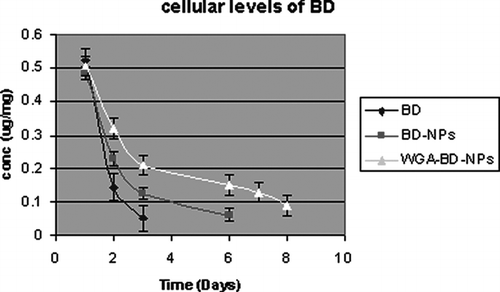
Cellular drug levels were estimated at different time intervals, after treating A549 cells with BD, BD-NPs and WGA-BD–NPs. The drug levels dropped rapidly in the cells that were treated with the drug solution on day 2 of the study (). NPs-treated cells maintained sustained intracellular drug levels, and at all time points, cells treated with WGA-BD–NPs showed significantly higher (p < 0.005) drug levels than unconjugated NPs. Intracellular drug levels were detectable for 6 days and up to 8 days with unconjugated NPs and WGA-conjugated NPs, respectively.
In Vitro Antiproliferative Studies
As demonstrated in our earlier studies (Naazneen et al. Citation2007), the BD-treated cells demonstrated a transient inhibition of cell proliferation compared with untreated cells. Significantly higher (p < 0.05) and prolonged (up to 7 days) inhibition of cell proliferation was observed when cells were treated with BD-PLGA NPs and WGA-BD-PLGA NPs When the results of in vitro antiproliferative activities of unconjugated and conjugated BD-PLGA NPs were compared, increased in vitro antiproliferative activity was observed with conjugated BD-PLGA NPs.
Cell Cycle Analysis
Cell cycle analysis demonstrated that the cells treated with BD loaded NPs had a higher proportion of cells in the G0/G1 phase and a concurrent lower proportion of cells in the proliferative S phase on day 3 of treatment than the cells treated with the drug in solution (). Between the nanoparticle treatment group, though there was marginal increase in the proportion of cells in the G0/G1 phase, conjugated nanoparticles had a relatively lower percentage of cells in the S phase. Blank nanoparticles had no effect on the cell-cycle distribution, and the results were similar to the results with the untreated control.
TABLE 2 Effect of budesonide treatments on the cell cycle distribution in A549 cells
Confocal Microscopy
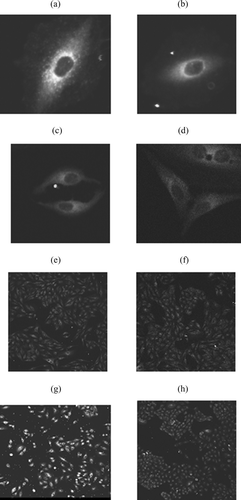
The 6-coumarin-loaded unconjugated NPs and WGA-conjugated NPs had a particle size of 245 ± 4 nm and 335 ± 5 nm and zeta potential of −19.2 ± 1.7 mV and −6.5 ± 1.2 mV, respectively. WGA-conjugated NPs had 10.6 μ g WGA /mg of nanoparticles. Confocal images at different time intervals showed that the NPs are taken up by the cells within 5 min of treatment with 6-coumarin-loaded NPs. At all time points the WGA-conjugated NPs are associated to a greater extent than unconjugated NPs ().
Budesonide Levels in BAL and Lung Tissue
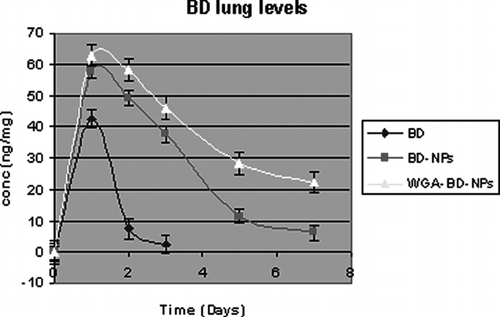
Following a single intratracheal instillation of BD, BD-NPs, and WGA-BD–NPs (equivalent to 150 μ g BD), BD levels in lung and BAL tissue were measured at different time intervals (day 1–7). Various pharmacokinetic parameters are listed in . The area under the lung BD concentration time curve (AUC) for WGA-BD-NPs and BD-NPs was 306.85 and 217.5 ng/mg/day, respectively, which is six and four times higher than AUC for plain BD. The elimination rate constant for plain BD was found to be 1.09 that is 6.4 and 3.51 times higher than WGA-BD-NPs and BD-NPs, respectively.
TABLE 3 Pharmacokinetic parameters after intratracheal instillation in rats
BD was below the limit of detection in the lung after 3 days for the BD group (). After 1 week, the lung levels were 22.5 ± 3.2 ng/mg and 6.25 ± 2.5 ng/mg and the BAL BD levels were 37.6 ± 3.7 ng/ml and 10.8 ± 2.4 ng/ml in the WGA-BD-NPs- and BD-NPs-treated group, respectively.
DISCUSSION
Emulsion-solvent evaporation method yielded spherical NPs with smooth surfaces. WGA conjugation on to the surface of NPs marginally altered the particle size of NPs, but there was significant change in the surface charge that reduced from −14.2 ± 1.9 to −4.59 ± 2.1. Negative charge is attributed to the presence of uncapped end carboxyl groups of the polymer at the particle surface. Coating of nanoparticles with some amphiphilic polymers normally decreases the zeta potential because the coating layers shield the surface charge and move the shear plane outward from the particle surface (Sahoo et al. Citation2002). Cellular drug levels revealed the presence of drug up to day 8 and 6 after treatment with conjugated and unconjugated NPs, respectively, and the drug levels were higher after WGA-NPs treatment at all time points. Specific binding of WGA to N-acetyl-D-glucosamine residues located at the surface of A549 cells followed by internalization of NPs may be the reason for higher cellular levels. Improved cytoassociation of the NPs grafted with WGA to Caco-2 cells has been reported by Wirth et al. as compared with the unmodified ones (Wirth et al. Citation1998).
In our earlier studies we found that the in vitro antiproliferative activity improved and prolonged significantly after WGA conjugation of BD-NPs. Cell cycle arrest was confirmed by flow cytometry. The mechanism of inhibition of cell proliferation was mediated through inhibition of cell-cycle progression with a relatively higher percentage of cells in the G0/G1 arrest phase in the group that was treated with WGA-BD-NPs compared with BD-NPs and drug in solution. On the basis of intracellular drug levels, we concluded that more drug is available at the site of action for a sustained period of time in case of NPs, more so with WGA-conjugated NPs, than in the drug in solution and resulting in greater antiproliferative activity.
The particle size and zeta potential of 6-coumarin-loaded NPs were similar to the drug-loaded NPs, so their cellular uptake can be expected to be similar to the drug loaded NPs. The images at all time points clearly indicate higher cellular association of WGA-conjugated NPs as compared with unconjugated NPs. This may be due to the higher endocytosis through WGA receptor.
Lung tissue levels and BAL levels of BD were measured after intratracheal instillation of BD, BD-NPs, and WGA-BD-NPs. Significantly higher AUC in lung for nanoparticles indicates higher retention of drug levels in the lung with NPs as compared with plain drug. Between the two NPs, AUC in the lung was 306.85 and 215.7 ng/mg/day for conjugated and unconjugated NPs, respectively. This further indicates higher retention of conjugated NPs than unconjugated NPs.
CONCLUSION
Our results demonstrated significantly higher intracellular drug levels for prolonged period, cellular uptake, and bioavailability with WGA-BD-NPs compared with BD-NPs or BD solution. Thus, WGA-modified BD-PLGA NPs can play a promising role in alleviating problems associated with asthma treatment, such as short-lived action, nocturnal exacerbations, and hospitalizations of patients, and increae in dose with time. However, the role in clinical practice of developed NPs from this investigation may be realized only after more extensive animal studies and followed by clinical investigations.
The authors are thankful to the Department of Science and Technology, government of India, for providing financial support under the Women Scientist Scheme (WOS-A). The authors also are grateful to Dr. T. Bagchi, Department of Microbiology and Biotechnology, Maharaja Sayajirao University of Baroda, and Dr. B. S. Dwarkanath, Scientist F, Institute of Nuclear Medicine and Allied Sciences, New Delhi, for helping us in cell culture studies.
REFERENCES
- Abu-Dahab R., Schafer U. F., Lehr C. M. Lectin-functionalized liposomes for pulmonary drug delivery: effect of nebulization on stability and bioadhesion. Eur. J. Pharm. Sci. 2001; 14(1)37–46
- Anderson J. M., Shive M. S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Delivery Rev. 1997; 28: 5–24
- Bandi N., Kompella U. B. Budesonide reduces vascular endothelial growth factor (VEGF) secretion and expression in airway (Calu-1) and alveolar (A549) epithelial cells. Eur. J. Pharmacol. 2001; 425(2)109–16
- Brown R. A., Schanker L. S. Absorption of aerosolized drugs from the rat lung. Drug Metab. Distrib. 1983; 11: 355–360
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv. Carbohydr. Chem. Biochem. 1978; 35: 127–340
- Konduri K. S., et al. Efficacy of liposomal budesonide in experimental asthma. J. Allergy Clin. Immunol. 2003; 111: 321–327
- Lai Y. L., et al. Sustained bronchodilation with soproterenol poly(glycolide-co-lactide) microspheres. Pharm. Res. 1993; 10: 119–125
- Lehr C. M. Lectin–mediated drug delivery: the second generation of bioadhesives. J. Control. Rel. 2000; 65(1–2)19–29
- Mo Y., Lim L. Y. Mechanistic study of the uptake of wheat germ agglutinin-conjugated PLGA nanoparticles by A549 cells. J. Pharmaceu. Sci. 2004; 93: 20–28
- Naazneen S., et al. Assessment of in-vitro antiproliferative activity after intracellular drug delivery from wheat germ agglutinin-conjugated budesonide nanoparticles. J. Biomed. Nanotechnol. 2007; 3: 61–67
- Sahoo S. K., Panyam J., Prabha S., Labhasetwar V. Residual polyvinyl alcohol associated with poly (lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Rel. 2002; 82(1)105–114
- Suarez S., et al. Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J. Antimicrob. Chemother. 2001; 48: 431–434
- Wirth M., et al. Lectin-mediated drug targeting: preparation, binding characteristics and antiproliferative activity of wheat germ agglutinin conjugated doxorubicin on Caco-2 cells. Pharm. Res. 1998; 15: 1031–1037