Abstract
The aim of our present study was to evaluate the in vitro percutaneous absorption of atenolol, a well-known antihypertensive, from a series of formulations containing various penetration enhancers. Particularly the promoting effect of EPA and DHA, two polyunsaturated fatty acids (PUFAs), has been studied, and drug permeation data have been compared with those obtained with the other formulations containing “classic” penetration enhancers such as transcutol, d-limonene, and PG. Not all the penetration enhancers tested were effective in increasing atenolol percutaneous flux and the best permeation profile for atenolol was obtained with the formulation containing transcutol (B) and PUFA (E). To explain the enhancer mechanism, the atenolol diffusion and partitioning coefficients from the different formulations were calculated. The results indicated that PUFAs increased the apparent diffusion coefficient of the drugs but did not affect their apparent stratum corneum (SC)/vehicle partition coefficient (Km). At this same time transcutol exerted its enhancer effect increasing significantly the apparent SC/vehicle partition coefficient (Km) and in a minor amount the apparent diffusion coefficient of skin permeation process (Dm). The potential application of formulations B and E in atenolol percutaneous absorption was determined from the calculation of the steady-state plasma concentrations (Css). These values resulted within the drug therapeutic range and suggest that atenolol transdermal delivery could be feasible.
The dermal or transdermal administration of drugs for the treatment of local or systemic conditions overcomes several important limitations associated with more conventional forms of drug delivery such as gastrointestinal/hepatic first-pass metabolism and inadvertent systemic drug absorption. However, because of the low permeability of the stratum corneum, different physical and chemical approaches have been developed to overcome the skin barrier and to have better control of drug transport across the skin (Muller, Radtke, and Wissing Citation2002; Williams and Barry Citation2004; Doukas and Kollias Citation2004; Puglia et al. Citation2006). Among them, penetration enhancers are one of the most convenient methods and show relatively high effects (Hadgraft Citation2001; Williams and Barry Citation2004), interacting with skin constituents to increase drug flux. Unfortunately, many chemical enhancers are toxic, skin irritating, or allergenic at some degree or another depending on their concentration and the frequency of their treatment. Therefore, the development of a new relatively safer enhancer is desiderable.
Fatty acids have been shown to accelerate skin permeation of drugs probably modifying intercellular lipid packing in the stratum corneum lipid domains and so reduce the barrier function (Santoyo and Ygartua Citation2000; Tanojo et al. Citation1997). Some scientific works (Heard et al. Citation2003; Nanayakkara et al. Citation2005; Puglia et al. Citation2005; Richards et al. Citation2006) outlined a high epithelial penetration ability of polyunsaturated fatty acids (PUFAs) and an interesting enhancement effect in the skin drug permeation.
Heard and co-workers (Citation2003), for instance, demonstrated in vitro the feasibility of the simultaneous permeation of ketoprofen, ibuprofen, and essential fatty acids, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) from a formulation containing fish oil (Heard et al. Citation2003). In a study performed in our research laboratories (Puglia et al. Citation2005), a formulation containing ketoprofen and a fish oil extract proved to possess, in an in vivo model, higher anti-inflammatory activity compared with a formulation containing ketoprofen alone. This evidence appeared to correlate to a possible penetration enhancer effect of EPA and DHA, which represented the PUFAs most abundant species in the fish oil extract. Both ω -3 increased ketoprofen flux through human volunteer skin guaranteeing an appreciable anti-inflammatory effect against the erythema induced by ultraviolet B (UVB) radiations.
Atenolol is a beta-adrenergic receptor blocking agent without membrane stabilizing or intrinsic sympathomimetic activities and has been used for the treatment of hypertension (Gennaro Citation1990). In case of oral administration, it can induce such side effects as diarrhea, nausea, ischemic colitis, and mesenteric arterial thrombosis. In recent years, owing to the advantages offered by transdermal administration and the extensive use of atenolol in the treatment of various cardiovascular disorders, several authors have proposed different strategies to enhance penetration through the skin of atenolol (Cho and Shin Citation2004; Gupta and Jain Citation2006). Unfortunately the best part of these works were developed evaluating the percutaneous absorption of atenolol by means of synthetic membranes or animal skins that could not provide a real prediction of in vivo drug transdermal administration in humans (Scott, Walker, and Dugard Citation1986).
In this study, to evaluate the feasibility of atenolol transdermal administration, we studied its in vitro percutaneous absorption through excised human skin from different topical formulations. Particularly the promoting effect of EPA and DHA has been studied and drug permeation data have been compared with those obtained with the other formulations containing “classic” penetration enhancers such as transcutol, d-limonene, and propylene glycol (PG).
MATERIALS AND METHODS
PPG-15 stearyl ether, isohexadecane/PPG-15 stearyl ether, steareth 2, steareth 21, stearic acid, cetylstearylic acid, xanthan gum, transcutol CG (ethoxydiglycol), and undebenzophenon were purchased from Gattefossè (Gattefosse' Italia S.r.l., Milan, Italy). Propylene glycol (PG) was obtained from Sigma Chemicals (St. Louis, MO, USA). D-limonene was obtained from Carlo Erba (Italy). EPA and DHA ethyl esters (85%, ratio 0,9-1,5) were obtained from Seacor® (Società prodotti antibiotici S.p.A, Milan, Italy). All other materials were of analytical grade.
Formulations
Five different o/w emulsions containing atenolol (1%) and different penetration enhancers (see ) were prepared (Puglia et al. Citation2005) by slowly adding the aqueous phase to the oily phase and to the blend of surfactants under continuous agitation. The phases were kept to 70°C. This mixture was stirred until it was cool, thus forming the emulsion formulation.
TABLE 1 Composition of the topical formulations containing atenolol 1%
Skin Membrane Preparation
For in vitro diffusion studies, samples of adult human skin (mean age 36 ± 8 years) from breast reduction operations were processed to obtain stratum corneum/epidermis (SCE) membranes. To prepare the membranes, subcutaneous fat was carefully trimmed and the skin was immersed in distilled water at 60 ± 1°C for 2 min (Kligman and Christophers Citation1963), after which the membranes were removed from the dermis using a dull scalpel blade. The epidermal membranes were dried in a desiccator at ∼25% relative humidity (RH).
The dried samples were wrapped in aluminum foil and stored at 4 ± 1°C until use. Preliminary experiments were carried out to asses SCE samples for barrier integrity by measuring the in vitro permeability of [3H]water through the membranes using the Franz cells described below. The value of the permeability coefficient (Pm) for tritiated water was found to be 1.6 ± 0.2 × 10−3 cm/h which agreed well with those for tritiated water reported by others using human SCE samples (Bronaugh, Stewart, and Simon Citation1986).
In Vitro Skin Permeation Experiments
Samples of dried SCE were rehydrated by immersion in distilled water at room temperature for 1 hr before being mounted in Franz-type diffusion cells supplied by LGA (Berkeley, CA, USA). The exposed skin surface area was 0.75 cm2 and the receiver compartment volume was 4.5 mL. The receptor compartment contained a water-ethanol solution (50:50) to allow the establishment of the “sink condition” and to maintain permeant solubilization (Touitou and Fabin Citation1988a). The solution was stirred with a magnetic bar at 500 rpm and thermostated at 32 ± 1°C during all the experiments (Siewert et al. Citation2003).
After placing ∼300 mg of each formulation on the skin surface, the donor compartment was sealed to avoid evaporation. Each experiment was run in duplicate for 36 hr using three different donors (n = 3). At intervals (t = 0, 2, 4, 6, 8, 12, 18, 24, and 36 hr) samples (200 μ l) of receiving solution were withdrawn and replaced with fresh solution. The samples were analyzed for atenolol content by HPLC.
Atenolol flux through the skin was calculated by plotting the cumulative amount of drug penetrating the skin against time and determining the slope of the linear portion of the curve and the χ -intercept values (lag time) by linear regression analysis.
Drug flux (μ g/cm2 per hr), at steady-state, was calculated by dividing the slope of the linear portion of the curve by the area of the skin surface through which diffusion took place. Statistical analysis of data was performed using Student's t-test.
The effectiveness of penetration enhancers was determined by comparing atenolol flux in the presence and absence of enhancers, and their ratio was defined as the enhancement factor (EF):
HPLC Determination of Atenolol
The HPLC apparatus consisted of Shimadzu LC10 AT Vp (Shimadzu, Milan, Italy) equipped with a 20 μ l loop and a SPD-M 10 A Vp Shimadzu UV photodiode array detector. Chromatography was performed following a method reported by Kim and Shin (Citation2004) and using a Jupiter Phenomenex C18R.P. (particle size 5 μ m; 25 cm × 4.6 mm i.d; Phenomenex, Torrance, CA, USA). The mobile phase for atenolol was a combination of methanol/acetonitrile/pH 3 buffer (1:1:4) at a flow rate of 1.0 ml/min. UV detector was set at the wavelength of 224 nm. Under these conditions, atenolol peak appeared at the retention time of 6.5 min.
RESULTS AND DISCUSSION
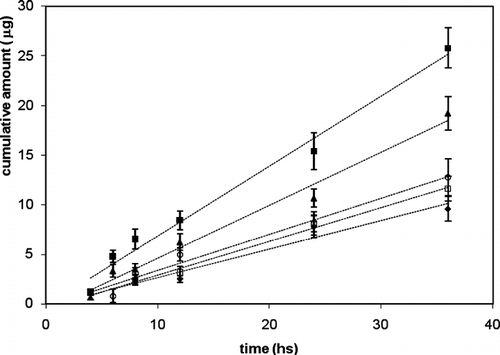
In , the plot of the cumulative amount of atenolol permeated through human SCE membranes as a function of time are reported. Drug flux values (JSS) from A–E formulations calculated from the linear segments at the steady-state are reported in . flux value at the steady-state of atenolol from A formulation () was found to be 0.38 ± 0.07 μ g/cm2 per hr. To improve the skin permeation of atenolol, we carried out in vitro experiments applying this drug with some penetration enhancers such as d-limonene, transcutol, and PG extensively used to increase drug percutaneous absorption (Hadgraft Citation2001; Williams and Barry Citation2004; Puglia et al., Citation2001).
TABLE 2 Atenolol steady-state flux through excised human skin, lag time, permeability (Pm), partition coefficient (Km), and diffusion coefficient (Dm)
FIG. 1 permeation profiles of atenolol through SCE membranes from different formulations (A–E). (♦) = A; (▪) = B; (□) = C; (ˆ) = D; (▴) = E.
To evaluate the feasibility of EPA and DHA in promoting atenolol percutaneous absorption, we have formulated an emulsion containing 20% of these PUFAs (formulation E). We chose to use this concentration because in a previous study (Puglia et al. Citation2005) we showed the suitable skin penetration ability of these substances. As regards PG concentration (20%), it was selected because different scientific evidences indicate it as the most suitable to guarantee an enhancing effectiveness of this substance. Similar considerations were used to select the appropriate concentration of d-limonene (1%) and transcutol (20%).
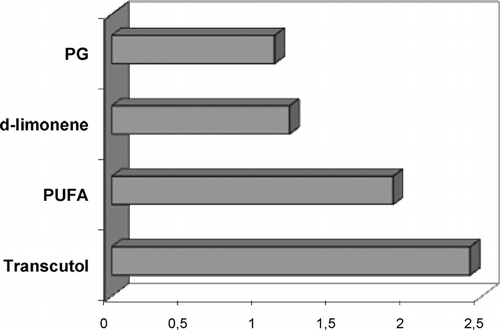
From the flux values obtained (), not all the penetration enhancers tested in this study produced an increase of percutaneous flux of atenolol compared with control formulation. Although PG and d-limonene are successfully used elsewhere to promote percutaneous absorption of drugs through human skin, in this study they appeared inefficacious in increasing percutaneous absorption of atenolol (p > 0.05).
The best permeation profile was obtained when we applied the drug together with transcutol (p < 0.05). Formulation B, in fact, was able to increase atenolol flux about 2-fold compared with the free enhancer formulation (), whereas PUFAs proved to be less efficacious, compared with transcutol, in promoting the skin permeation of atenolol.
FIG. 2 Atenolol enhancement factor (EF) from formulation containing PG, d-limonene, PUFAs, and transcutol.
reports other important drug permeation parameters such as the lag time (TL), diffusion coefficient (D), permeability (P), and the partition coefficient (K) calculated for different formulations tested in the present study (A–E). Particularly, the lag time represents the time period required to establish steady-state diffusion and it corresponds to the nonlinear portion of a permeation profile, whereas the other parameters were obtained using the equations reported below:
where h is the barrier thickness for human skin and C is the donor phase concentration. The h value is 16.8 μ m assuming that the stratum corneum represents the main rate-limiting barrier. Coefficient D, particularly, reflects the facility for the molecules to move through the membrane strata and it is a function of the molecular structure of the diffusant (Röpke et al. Citation2002). The relative affinity of the drug for skin and vehicle is presented by the partition coefficient K. Generally a high K value indicates that the vehicle has a poor affinity for the drug. A low K value, indicating a high degree of mutual interaction, reflects the tendency of the drug to remain in the vehicle.
Due to the excellent barrier function of the skin, the choice of an appropriate enhancer is particularly important in the development of a transdermal delivery system. In spite of extensive studies conducted regarding the effect of various enhancers on percutaneous absorption, their mechanism of action still remains unclear and it is not possible to predict a priori their effectiveness for a given drug in given topical formulation. However, it is now well established that enhancers can have two possible modes of action to improve the topical bioavailability of a drug (Kadir et al., Citation1987). The first, known as the “push” effect, is to increase the solubility of the solute in the formulation and hence its concentration gradient in solution. The second, the “pull” effect, is related to the flux of the enhancer itself into and through the skin, which can induce structural transformations of the skin barrier. This then reduces its diffusional resistance and promotes transdermal delivery of pharmacological substances.
As shown in , PUFAs increased the apparent diffusion coefficient of the drugs but did not affect their apparent SC/vehicle partition coefficient (K). These results are not surprising since it is well known that the mechanism of enhancement by fatty acids is reduction of skin resistance as a permeability barrier by disruption of tightly packed lipid regions of stratum corneum (Wang, Yang, and Heng Citation2004).
Findings from our study indicate that transcutol exerted the best effect in increasing skin permeation of atenolol. Regarding its enhancement mechanism, in our experiments, it increased significantly the apparent SC/vehicle partition coefficient (K) and in a minor amount the apparent diffusion coefficient of skin permeation process (Dm) (). The mechanisms involved in the enhancement of drug permeation mainly appear to be related to the solubilizing properties of transcutol, combined with its ability to increase drug cutaneous retention. This evidence is consistent with the findings of other authors (Harrison et al. Citation1996) who reported the ability of this compound to promote drug skin absorption by an increased SC/vehicle drug partitioning and an higher drug retention in the skin.
To assess the feasibility of atenolol transdermal administration in human, blood levels of this drug following application of transdermal formulations were predicted by using the flux values from the in vitro experiments on excised human skin (). Steady-state plasma concentration (CSS) can be calculated by means of the following equation (Touitou et al. Citation1988b):
where JSS is the slope of the linear section of the cumulative amount permeated per unit area versus time plot; A denotes the area of application to the skin, Vd (ml) is the volume of distribution; and Ke (h) represents the elimination constant. Vd and Ke of atenolol in humans are 1000 ml and 2.33× 10− 2/hr (Clarke Citation1986).
Therefore, for the formulation without penetration enhancers having a flux of 0.38 μ g cm− 2 per hr, blood concentration of 0.16 μ g/ml is predicted for atenolol using an area of application to the skin of 10 cm2 for 70 kg human. This is only an approximate estimation since biotransformation of atenolol in its penetration through the excised human skin is not known. As can be noted from the comparison of theoretical therapeutic concentration (CT) with the experimental data (), the CSS value was not suitable for transdermal administration of atenolol.
FIG. 3 Atenolol plasma concentration (CSS) and theoretical therapeutic concentration (CT) from control formulation (A), formulation containing transcutol (B), and formulation containing PUFAs (E).
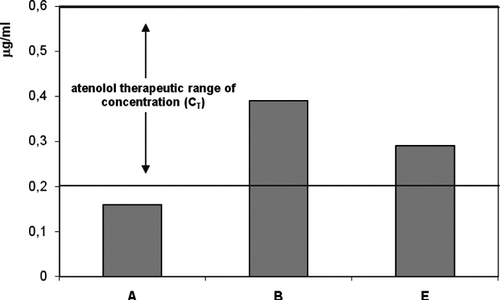
To verify the potential application of enhancer formulations B and E, which presented the better skin permeation profiles for atenolol, we calculated the corresponding CSS using the percutaneous fluxes obtained from these vehicles (). The results suggested that both formulations could ensure, although at different degrees, blood concentrations within the therapeutic range and so the successful transdermal administration of the drug. The results obtained from EFA and DHA topical application outlined an interesting enhancer effect of these substances on atenolol permeation profile. Notwithstanding that transcutol appeared more efficacious in increasing atenolol flux through SCE membranes compared with the formulation containing PUFAs, we think that the result is important considering the “safe profile” of these substances. Furthermore our previous in vitro and in vivo studies regarding EPA and DHA percutaneous absorption revealed an intrinsic therapeutic activity of these fatty acids (Puglia et al. Citation2005) which could represent a plus value for the results of the present work.
CONCLUSION
Not all the penetration enhancers tested in this study were effective in increasing atenolol percutaneous flux. Only the formulations containing transcutol (B) and PUFAs (E) gave the best enhancing effect on atenolol permeation profile and guaranteed blood concentration of the drug within the therapeutic range. On the basis of these results, we believe that atenolol in association with transcutol or PUFAs can be regarded as a successful candidate for transdermal administration in humans, even if clinical in vivo studies are needed to validate the results of the present study.
REFERENCES
- Bronaugh R. L., Stewart R. F., Simon M. Methods for in vitro percutaneous absorption studies VII: use of excised human skin. J. Pharm. Sci. 1986; 75: 1094–1097
- Cho C. W., Shin S. C. Enhanced transdermal delivery of atenolol from the ethylene-vinyl acetate matrix. Int. J. Pharm. 2004; 287: 67–71
- Clarke S. Atenolol analytical and toxicological data. Isolation and Identification of Drugs in Pharmaceuticals, Body Fluids, and Postmortem Material, 2nd ed., A. C. Moffat, J. V. Jackson, M. S. Moss, B. Widdop. The Pharmaceutical Press, London 1986; 362–363
- Doukas A. G., Kollias N. Transdermal drug delivery with a pressure wave. Adv. Drug Deliv. Rev. 2004; 56: 559–579
- Gennaro A. R. Remington: The Science and Practice of Pharmacy, 18th ed. Mack Publishing Company, Easton, PA 1990; 900–901
- Gupta S. P., Jain S. K. Transdermal atenolol releasing system: an approach towards its development. J. Drug Target. 2006; 14: 607–613
- Hadgraft J. Skin, the final frontier. Int. J. Pharm. 2001; 224: 1–18
- Harrison J. E., Watkinson A. C., Green D. M., Hadgraft J., Brain K. The relative effect of Azone and Transcutol on permeant diffusivity and solubility in human stratum corneum. Pharm. Res. 1996; 13: 542–546
- Heard C. M., Gallagher S. J., Harwood J., Maguire P. B. The in vitro delivery of NSAIDs across skin was in proportion to the delivery of essential fatty acids in the vehicle—evidence that solutes permeate skin associated with their solvation cages?. Int. J. Pharm. 2003; 261: 165–169
- Kadir R., Stempler D., Liron Z., Cohen S. Delivery of theophylline into excised human skin from alkanoic acid solutions: a “push-pull” mechanism. J. Pharm. Sci. 1987; 76: 774–779
- Kim J., Shin S. C. Controlled release of atenolol from the ethylene-vinyl acetate matrix. Int. J. Pharm. 2004; 273: 23–27
- Kligman A. M., Christophers E. Preparation of isolated sheets of human stratum corneum. Arch. Dermatol. 1963; 88: 702–705
- Muller R. H., Radtke M., Wissing S. A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002; 54: S131–155
- Nanayakkara G. R., Bartlett A., Forbes B., Marriott C., Whitfield P. J., Brown M. B. The effect of unsaturated fatty acids in benzyl alcohol on the percutaneous permeation of three model penetrants. Int. J. Pharm. 2005; 301: 129–139
- Puglia C., Bonina F., Trapani G., Franco M., Ricci M. Evaluation of in vitro percutaneous absorption of lorazepam and clonazepam from hydro-alcoholic gel formulations. Int. J. Pharm. 2001; 228: 79–87
- Puglia C., Filosa R., Peduto A., de Caprariis P., Rizza L., Bonina F., Blasi P. Evaluation of alternative strategies to optimize ketorolac transdermal delivery. AAPS Pharm. Sci. Tech. 2006; 7: 64
- Puglia C., Tropea S., Rizza L., Santagati N. A., Bonina F. In vitro percutaneous absorption studies and in vivo evaluation of anti-inflammatory activity of essential fatty acids (EFA) from fish oil extracts. Int. J. Pharm. 2005; 299: 41–48
- Richards H., Thomas C. P., Bowen J. L., Heard C. M. In-vitro transcutaneous delivery of ketoprofen and polyunsaturated fatty acids from a pluronic lecithin organogel vehicle containing fish oil. J. Pharm. Pharmacol. 2006; 58: 903–908
- Röpke C. D., Kaneko T. M., Rodrigues R. M., da Silva V. V., Barros S., Sawada T. C., Kato M. J., Barros S. B. Evaluation of percutaneous absorption of 4-nerolidylcathecol from four topical formulations. Int. J. Pharm. 2002; 249: 109–116
- Santoyo S., Ygartua P. Effect of skin pretreatment with fatty acids on percutaneous absorption and skin retention of piroxicam after its topical application. Eur. J. Pharm. Biopharm. 2000; 50: 245–250
- Scott R. C., Walker M., Dugard P. H. A comparison of the in vitro permeability properties of human and some laboratory animal skins. Int. J. Cosm. Sci. 1986; 7: 189–194
- Siewert M., Dressman J., Brown C. K., Shah V. P. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS Pharm. Sci. Tech. 2003; 4, article 7
- Tanojo H., Bouwstra J. A., Junginger H. E., Bodde H. E. In vitro human skin barrier modulation by fatty acids: skin permeation and thermal analysis studies. Pharm. Res. 1997; 14: 42–49
- Touitou E., Fabin B. Altered skin permeation of a highly lipophilic molecule: tetrahydrocannabinol. Int. J. Pharm. 1988a; 43: 17–22
- Touitou E., Fabin B., Dany S., Almog S. Transdermal delivery of tetrahydrocannabinol. Int. J. Pharm. 1988b; 43: 9–15
- Wang M. Y., Yang Y. Y., Heng P. W. Role of solvent in interactions between fatty acids-based formulations and lipids in porcine stratum corneum. J. Control. Rel. 2004; 94: 207–216
- Williams A. C., Barry B. W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004; 56: 603–618