Abstract
Our objective was to investigate the controlled release and transport of leuprolide acetate polypeptide in the colon using novel combinations of rate-controlling polymers. Polymer swelling, disintegration, drug release, and transport characteristics were measured using different polymers, carbopol, chitosan and polyox, alone and in combination. Studies demonstrated Carbopol® -containing combination formulations had maximum swelling and the slowest disintegration properties. A decrease in dissolution rate was observed from all combination formulations when compared with their individual counterparts. Carbopol® combinations showed the slowest overall release. Drug transport studies using the everted sac technique demonstrated good correlation to the swelling, disintegration, and dissolution studies. Thus, novel polymer combinations can be used to deliver polypeptide drug to the colon effectively compared with individual polymers.
Oral delivery of peptides and proteins continues to pose challenges to pharmaceutical scientists. Despite this, various research groups are continuing to make attempts to deliver these entities via the gastrointestinal (GI) tract, with moderate success. Strategies for delivery include site-directed delivery particularly in the colon and use of mucoadhesive polymers and liposomal formulations (Liu et al. Citation2006). The challenges to oral peptide and protein delivery are several, ranging from poor absorption due to degradation by proteolytic enzyme in the GI tract to high hydrophilicity and large molecular size. All these factors severely limit the use of the oral route for delivery of these entities (Vyas et al. Citation1997; Wilding, Davis, and O'Hagan Citation1994). However, studies demonstrate that an advantageous region for the absorption of peptide or protein drugs may exist in the digestive tract, specifically, the colon (Zheng et al. Citation1999). The colon offers a good site for absorption due to its less hostile environment compared with the stomach and small intestine, presence of fewer enzymes, and longer retention times. As some proteases and peptidases appear to act as barriers limiting the intestinal absorption of peptides, the colon may be a preferred absorption site where the enzymatic activity is relatively low and provides a safe environment for perorally applied undigested, unchanged, and fully active peptides (Chourasia and Jain Citation2003).
With growing evidence of the colon identified as an ideal target site for delivery of peptides, studies are devising new site-directed delivery strategies to enhance oral absorption. The use of mucoadhesive and hydrogelling polymers such as chitosan (Fetih Citation2006), polyethylene glycol (PEG)-chitosan mixtures (Prego et al. Citation2006a, Citation2006b), polyacrylic acid carbomers (Green et al. Citation1999) and polyox resins (Rowe Citation2003) have attracted considerable recent attention for delivering drugs to the intestine. Various approaches have been studied to deliver dosage forms to the colon; these include pH gradient (Dew, Ryder, and Evans 1983; Ashford et al. Citation1993; Cole et al. Citation2002), colonic bacterial enzymes (Ofori-Kwakye, Fell, and Sharma Citation2004; Wilson and Basit Citation2005), GI transit time (Gazzaniga et al. Citation1994; Steed et al. Citation1997), and pressure arising from intestinal contractions (Takaya, Ikeda, and Imagawa Citation1995).
The pH-dependent approach for colonic drug delivery utilizes the pH differential along the GI tract where values range from pH 1 to 2.5 in the stomach and pH 6.6 to 7.5 in the proximal small bowel progressively increasing in the terminal ileum and finally ranging around 5.5–7 in the colon region (Evans Citation1988; Shargel et al. 2006). The use of polymeric carriers that are able to withstand the acid environment of the stomach and dissolve at neutral pH of the distal gut has given rise to ileocolonic drug delivery systems. The most common polymer used is the commercially available Eudragit-S (Rohm Pharma, Darmstadt, Germany) that solubilizes at a pH > 7.0 (Chourasia and Jain Citation2003). The composition of the Eudragit-S polymer is 1:2 ratios of methacrylic acid and methyl methacrylate ester copolymer, usually dissolved in an organic solvent to yield a coating dispersion. Newer polymers are now available have been reported to have similar pH dissolution thresholds as Eudragit-S and are freely soluble in aqueous media (Ibekwe et al. Citation2006). However, Eudragit-S remains the most tried and tested polymer for colonic delivery as the new polymers have yet to be fully investigated.
Leuprolide acetate (LA) is a synthetic nonapeptide analog of naturally occurring porcine or ovine gonadotropin releasing hormone (GnRH). It is used in the treatment of prostate cancer, endometriosis, precocious puberty, and uterine leiomyomata. It is currently available as an intramuscular or subcutaneous injection (Okada et al. Citation1988). Leuprolide acetate was used as a model peptide in this study.
Thus, the purpose of this study was to assess and compare the controlled release and drug transport characteristics of the model polypeptide, LA, when placed in tablet formulations containing novel combinations of rate-controlling polymers and coated with a commercially available colonic release polymer, Eudragit-S. The controlled release polymers used were chitosan, a cationic polysaccharide; carbopol, a polyacrylic acid polymer; and, polyox, a neutrally charged polymer. Chitosan is poly (N-acetyl-2-amino-2-deoxy-D-glucopyranose) linked by (1 → 4)-β -glycosidic bonds (Roberts Citation1992). Lueβ en et al. (Citation1996) showed that the intestinal absorption of buserelin, a synthetic nonapeptide was enhanced in rats by chitosan. Since chitosan did not inhibit proteolytic enzyme activity, it was assumed that the opening of intercellular junctions by chitosan could lead to the enhancement of peptide absorption across the mucosa (Luβ en et al. Citation1996). Chitosan has a high charge density at a pH < 6.5; it also forms gels when combined with polyanions (Sanford Citation1990).
Carbopol-974F is a high molecular weight polymer mainly used in liquid or semisolid pharmaceutical formulations as suspending or viscosity-increasing ingredients (Rowe et al. Citation2003). In tablet formulations, carbopol is used wet or as a dry binder and as a rate controlling excipient. Polyox WSR 303-NF, also known as polyethylene oxide, is a nonionic, homopolymer of ethylene oxide. Polyox is used as a tablet binder that provides delayed drug release via the hydrophilic matrix approach (gel-forming) and has shown to be an excellent mucoadhesive polymer (Rowe et al. Citation2003).
Tablet formulations of individual polymers and their combinations were made and tested by in vitro methods. The swelling index was investigated to determine the amount of water intake within each tablet formulation since this determines the polymers' ability to create porous channels and control the release of the drug over a period of time (Augst, Kong, and Mooney Citation2006). There data were correlated to in vitro dissolution behavior wherein dissolution tests were performed to study drug release from the tablet formulations. Drug transport was studied using the everted sac technique (Guo et al. Citation2004). This system provides quantitative information on drug absorption mechanisms through testing the drug content in the intestinal sac. The everted sac has been used to study the uptake of lipids vesicles, proteins, and macromolecules with oral drug delivery potential, bioadhesive lectins, and synthetic nondegradable polymers (Rowland and Woodley Citation1981).
MATERIALS AND METHODS
Leuprolide acetate (lot #0111-029) was obtained from Polypeptide Laboratories (Torrance, CA, USA). Chitosan (low mw) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Carbopol 974P-NF (Noveon Inc., Cleveland, OH, USA) was donated by Lipscomb Chemical (Long Beach, CA, USA). Sentry polyox WSR 303-NF was donated by Dow Chemical (Midland, MI, USA). Avicel pH 101 was donated by FMC Biopolymer (Newark, DE, USA); and Eudragit S-100 was donated by Degussa (Germany). Magnesium stearate and talc were purchased from Sigma-Aldrich. All chemicals used in these studies were of pure quality, analytical grade.
Preparation of Core LA Tablets
First, 200 mg weight tablet cores each containing 5 mg LA polypeptide were formulated with individual polymers to yield the following formulations: carbopol (F1), polyox (F2), and chitosan (F3) and their combinations in a 1:1 ratio to make chitosan:polyox (F4), carbopol:polyox (F5), and carbopol:chitosan (F6). Other excipients such as Avicel pH 101, talc, and magnesium stearate were used as per protocol. Second, single punch Carver tablet press was used to directly compress the tablets to 6 kg/cm2 hardness. The core tablets were evaluated for weight variation, hardness, and disintegration.
Enteric Coating
Subsequent to the preparation of core tablets, all tablets were subjected to enteric coating using Eudragit S-100 to avoid the gastric and small intestinal environment of the GI tract. Eudragit S-100 dissolves at pH 7 or higher making it an ideal polymer for enteric coating and targeted drug delivery to the ileocaecal region of the GI tract. The tablets were coated with a 20% (w/v) Eudragit S-100 polymer-alcohol solution and subsequently dried under a constant air stream. An average of a 15% weight gain was noted in the coated tablets.
Polymer Swelling
Swelling studies were conducted to obtain information on water uptake and to correlate these data to drug release mechanisms in the milieu of the GI tract. All the formulations (F1)–(F6) were subjected to swelling studies. Each core tablet was preweighed and placed on top of a mesh sieve that was subsequently lowered into a dish containing 70 ml of pH 7.4 phosphate buffered saline (PBS) solution. Temperature of the water bath was maintained at 37° C. At designated time intervals the sieve was removed and excess water was wiped off from the mesh and surrounding area. The water uptake was determined by the following equation:
where Q = water uptake (%), Wd = weight of dry tablet, and Ws = weight of swollen tablet. All swelling studies were performed in triplicate.
Disintegration Time
The disintegration time study was conducted in a USP/NF disintegration apparatus, Electrolab ED-2L. Triplicate samples of uncoated core tablets of each formulation (F1)–(F6) were placed in the cylinder baskets of the apparatus and lowered into a beaker filled with 900 ml of PBS pH 7.4 at 37°C. The time of disintegration was recorded.
In Vitro Dissolution
Dissolution studies on the Eudragit-S-100-coated LA tablets were conducted using a USP Type II Paddle Apparatus (Vankel). The paddle speed was maintained at 50 rpm and temperature set at 37°C. The initial drug release study were conducted in 300 ml 0.1N HCl (pH 1.5) for 2 hr to simulate gastric conditions, followed by substitution with sodium phosphate tribasic buffer solution at an adjusted pH 6.8 for an additional 3 hr to simulate the environment of the small intestine. Finally, the phosphate buffer was adjusted to pH 7.4 to simulate an ileocaecal environment and the study was continued for an additional 5 hr. Sampling was conducted at various predetermined intervals for 10 hr. Drug release was measured by a UV-Vis spectrophotometer (Shimadzu, UV-2401PC) and analyzed using the Lowry colorimetric assay at a wavelength of 750 nm. All dissolution studies were performed in triplicate.
In Vitro Drug Transport
The everted sac technique was used to determine drug transport across the rat colon. Female Sprague-Dawley rats (Harlan, San Diego, CA, USA) were anesthetized using halothane and medical grade oxygen gas as per institution-approved protocols. The colonic segment of the rat was removed and rinsed with 0.9% ice-cold saline. The colon was cut into 4 cm segments, everted on a glass rod to expose the serosal side, and tied at one end with a suture thread. The suture was then tied to a small washer and the open end of the colon was fitted over the tip of the glass pipette using Teflon tape for securing purposes. The colon was then immersed into a 16 × 100 mm test tube containing 10 ml of oxygenated Tyrode buffer wherein the La tablet placed. The tube was placed in an Erlenmeyer flask with 200 ml water and placed into a moving water bath, at 37°C, 50 rpm speed. Next, 500 μ l of Tyrode buffer was added to the mucosal side of colon sac and samples were withdrawn and replaced through the opening with a pipette at predetermined intervals. Samples were analyzed using Lowry colorimetric assay at a spectrophotometric wavelength of 750 nm. All everted sac experiments were performed in triplicate.
RESULTS AND DISCUSSION
Polymer Swelling Studies
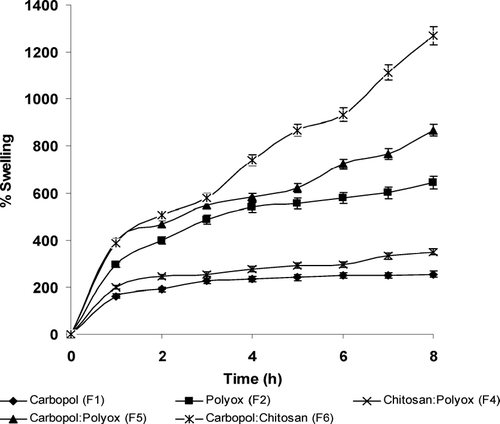
As shown in , carbopol-and-chitosan-combination formulation (F6) showed the highest percent of water uptake among all formulations, with a 13-fold increase compared with the size of the original tablet. The resulting hydrogel form remained intact as swelling occurred during an 8-hr period. The opposite charge effects of both components, carbopol (-ve) and chitosan (+ ve), resulted in an electrostatic interaction likely causing strong attraction between the two components and further facilitating improved swelling characteristics and maintaining the integrity of the tablet. However, the carbopol formulation (F1) by itself showed low swelling characteristics of only 200% whereas the directly compressed chitosan formulation (F3) disintegrated within 1 hr and was discarded. Thus, combination polymers seem to be better suited for measuring the swelling characteristics of polymers.
When carbopol was mixed with polyox (F4), a 9-fold (900%) increase was observed; however, polyox by itself (F2) showed a swelling index of approximately 600%. The presence of carbopol in combined formulations of chitosan (F6) and polyox (F5) had strong influence on the overall swelling properties of these hydrogels. When chitosan and polyox were mixed (F4), the resulting swelling index was significantly lower than other combinations thereby indicating low interaction between the two polymers. Polyox water soluble resins are very hydrophilic polymers, essentially nonionic in nature, hydrating rapidly to form a gel layer on the tablet surface for the release of actives. Due to their nonionic nature, no interaction between drugs or other polymers can be expected. Formulation (F2) containing only polyox showed an intermediate swelling effect (600% increase) compared with combinations containing carbopol that were significantly higher. By itself, polyox showed the highest swelling index of all individual polymers tested. Overall, the presence of carbopol in combination with other polymers (F5, F6) appeared to influence swelling behavior than when used alone (F1).
Disintegration Time Studies
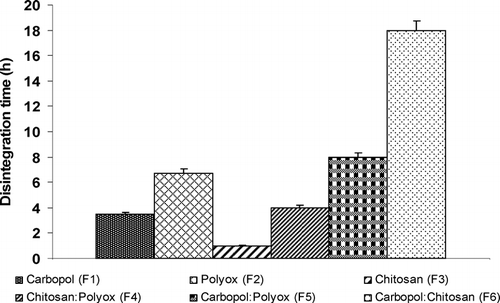
A study of disintegration time for all six formulations demonstrated correlation with the swelling indices of the formulations tested. As shown in , chitosan (F3) had the fastest disintegration of < 1 h which correlated with negligible swelling characteristics. A mixture of carbopol and chitosan (F6) showed disintegration time of over 18 hr and correspondingly correlated to the high swelling index of this formulation. Again, the charge interaction of carbopol and chitosan maintained the integrity of the tablet thus preventing their disintegration for several hours. Despite significant swelling (900%) of formulation (F5) containing carbopol and polyox, a disintegration time of ∼ 8 hr was significantly lower than formulation (F6). Formulations (F1), (F2), and (F4) also correlated well with their swelling indices. Thus, a clear correlation of swelling and disintegration was evident across all formulations tested. Further studies were conducted to assess dissolution behavior and its correlation, if any, to the swelling characteristics of these formulations.
In Vitro Dissolution Studies
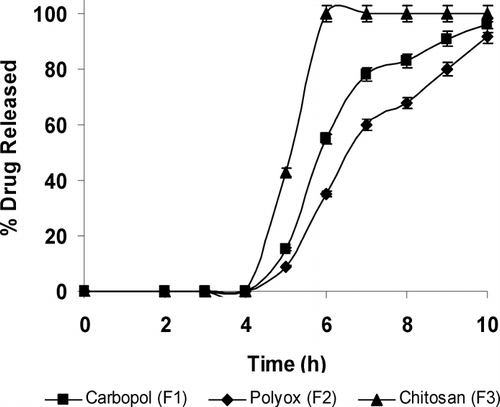
and show the release of LA from individual and combination polymers in colonic media, respectively. As shown in , chitosan formulations (F3) showed the quickest drug release with approximately 100% LA released within 1 hr of being in a colonic environment. The relatively quick release of LA correlated well with the swelling indices and disintegration time thereby indicating that chitosan polymer by itself was unsuitable for our studies. Chitosan is soluble in acidic media; however, due to the Eudragit S-100 coat, the gastric medium was bypassed and the chitosan surface was eventually exposed to a neutral pH environment of the intestine where it was rendered insoluble and disintegrated before swelling could occur. This result correlated with swelling and disintegration studies conducted earlier whereby chitosan in a neutral pH medium exhibited virtually no swelling and quick disintegration characteristics.
Comparatively, LA from carbopol (F1) and polyox (F2) demonstrated only 17% and 9% release at 5 hr, respectively. These two formulations containing LA were much slower in release and showed good correlation to swelling and disintegration characteristics. The slower release of LA from formulation (F2) was attributed to the higher swelling efficiency of polyox observed in our experiments that created longer channels within the swollen matrix. This allowed the embedded drug to release slower into the colonic media. Therefore, our data for LA release from individual polymers demonstrated a good correlation among swelling, disintegration, and dissolution.
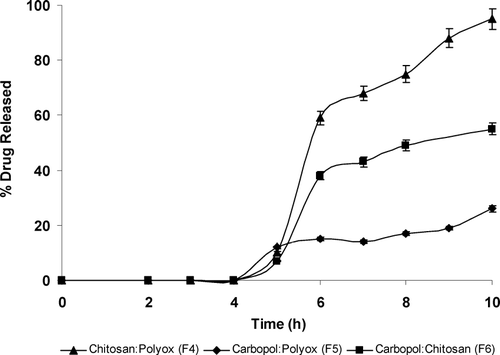
compared the release of LA polypeptide from combinations of polymers (F4–F6). At 5 hr, minimal drug release was observed from all three formulations. Beyond this time frame, the chitosan:polyox combination (F4) demonstrated a faster release of the LA drug compared with the other two formulations. This was due to the lower percentage of water uptake compared with the other two combination polymers that led to decreased amount of swelling and hence faster drug release. These data correlated well with the swelling and disintegration studies. Formulation (F4) released approximately 60% of the LA polypeptide within 6 hr of the dissolution run and almost 100% released within 10 hr. Carbopol:polyox combination (F5) showed the slowest rate of release with ∼ 15% within 5 hr and only 25% within a 10-hr period. Since both of these polymers are bioadhesive with swelling and gelling properties, their combination controlled the release of drug well.
Carbopol:chitosan combination (F6) showed drug release after 5 hr of dissolution indicating that the drug had started to release in the colon. There was ∼ 35% release in 5 hr and ∼ 55% release by 10th hr. This observation did not correlate well with the data from swelling and disintegration studies. However, overall the drug release rate from both carbopol combinations (F5, F6) was significantly slower than release from individual polymers (F1, F2, F3) and the chitosan:polyox (F4) combination.
In Vitro Drug Transport Studies
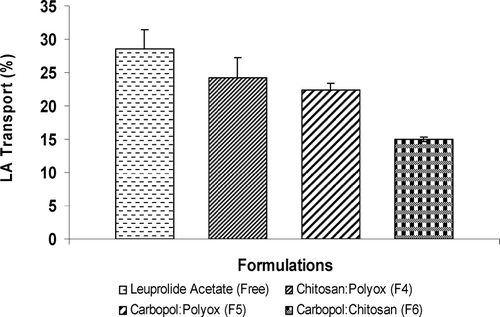
The everted colonic sacs were performed on the day the rats were sacrificed. The three combination formulations (F4–F6) were used in these experiments due to their superior drug release compared with individual polymers. shows drug transport from three formulations (F4-F6) compared with a control (free drug). Formulation (F6), a mix of carbopol and chitosan, had the lowest drug transport, with 15% transported across the everted colonic sac. Next, formulation (F5), a mix of carbopol and polyox, showed ∼ 22% drug transport moving across the mucosal lining. Similar results were observed with formulation F4, combination of chitosan and polyox, whereby the highest amount of drug release of ∼ 24% was transported across the mucosal lining of the everted colonic sac. The overall drug transport characteristics correlated well with earlier data obtained from swelling and disintegration studies. Since swelling of the tablet helps control drug release, we can predict that the more swelling the longer it will take for drug release and therefore the drug transport also would be lower. The formulations containing chitosan did not seem to enhance drug permeation partly because chitosan was not in its soluble form in media of pH 6.8. This prevented the formation of a hydrogel capable of opening the tight junctions such that more drug could be transported.
CONCLUSION
Overall, polymer combinations show better drug release kinetics than individual polymer formulations. In addition, swelling and disintegration studies of these polymer combinations correlated with drug transport across the mucosal barrier. Thus, we concluded that combinations of polymers are better suited for protein and peptide drug delivery into the colon than individual polymers. The carbopol combination formulations proved to be superior to all other combinations and individual polymers.
REFERENCES
- Ashford M., Fell J., Attwood D., et al. An in vitro investigation into the suitability of pH dependent polymers for colonic targeting. Int. J. Pharmaceut. 1993; 91: 241–245
- Augst A. D., Kong H. J., Mooney D. J. Alginate hydrogels as biomaterials. J. Pharmaceut. Pharmacol. 2006; 6(8)623–633
- Chourasia M. K., Jain S. K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharmaceut. Sci. 2003; 6(1)33–66
- Cole E. T., Scott R. A., Connor A. L., et al. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharmaceut. 2002; 231: 83–95
- Dew M. J., Ryder E. J., Evans N. Colonic release of 5-aminosalicylic acid from an oral preparation in active ulcerative colitis. Br. J. Clin. Pharmacol. 2002; 16: 185–187
- Evans D. F., Pye G., et al. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988; 29: 1035–1041
- Fetih G., Fausia H., et al. Colon specific delivery and enhanced colonic absorption of Asu(1,7) eel calcitonin using chitosan capsules containing various additives in rats. J. Drug Target. 2006; 14(3)165–172
- Gazzaniga A., Iamartino P., Maffione G., et al. Oral delayed release system for colon specific delivery. Int. J. Pharmaceut. 1994; 108: 77–83
- Green J. T., Evans B. K., et al. An oral formulation of nicotine for release and absorption in the colon: its development and pharmacokinetics. Br. J. Clin. Pharmacol. 1999; 48: 485–493
- Guo J., Ping Q., Jiang G., Dong J., Li S., Feng L., Li Z., Li C. Transport of leuprolide across rat intestine, rabbit intestine and Caco-2 cell monolayer. Int. J. Pharmaceut. 2004; 278: 415–422
- Ibekwe V. C., Fadda H. M., et al. A comparative in vitro assessment of the drug release performance of pH-responsive polymers for ileo-colonic delivery. Int. J. Pharmaceut. 2006; 308: 52–60
- Liptay S., Weidenbach H., Alder G., Schmid R. M. Colon epithelium can be transiently transfected with liposomes, calcium phosphate precipitation and DEAE dextran in vivo. Digestion 1998; 59: 142–147
- Liu L. S., Kende M., et al. Pectin/Zein beads for potential colon-specific drug delivery: synthesis and in vitro evaluation. Drug Delivery 2006; 13(4)1–7
- Lußen H. L., de Leeuw B. J., Langemeyer M. W. E., de Boer A. G., Verhoef J. C., Junginger H. E. Mucoadhesive polymers in peroral peptide drug delivery. IV. Carbomer and chitosan improve the intestinal absorption of the peptide drug burselin in vivo. Pharmaceut. Res. 1996; 13: 1668–1672
- Ofori-Kwakye K., Fell J. T., Sharma H. L. Gamma scintigraphic evaluation of film coated tablets intended for colonic or biphasic release. Int. J. Pharmaceut. 2004; 270: 307–313
- Okada H., Heya T., Ogawa Y., et al. One month release injectable microcapsules of a lutenizing hormone-releasing hormone agonist (leuprolide acetate) for treating experimental endometriosis in rats. J. Pharmacol. Exp. Therap. 1988; 244(2)744–750
- Prego C., et al. Efficacy and mechanism of action of chitosan nanocapsules for oral peptide delivery. Pharmaceut. Res. 2006a; 23(3)549–556
- Prego C., Torres D., et al. Chitosan-PEG nanocapsules as new carriers for oral peptide delivery: effect of chitosan pegylation degree. J. Control. Rel. 2006b; 111: 299–308
- Rha C. K., Rodriguez-Sanchez D., Kienzle-Sterzer C. Novel applications of chitosan. Biotechnology of Marine Polysaccharides, R. R. Colwell, E. R. Pariser, A. J. Sinskey. McGraw Hill, New York 1985; 283–312
- Roberts G. A. F. Structures of chitin and chitosan. Chitin Chemistry, G. A. F. Roberts. MacMillan, London 1992; 1–53
- Rowe R. C., Sheskey P. J., Weller P. J. Handbook of Pharmaceutical Excipients, 4th ed. Pharmaceutical Press and American Pharmaceutical Association, Bath, UK 2003; 447–450
- Rowland R. N., Woodley J. F. The uptake of distearyphosphatidylcholine/ cholesterol liposomes by rat intestinal sac in vitro. Biochem. Biophys. Acta 1981; 673: 399–406
- Sanford P. A. Chitosan and alginate: new forms of commercial interest. Am. Chem. Soc. Div. Polymer Chem. 1990; 89: 151–165
- Shargel L. Encyclopedia of Pharmaceutical Technology, J. Swarbrick. Informa Healthcare, London, UK 2000; 208
- Steed K., Hooper G., Monti N., et al. The use of pharmacoscintigraphy to focus the development strategy for a novel 5-ASA colon targeting system (TIME CLOCK® system). J. Control. Rel. 1997; 49: 115–122
- Takaya T., Ikeda C., Imagawa N. Development of a colon specific delivery capsule and the pharmacological activity of recombinant human granulocyte colony stimulating factor (rhG-CSF) in beagle dogs. J. Pharm. Pharmacol. 1995; 47: 474–478
- Tozaki H., Komoike J., Tada C., Maruyama T., Terabe A., Suzuki Yamamoto A., Muranishi S. Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon. J. Pharmaceut. Sci. 1997; 86: 1016–1021
- Vyas S. P., Venugopalan P., Sood A., Mysore N. Some approaches to improve bioavailability of peptides and proteins through oral and other mucosal routes. Pharmazie 1997; 52: 339–345
- Wilding I. R., Davis S. S., O'Hagan D. T. Targeting of drugs and vaccines in the gut. Pharmacol. Therap. 1994; 62: 97–124
- Wilson P. J., Basit A. W. Exploiting gastrointestinal bacteria to target drugs to the colon: an in vitro study using amylase coated tablets. Int. J. Pharmaceut. 2005; 300: 89–94
- Zheng Y., Qiu Y., et al. Permeability and absorption of leuprolide from various intestinal regions in rabbits and rats. Int. J. Pharmaceut. 1999; 185: 83–92