Abstract
This article describes buccal permeation of chlorpheniramine maleate (CPM) and its transbuccal delivery using mucoadhesive buccal patches. Permeation of CPM was calculated in vitro using porcine buccal membrane and in vivo in healthy humans. Buccal formulations were developed with hydroxyethylcellulose (HEC) and evaluated for in vitro release, moisture absorption, mechanical properties, and bioadhesion, and optimized formulation was subjected for bioavailability studies in healthy human volunteers. In vitro flux of CPM was calculated to be 0.14 ± 0.03 mg.h−1.cm−2 and buccal absorption also was demonstrated in vivo in human volunteers. In vitro drug release and moisture absorbed were governed by HEC content and formulations exhibited good tensile and mucoadhesive properties. Bioavailability from optimized buccal patch was 1.46 times higher than the oral dosage form and the results showed statistically significant difference.
Buccal delivery of drugs provides an attractive alternate to the oral route of drug administration, particularly in overcoming deficiencies associated with the oral administration. Buccal mucosa has excellent accessibility, an expanse of smooth muscle, and relatively immobile mucosa, hence suitable for administration of retentive dosage forms. The direct entry of the drug into the systemic circulation avoids the first-pass hepatic metabolism leading to increase in bioavailability (Senel and Hincal Citation2001; Choi et al. Citation2000). Other advantages such as low enzymatic activity, painless administration, easy drug withdrawal, facility to include permeation enhancers/enzyme inhibitors or pH modifiers in the formulation, and versatility in designing as multidirectional or unidirectional release systems for local or systemic actions offer buccal adhesive drug delivery systems as a promising option for continued research.
Various mucoadhesive formulations were suggested for buccal delivery that includes buccal patches (Anders and Merkle Citation1989; Vamshi et al. Citation2007), adhesive tablets (Owens et al. Citation2005; Jafar et al. Citation2004), and adhesive gels (Ishida et al. Citation1983). However, buccal films are preferred over adhesive tablets in terms of flexibility and comfort (Peh and Wong Citation1999).
Chlorpheniramine maleate (CPM) is a histamine H1 receptor antagonist widely used for the treatment of various allergic conditions (Koch et al. Citation1998). It is a low molecular weight (274.79) amphiphile with pKa 9.1, characterized by the presence of a hydrophobic ring and a hydrophilic side chain (Ceschel et al. Citation1999). It was selected as the model drug for investigation as it undergoes extensive first-pass metabolism resulting in low bioavailability of 25–50% (Kotzan et al. Citation1982). Furthermore, the low dose (4 mg) is convenient for loading into a patch system.
Hydroxyethylcellulose (HEC 10) is a nonionic water-soluble polymer derived from cellulose. It dissolves readily in cold or hot water and the resulting solutions maintain a pH of ∼7, which is close to the physiological salivary pH (5.8–7.4). It has been used to prepare various mucoadhesive dosage forms resulting in a good mucoadhesive performance with excellent release characteristics (Nafee et al. Citation2003; Perioli et al. Citation2004). The present study describes in vitro permeation of CPM, formulation, and in vitro and in vivo evaluation of mucoadhesive buccal patches for the enhancement of bioavailability.
MATERIALS AND METHODS
Chlorpheniramine maleate was a gift from Zydus Cadila Healthcare Ltd. (Ahmedabad, India). Brompheniramine maleate was generous gift from Granules India (Hyderabad, India); hydroxyethylcellulose 10 cps was obtained from Loba Chemie Pvt. (Mumbai, India) and polyester backing membrane was gifted by 3M (St. Paul, MI, USA). Mucin (Crude Type II) was procured from Sigma-Aldrich (Germany) and was used without further purification. Phenol red was obtained from Hi Media Laboratories Pvt. (Mumbai, India). All reagents were used of analytical grade.
In vitro Drug Permeation Studies
In vitro permeation studies were performed with porcine buccal membrane using vertical diffusion cells. Buccal tissue from domestic pigs was obtained from a local slaughterhouse and used within 2 hr of slaughter. The tissue was stored in Krebs buffer at 4°C upon collection. Epithelium was separated from the underlying connective tissue using surgical technique and the membrane was allowed to equilibrate for ∼1 hr in receptor buffer to regain the lost elasticity. The membrane was clamped between donor and receiver chambers of the vertical diffusion cell.
Temperature was maintained at 37°C by a jacket surrounding the receiver chamber, and mixing in the receiver chamber was continued with a magnetic bead stirring approximately at 400 rpm. The mounted cells were allowed to equilibrate for 30 min and later donor chamber was charged with 2 ml of phosphate-buffered saline (PBS) pH 6.6 containing 4 mg CPM and phenol red (20 μg/ml). Receiver chamber contained 12 ml of PBS pH 7.4. Samples (1 ml) were collected at preset intervals and replenished with fresh medium.
Analysis of samples was performed with a Shimadzu HPLC system equipped with LC-10AT pump and UV-Vis spectrophotometric detector (SPD-10A). CPM was quantified according to a reported method (Huang et al. Citation1982). Samples were eluted on a C18 column (Phenomenex; 25 cm × 4.6 mm; 5 μm) using a mobile phase consisting of phosphate buffer (0.1 M ammonium dihydrogen orthophosphate adjusted to pH 2.5 with orthophosphoric acid) and acetonitrile (70:30). Sample preparation briefly involved addition of 300 μl of acetonitrile to 100 μl of sample, vortexed, centrifuged to precipitate the proteins, and 20 μl of supernatant was spiked. A flow rate of 1 ml/min was maintained and the detection wavelength was 265 nm.
Phenol red was estimated spectrophotometrically by alkalinizing with sodium hydroxide. First a 250 μl of acetonitrile was added to 250 μl of sample to precipitate the proteins, vortexed, and followed by addition of 1 ml of 0.2 M sodium hydroxide. Second, the solution was then made up to 5 ml, vortexed, centrifuged, and absorbance of supernatant was measured at 563 nm using UV-Vis spectrophotometer.
Buccal Absorption Studies
Buccal absorption test was performed for solution of CPM in 8 healthy male volunteers aged between 24 and 29 years and weighing between 59 to 75 kg. The ethics committee of the University College of Pharmaceutical Sciences, Kakatiya University, India, approved the protocol. Volunteers participated in the study after signing informed consents. This method uses phenol red, a nonabsorbable marker for determining saliva volumes. We assumed that phenol red is lost neither by absorption nor by swallowing (Schurmann and Turner Citation1978; Tucker Citation1988). Before the test, volunteers were asked to moisten their mouth with 20 ml of buffer solution. Phosphate buffer solution (20 ml, pH 6.6) containing 4 mg CPM and phenol red (20 μg/ml) was given to the volunteers and they were asked to swirl the solution about 60 swirling/min. The samples of 1 ml were collected from the floor of the mouth at 2, 4, 6, 8, 12, 14, and 16 min using a micropipette. While collecting the samples, volunteers were asked to stop swirling momentarily and draw solution to the floor of the mouth. After the last sample was collected, all the solution was expelled into a beaker. Volunteers were asked to rinse their mouth twice with 20 ml of phosphate buffer (pH 6.6) and the washings were pooled with the original sample. Volume was noted and the quantity of CPM present in the samples was estimated by HPLC. Phenol red was estimated as described in the previous section.
Preparation of Adhesive Polymeric Patches
Buccal patches were prepared using solvent casting technique with HEC as polymer and propylene glycol as plasticizer. Polymer was added to 20 ml of distilled water and allowed to stand for 6 hr to swell. Propylene glycol and CPM were dissolved in 5 ml of distilled water and added to the polymer solution. This was set aside for 2 hr to remove entrapped air, transferred to a Petri plate, and dried at 40°C in an oven. The formed patches were removed carefully, cut to size, and stored in a desiccator. The composition of the patches is shown in . Patches were subjected to weight variation, thickness variation, and content uniformity tests; resultant patches had a thickness ranging from 203 ± 8.91 μm to 389 ± 15.40 μm. Patches with any imperfections, entrapped air, differences in weight, or CPM content were excluded from further studies.
TABLE 1 Formulation ingredients of mucoadhesive buccal patches of chlorpheniramine maleate
In vitro Release Studies
The drug release from buccal patches was studied using the USP 28, type II dissolution test apparatus (Lab India dissolution test apparatus Disso 2000) equipped with an auto sampler and fraction collector for the collection and replenishment of the sample and dissolution medium, respectively. Patches (1.27 cm2) were meant to release the drug from one side only; therefore, an adhesive impermeable polyester backing layer was placed on the other side of the patch. The assembly for release studies was prepared by sandwiching the patch between dialysis membrane tubing with a cut off molecular weight of 5000 (Hi Media). A piece of glass slide was placed as support to prevent the assembly from floating.
The dialysis tubing with patch inside was secured from both ends using dialysis closure clips and placed in the dissolution apparatus. The dissolution medium was 500 ml of phosphate buffer pH 6.6. Mixing rate was 25 rpm and temperature was maintained at 37 ± 0.5°C. Samples of 3 ml were collected at different intervals and analyzed spectrophotometrically at 265 nm. Release studies were performed in 6 replicates and mean values were taken.
Moisture Absorption Studies
Moisture absorption studies were performed in accordance with a procedure reported earlier (Vamshi et al. Citation2007). Briefly, 5% w/v agar in distilled water, which in hot condition was transferred to Petri plates and allowed to solidify. Then 6 patches from each formulation were weighed and placed over the surface of the agar and left for 1 hr at 37°C and the hydrated patch was weighed again. The percentage of moisture absorbed was calculated using the formula:
Measurement of Mechanical Properties
Mechanical properties of the patches were evaluated using a microprocessor based advanced force gauze with a motorized test stand (Ultra Test, Mecmesin, West Sussex, UK) and outfitted with a 25 kg load cell. Strips from the patch with dimensions of 60 × 10 mm and no visual defects were cut and positioned between two clamps separated by a distance of 3 cm. Clamps were designed to secure the patch without crushing it. During the test, the lower clamp was held stationary and the strips were pulled apart by the upper clamp moving at a rate of 2.0 mm/sec until the strip broke. The force and elongation of the film at the point when the strip broke were recorded. The tensile strength and elongation at break values were calculated using the formula:
In vitro Bioadhesion Measurement
The adhesive binding of the patches containing CPM to porcine buccal mucosa was studied in quadruplicate with the same equipment as the one used for measurement of mechanical properties except that a load cell of 5 kg was used for this study. The procedure of the test was in accordance with the method reported earlier in the literature (Wong et al. Citation1999). In this test, porcine buccal membrane was secured tightly to a circular stainless steel adaptor and the buccal patch to be tested was adhered to another cylindrical stainless steel adaptor similar in diameter using a cyanoacrylate adhesive. During the test, 100 μl of 1% mucin solution was spread over the surface of the buccal mucosa and the patch was immediately brought into contact. A force of 0.5 N was applied for 180 sec to enhance the contact of the patch with the mucosa. At the end of the contact time, upper support was withdrawn at 0.5 mm/sec until the patch was completely detached from the mucosa. The work of adhesion was determined from the area under force-distance curve while the peak detachment force was the maximum force required to detach the patch from the mucosa.
In vivo Bioavailability Study
In vivo bioavailability study was conducted in 6 healthy male volunteers and a randomized crossover design was employed. The ethics committee of the University College of Pharmaceutical Sciences, Kakatiya University, India, approved the study and subjects were determined to be in good health before commencement of the study. The bioavailability of CPM (4 mg) from buccal patch was compared with a standard marketed conventional release tablet containing 4 mg CPM (chlorpheniramine maleate tablets IP, Cortex Laboratories Private, India). The volunteers who participated in the study were nonalcoholic and had no medication for 2 weeks prior to the study. They refrained from eating and drinking for initial 6 hr during the study with buccal patch. The patches (AA4) the with adhesive impermeable polyester backing layer remained in the buccal position of the oral cavity with polymer side facing buccal mucosa. Blood samples (5 ml) from anticubetal vein were withdrawn at preset intervals of 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 hr and were allowed to coagulate. For the crossover study, the tablet was administered to volunteers along with 200 ml of water and blood samples were collected at similar intervals. Whole blood samples were centrifuged and serum was separated and stored at –20° C until analyzed. Brompheniramine maleate was used as an internal standard and chlorpheniramine in serum was analyzed by HPLC method as described previously.
Serum (2.0 ml) was transferred into test tubes and 25 ng of brompheniramine maleate was added and vortexed, followed by 300 μl of 1 N NaOH. After vortexing, 5 ml of diethyl ether was added and shaken for 15 min. Tubes were centrifuged and the diethyl ether layer was separated. The extraction procedure was repeated and organic layers were pooled. They were then dried under vacuum and after complete drying, 1 ml of diethyl ether was added to each of the test tubes and vortexed for 2 min. The solution was transferred into microcentrifuge tubes and to each of these 100 μl of 0.5% orthophosphoric acid was added and vortexed for 5 min. The samples were frozen at −80°C and diethyl ether layer was discarded. The frozen aqueous layer was vacuum dried for 5 min to remove traces of diethyl ether. Samples were brought to room temperature and 20 μl was injected onto HPLC column.
Peak serum concentration (Cmax), time to reach peak serum concentration (Tmax) and area under serum concentration time curve (AUC) were obtained for each subject from serum concentration versus time profile using Kinetica™ software (InnaPhase Corp., 2000). All data was statistically analyzed using Sigmastat software package (Jandel Corp., CA, USA). Paired t-test was used for comparison of pharmacokinetic parameters. A value of p < 0.05 was considered to be significant and results were expressed as mean ± SD.
RESULTS AND DISCUSSION
Drug Permeation Studies
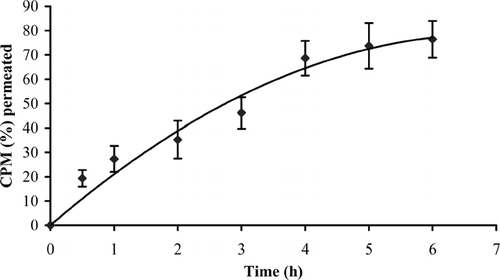
Porcine buccal mucosa has been the most frequently chosen model for in vitro permeation studies because of its similarity to human tissue and is available in large quantities from slaughterhouses (de Vries et al. Citation1991; Squier and Hall Citation1985). Cumulative amount of CPM permeated through the porcine buccal epithelium is shown in . The isolated membrane was intact as no detectable level of phenol red, which was used as a nonabsorbable marker compound, was found in the receiver compartment. The thickness of the isolated membrane, measured with a digital micrometer (Mitutoyo, Japan), ranged from 1040 to 1880 μm. Cumulative amount of CPM permeated in 6 hr was ∼76.43% and flux was calculated to be 0.14 ± 0.03 mg.h−1.cm−2. Le Brun et al. (Citation1989) reported buccal absorption of compounds with similar physicochemical properties and pKa values. Although buccal absorption of CPM in rabbit buccal mucosa was reported (Alur et al. Citation1999), others mention a considerable degree of parakeratinization in rabbit buccal epithelium (Squier and Hall Citation1984). Thus, studies on porcine buccal epithelium may yield more reliable results and can be extrapolated to humans.
Buccal Absorption Test
Buccal absorption test was conducted to substantiate the results from the in vitro permeation studies. In addition, it gives information regarding the irritant nature of the drug to oral mucosa. The results of buccal absorption study () revealed that CPM could be absorbed through the oral mucosal membranes. We found that ∼45.90% of the drug was absorbed in 16 min. The drug was absorbed at a rapid rate for the first 4 min, after which the drug absorption continued at a uniform rate. The volunteers did not swallow the solution. This was evident from the observation that the total quantity of phenol red (392.40 ± 5.31 μg) calculated from the expelled solution after 16 min and quantity of phenol red present in the 8 collected samples was nearly equivalent to the initial quantity of phenol red (400 μg) in solution given to the volunteers for swirling. The loss of phenol red (∼7.60 μg) may be due to binding of phenol red to the oral mucosa. This was calculated from the change in the phenol red concentration. Volunteers did not report any discomfort or irritation. Visual observation of the mucosa after the test did not show any evidence of mucosal irritation or damage.
FIG. 2 Buccal absorption of CPM (4.0 mg) in healthy human volunteers; values represented as mean ± S.D. (n = 8).
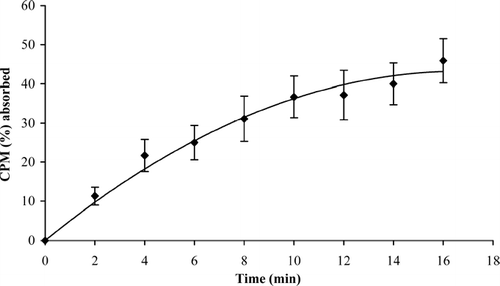
The results were in accordance with the results of in vitro permeation studies and show that CPM could permeate through the human buccal membrane. Hence there is a scope for the development of a buccal dosage form for CPM.
In vitro Drug Release Studies
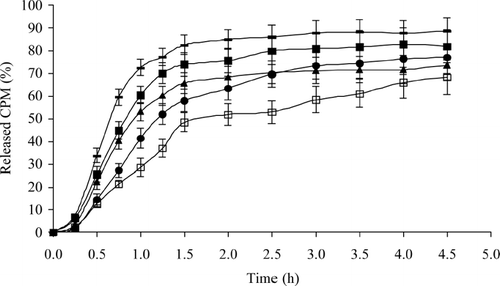
shows the drug release profiles of CPM patches containing various ratios of polymer and drug. It is apparent from the plots that drug release is governed by HEC content. A short lag time of ∼15 min was observed. The patch AA1 released the drug at a faster rate than rest of the formulations with T50 value less than 45 min, and more than 70% of the drug was released in the first 1 hr. Approximately 70% of the drug was released from the formulations AA2, AA3, and AA4 within the first 2.50 hr. As the polymer concentration was increased further, the release decreased. This may be evident from the fact that, on an average, only ∼53.02% of the drug was released from AA5 in the first 2.50 hr and release was ∼68.32% after 4.50 hr. In all formulations, a zero-order equation showed better fit than the first order as calculated by the r2 values. When the patches were subjected to dissolution test, rate of permeation of the dissolution medium into the patches determined the drug release rate. Increasing the quantity of polymer in the patches produces a water-swollen gel-like state that can substantially reduce the permeation of the dissolution medium into the patches and thus retard the drug release.
Moisture Absorption Studies
Moisture absorption studies evaluated the integrity of the formulation upon exposure to moisture. Results of moisture absorption studies () revealed that the percentage of moisture absorbed varied from 148.34 ± 7.60 to 205.31 ± 7.54 w/w for various formulations. The percentage of moisture absorbed increased with increase in polymer content of formulations. Formulation AA1 deformed within 30 min; hence lower polymer concentrations may not be suitable for formulation of buccal patches. Swelling followed by erosion during the test shows that the mechanism by which the drug was released involved swelling of the polymer followed by drug release from the swollen matrix by diffusion.
TABLE 2 Moisture absorption and mechanical properties of mucoadhesive buccal patches of chlorpheniramine maleate
Mechanical Properties of Patches
Ideal buccal film, apart from good bioadhesive strength, should be flexible, elastic, and strong enough to withstand breakage due to stress caused during its residence in the mouth. The tensile strength (TS) and elongation at break (E/B) shows the strength and elasticity of the film. A soft and weak polymer is characterized by a low TS and E/B; a hard and brittle polymer is defined by a moderate TS, and low E/B; a soft and tough polymer is characterized by a moderate TS and a high E/B; whereas a hard and tough polymer is characterized by high TS and E/B (Aulton et al. Citation1981). An ideal buccal film should have a relatively high TS and E/B (Peh and Wong Citation1999).
The results of the mechanical properties, i.e., TS and E/B, are presented in . TS increased with the increase in polymeric content but E/B values decreased with the increase in polymer content. Maximum TS was exhibited by AA5 patch (11.34 ± 1.32 kg.mm−2) and minimum was exhibited by AA1 (3.12 ± 0.90 kg.mm−2). There was no statistically significant difference in TS values for AA1 and AA2 patches (p < 0.05). Similar results were obtained with AA3 and AA4 as well as AA4 and AA5 and comparison between any other two formulations showed that they were significantly different.
With respect to E/B values, except formulations AA3 and AA4, all have shown statistically significant difference (p < 0.05). Maximum E/B was seen with AA1 (86.44 ± 5.28% mm−2) and the least was observed with AA5 (19.24 ± 3.73% mm−2).
In vitro Bioadhesion Measurements
In vitro bioadhesion measurements are routinely performed for mucoadhesive dosage forms, and the most commonly used technique for evaluation of buccal patches is the measurement of adhesive strength (He et al. Citation1998). Work of adhesion, calculated from area under the force distance-curve, is a measure of work that must be done to remove a patch or film from the tissue. Peak detachment force is the maximum applied force at which the patch detaches from tissue. The peak detachment force for the AA4 patch was calculated as 7.52 ± 0.74 N and work of adhesion was 2.70 ± 0.26 mJ. These values for bioadhesion and peak detachment force were within the range for suitable bioadhesion as reported for various films (Peh and Wong Citation1999). In addition, all the formulations were found to have similar values since the basic surface environment of the patch, which is essential for the bioadhesion, remains the same and it is only the thickness that varies. However, differences do exist due to change in the polymer type or composition of the film.
Selection of Formulation for Bioavailability Studies
Formulation AA4 was selected for the bioavailability studies due to its superior drug release properties in terms of percentage drug released (∼77.10% in 4.50 hr), its capacity to retain the structure in moisture absorption studies, and bioadhesion studies in vitro Although the final percentage of drug released from the formulation AA1 is much higher (about 88.48%), erosion in moisture absorption tests makes the formulation unsuitable to retain in the mouth and it is likely that the drug may be swallowed with the saliva before it gets absorbed through the buccal mucosa. The final percentage drug released in case of AA3 is low (∼73.65 %) and is further lower for AA5 (∼68.32%) as compared with AA4. TS of AA4 films (8.73 ± 2.34 kg.mm−2) was next to AA5 (11.34 ± 1.32 kg.mm−2). This indicated that AA4 patch possessed better film properties than other formulations. Bioadhesion values in vitro revealed that AA4 could be suitably used as a mucoadhesive buccal delivery system.
In vivo Bioavailability Studies
Analysis of serum samples by HPLC did not show any interfering peaks. Both chlorpheniramine and internal standard brompheniramine eluted without any interference. The retention times for chlorpheniramine and brompheniramine were 5.4 and 6.3 min, respectively. The method employed for the assay was sensitive and serum levels of chlorpheniramine could be detected for 24 hr. Results from the bioavailability study (, ) reveal that CPM is absorbed well by buccal route, as compared with oral route. The peak serum concentration (Cmax), time for peak serum concentration (Tmax) and the area-under-the-serum-concentration time profiles were compared. In four volunteers, Cmax was higher for buccal route than oral route and in the remaining volunteers Cmax for oral route was higher than the buccal route. This may be due to the high bioavailability variations (25–50%) for CPM.
FIG. 4 Serum levels of chlorpheniramine administered by oral (♦) and buccal (▪) routes; values represented as mean ± S.D. (n = 6).
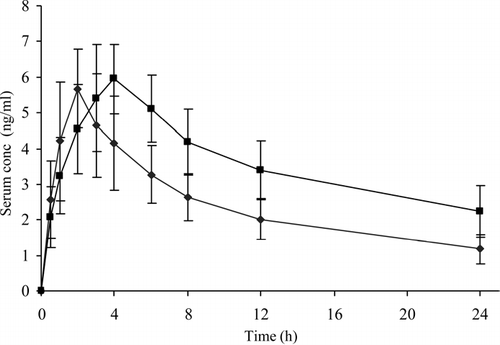
TABLE 3 Pharmacokinetic parameters of chlorpheniramine maleate (CPM) (4.0 mg) administered by oral and buccal routes in human volunteers
In all the volunteers Tmax values were higher for buccal administration than the oral administration and the difference was statistically significant (p < 0.05). This difference was because of matrix-type buccal formulation in which CPM is embedded in a polymeric matrix of HEC, in contrast, tablet administered by oral route is an immediate release dosage form. The overall mean value of AUC0 − t by buccal route was 1.46 times higher than that of oral route, and the difference was found to be statistically significant (p < 0.05) demonstrating improved bioavailability of CPM from buccal patch.
CONCLUSION
This study demonstrated that chlorpheniramine maleate could be delivered through the buccal route. The developed buccal formulation was efficacious for the delivery of hydrophilic drugs such as chlorpheniramine maleate. Results demonstrated that the dosage form was nonirritating and did not cause mucosal damage or irritation upon buccal administration. Hydroxyethylcellulose can be used as a bioadhesive polymer for buccal delivery resulting in films with favorable film properties, reasonable bioadhesion sufficient to retain the dosage form until the drug is absorbed, and superior release properties. Results of bioavailability study showed improved absorption of the drug from the buccal patch compared to oral tablet.
This research work was supported by All India Council for Technical Education (AICTE) and University Grants Commission (UGC) of India.
REFERENCES
- Alur H. H., Pather S. I., Mitra A. K., Johnston T. P. Transmucosal sustained-delivery of chlorpheniramine maleate in rabbits using a novel, natural mucoadhesive gum as an excipient in buccal tablets. Int. J. Pharm. 1999; 188: 1–10
- Anders R., Merkle H. P. Evaluation of laminated mucoadhesive patches for buccal drug delivery. Int. J. Pharm. 1989; 49: 231–240
- Aulton M. E., Abdul-Razzak M. H., Hogan J. E. The mechanical properties of hydroxypropylmethylcellulose films derived from aqueous systems: the influence of solid inclusions. Drug Dev. Ind. Pharm. 1981; 7: 649–668
- Ceschel G. C., Maffei P., Gentile M. Design and evaluation of a new transdermal formulation containing chlorpheniramine maleate. Drug Dev. Ind. Pharm. 1999; 25: 1035–1039
- Choi H., Jung J., Yong C. S., Rhee C., Lee M., Han J., Park K., Kim C. Formulation and in vivo evaluation of omeprazole buccal adhesive tablet. J. Contr. Rel. 2000; 68: 405–412
- de Vries M. E., Bodde H. E., Verhoef J. C., Junginger H. E. Developments in buccal drug delivery. Crit. Rev. Ther. Drug. Car. Syst. 1991; 8: 271–303
- He P., Davis S. S., Illum L. In vitro evaluation of mucoadhesive properties of chitosan microspheres. Int. J.Pharm. 1998; 166: 75–88
- Huang S. M., Athanikar N. K., Sridhar K., Huang Y. C., Chiou W. L. Pharmacokinetics of chlorpheniramine after intravenous and oral administration in normal adults. Eur. J. Clin. Pharmacol. 1982; 22: 359–365
- Ishida M., Nambu N., Nagai T. Highly viscous gel ointment containing carbopol for application to the oral mucosal. Chem Pharm Bull. 1983; 31: 4561–4564
- Jafar A., Ali N., Djavad F., Massoud A., Mohammad R. S. S., Majid S. Development and evaluation of buccoadhesive propranolol hydrochloride tablet formulations: effect of fillers. IL Farmaco 2004; 59: 155–16
- Koch K. M., O'Connor-Semmes R. L., Davis I. M., Yin Y. Stereoselective pharmacokinetics of chlorpheniramine and the effect of ranitidine. J. Pharm. Sci. 1998; 87: 1097–1100
- Kotzan J. A., Vallner J. J., Stewart J. T., Brown W. J., Viswanathan C. T., Needham T. E., Dighe S. V., Malinowski R. Bioavailability of regular and controlled-release chlorpheniramine products. J. Pharm. Sci. 1982; 71: 919–923
- Le Brun P. P. H., Fox P. L. A., de Vries M. E., Bodde H. E. In vitro penetration of some β -adrenoreceptor blocking drugs through porcine buccal mucosa. Int. J. Pharm. 1989; 49: 141–145
- Nafee N. A., Ismail F. A., Boraie N. A., Mortada L. M. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int. J. Pharm. 2003; 264: 1–14
- Owens T. S., Dansereau R. J., Sakr A. Development and evaluation of extended release bioadhesive sodium fluoride tablets. Int. J. Pharm. 2005; 288: 109–122
- Peh K. K., Wong C. F. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 1999; 2: 53–61
- Perioli L., Ambrogi V., Rubini D., Giovagnoli S., Ricci M., Blasi P., Rossi C. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J. Contr. Rel. 2004; 95: 521–533
- Schurmann W., Turner P. A membrane model of the human oral mucosa as derived from buccal absorption performance and physicochemical properties of the β -blocking drugs atenolol and propranolol. J. Pharm. Pharmacol. 1978; 30: 137–147
- Senel S., Hincal A. A. Drug penetration enhancement via buccal route: possibilities and limitations. J. Contr. Rel. 2001; 72: 133–144
- Squier C. A., Hall B. K. The permeability of mammalian nonkeratinized oral epithelia to horseradish peroxidase applied in vivo and in vitro. Arch. Oral Biol. 1984; 29: 45–50
- Squier C. A., Hall B. K. The permeability of skin and oral mucosa to water and horseradish peroxidase as related to the thickness of the permeability barrier. J. Invest. Dermatol. 1985; 84: 176–179
- Tucker I. G. A method to study the kinetics of oral mucosal drug absorption from solutions. J. Pharm. Pharmacol. 1988; 40: 679–683
- Vamshi Vishnu Y., Chandrasekhar K., Ramesh G., Madhusudan Rao Y. Development of mucoadhesive patches for buccal administration of carvedilol. Curr. Drug Del. 2007; 4: 27–39
- Wong C. F., Yuen K. H., Peh K. K. An in-vitro method for buccal adhesion studies: importance of instrument variables. Int. J. Pharm. 1999; 180: 47–57