Abstract
The aim of this investigation was to study the effect of an ethanol-water solvent system and ehtanolic solution of menthol on the permeation of ondansetron hydrochloride across the rat epidermis in order to select a suitable ethanol-water vehicle and optimal concentration of menthol for the development of a transdermal therapeutic system. The solubility of ondansetron hydrochloride in ethanol, water and selected concenetrtaion of ethanol-water vehicles (20:80 v/v, 40:60 v/v and 60:40 v/v) was determined. The effect of these solvent vehicles, containing 1.5% w/v of ondansetron hydrochloride, on the in vitro permeation of the drug was studied across the rat epidermis. The highest permeation was observed from 60% v/v of ethanol-water vehicle that showed highest solubilty. Hence, the hydroxypropyl cellulose (HPC) (2% w/w) gel formulations containing 1.5% w/w of ondansetron hydrochloride and selected concentrations of menthol (0, 2, 4, 8 and 10% w/w) were prepared using 60% v/v of ethanol-water vehicle, and subjected to in vitro permeation of the drug across rat epidermis. The transdermal permeation of ondansetron hydrochloride was enhanced markedly by the addition of menthol to HPC gel drug reservoir formulations. A maximum flux of ondansetron hydrochloride (77.85 ± 2.85 μ g/cm2.h) was observed with a mean enhancement ratio of 13.06 when menthol was incorporated at a concentration of 8% w/w in HPC gels. However, there was no significant increase in the drug flux with 10% w/w menthol when compared to that obtained with 8% w/w of menthol in HPC gel formulations. The results suggest that 2% w/w HPC gel drug reservoir formulation, prepared with 60% v/v ethanol-water, containing 8% w/w of menthol provides an optimal transdermal permeation of ondansetron hydrochloride.
INTRODUCTION
Transdermal drug delivery has many advantages, including the ability to bypass the first-pass metabolism, the option to administer drugs that are inactivated on oral administration, and the maintenance of relatively stable blood levels (Weiss et al. Citation1989). Despite the obvious advantages of transdermal drug delivery, this route presents unique challenges. The greatest obstacle is the stratum corneum, which provides a rate-limited step for drug absorption across the skin (Zettersten et al. Citation1997). It is composed of dead, flattened cells filled with keratin in the form of regular array of protein-rich cells embedded in an intercellular and multicellular lipid domain running parallel to the skin (Elias Citation1996). Many studies showed that lipoidal domains, the integral component of the transport barrier, must be reached if the drug is to be delivered transdermally at an appropriate rate (Gao and Singh Citation1998; Mao-Qiang et al. Citation1993). A popular technique to enhance the percutaneous absorption is the use of penetration enhancers that reversibly perturb the barrier function of the stratum corneum (Williams and Barry Citation2004). They include organic solvents (ethanol, propylene glycol, N-methypyrrolidine, dimethyl sulphoxide), fatty alcohols and acids, terpenes, and azone and its derivatives. Of these, terpenes are generally regarded as safe (GRAS) with few or no adverse effects when used in transdermal formulations (Williams and Barry Citation2004). The terpenes are incorporated in transdermal formulations either in dissolved or dispersed state in an aqueous vehicle containing water, ethanol, propylene glycol, or their cosolvents. Ethanol and propylene glycol are the most commonly used cosolvents to solubilise the drug that also work as permeation enhancer (Walker and Smith Citation1996). Ethanol, used as part of a cosolvent system with water, has been demonstrated to enhance the transdermal permeation of a variety of drugs (Obata et al. Citation1993; Takahashi et al. Citation1991; Berner et al. Citation1989). Menthol, a cyclic monoterpene, has been shown to enhance the transdermal transport of several hydrophilic and lipophilic drugs (Ho et al. Citation1998; Gao and Singh Citation1998; Kobayashi et al. Citation1993; Katayama et al. Citation1992; Hori et al. Citation1991; Okabe et al. Citation1990; Krishnaiah et al. Citation2002). In the light of this information, it was planned to develop a menthol-based transdermal therapeutic system for ondansetron hydrochloride.
Ondansetron hydrochloride is a novel and specific antagonist of 5-HT3 receptor indicated for the treatment of chemotherapy-induced nausea and vomiting in patients with cancer (Markham and Sorkin Citation1993). Intravenous and oral dosage forms of the drug are commercially available. Following oral administration, ondansetron hydrochloride is well absorbed and undergoes first-pass metabolism. The absolute oral bioavailability averages 67%. A single 8 mg dose administered either in tablet or in solution form produces peak plasma concentrations of about 0.03 to 0.04 μ g/ml after 1.5 to 2 hours of administration. The recommended oral dosing regimen of ondansetron hydrochloride for emeogenic neoplastic agents is 8 mg, three times a day. Sustained-release formulations may enable the ondansetron dosing-frequency to be reduced, and therefore increase patient compliance. Rectal (Hsyu et al. Citation1994) and nasal (Hussain et al. Citation2000) absorption of ondansetron have been reported. The transdermal administration of drugs bypasses the first-pass effect, minimizes inter- and intra-patient variation and provides steady-state plasma concentration of the drug and long-term therapy from a single dose. The most striking advantage is that the patient would be able to terminate the unwanted side-effects, if any, simply by removing the transdermal patch from the application site. All these advantages lead to improved patient compliance. Thus, it is aimed at developing transdermal therapeutic systems for ondansetron hydrochloride for their possible use in the treatment of chemotherapy-induced nausea and vomiting in patients with cancer. In this context, a few reports appear in the literature indicating the possibility of developing transdermal formulations for ondansetron hydrochloride (Takahashi and Rytting Citation2001; Gwak et al. Citation2003; Gwak et al. Citation2004; Dimas et al. Citation2004; Dimas et al. Citation2004a). Thus, ondansetron hydrochloride is a good model drug for developing a membrane-controlled transdermal therapeutic systems (TTS). The present study describes the penetration enhancing effect of selected compositions of ethanol-water cosolvent systems and ethanolic solution of selected concentrations of menthol on transdermal permeation of ondansetron hydrochloride using rat epidermis as a skin model.
MATERIALS AND METHODS
Ondansetron hydrochloride and menthol were obtained from M/s Natco Fine Chemicals Pvt. Ltd., Hyderabad, India, and M/s Merck-Schuchardt, Germany, respectively. Hydroxypropyl cellulose (HPC) was obtained from M/s Dow Chemical Company, USA and was of USP quality. Acetonitrile (HPLC grade) and water (HPLC grade) were obtained from M/s Qualigens Fine Chemicals, Mumbai, India. Other materials, used in the study, include ethanol and potassium dihydrogen phosphate of analytical reagent grade, and were obtained from M/s Qualigens Fine Chemicals, Mumbai, India.
Preparation of Ethanol-Water Solvent System and HPC Gel Drug Reservoir
Ethanol and water were mixed in different ratios so as to obtain binary solvent systems of 20:80 v/v, 40:60 v/v or 60:40 v/v of ethanol in water. The composition of the drug reservoir (2% w/w HPC gel) containing selected concentrations of menthol was given in . The HPC powder was added to 60% v/v ethanol-water solvent system while being stirred by means of a stirrer (M/s Remi Motors, India) at 2,500 rpm and the resulting mixture was mixed continuously at 37°C until the gel was formed (1 h). Ondansetron hydrochloride and menthol, in the required quantity, were added to HPC gel and mixed well for complete dissolution. The drug reservoir formulations were left overnight at room temperature (28 to 30°C).
TABLE 1 Composition of HPC gel drug reservoir containing ondansetron hydrochloride and selected concentrations of menthol
HPLC Analysis of Ondansetron Hydrochloride
The quantitative determination of ondansetron hydrochloride was performed by high-performance liquid chromatography (HPLC). A gradient HPLC (Shimadzu HPLC Class VP series) with two LC-10AT VP pumps, variable wave length programmable UV/VIS Detector SPD-10A VP, CTO-10AS VP Column oven (Shimadzu), SCL-10A VP system controller (Shimadzu) and RP C-18 column (250 mm × 4.6 mm I.D.; particle size 5 μ m; YMC, Inc., Wilmington, NC 28403, U.S.A) was used. The HPLC system was equipped with the Class-VP series version 5.03 software (Shimadzu).
The mobile phase consisted of a mixture of methanol and 0.02 M potassium dihydrogen phosphate in the ratio of 60:40 v/v. The mobile phase components were filtered, and pumped at a flow rate of 1.0 mL/min. The column temperature was maintained at 40°C. The eluent was detected by UV detector at 213 nm, and the data were acquired, stored and analyzed with the software Class-VP series version 5.03 (Shimadzu). A standard curve was constructed for ondansetron hydrochloride in the range of 0.01 to 50 μ g/mL. A good linear relationship was observed between the concentration of ondansetron hydrochloride and peak area of ondansetron hydrochloride with a high correlation coefficient (r = 0. 9999). The required studies were carried out to validate the precision and accuracy of this HPLC method.
Solubility Studies
Excess ondansetron hydrochloride was added to 10 mL of water, ethanol, selected concentrations of ethanol-water solvent systems or 60% v/v ethanol-water containing selected concentrations of menthol and vortexed. The mixture was immersed in a water bath at 37°C and allowed to equilibrate. The samples (0.5 mL) were obtained as function of time (12 h, 24 h and 36 h) and filtered through 0.4 μ m membrane filter, the filtrate was suitably diluted and the concentrations of ondansetron hydrochloride were estimated by HPLC method as described above.
Quantitative Determination of Ondansetron Hydrochloride in HPC Gel Formulations
One gram of the HPC gel drug reservoir formulation was accurately weighed, placed in 100 mL volumetric flask containing 30 mL of mobile phase (60:40 v/v of methanol: 0.02 M KH2PO4), stirred for 30 min and made upto volume with mobile phase. The resultant mixture was filtered through 0.45 μ m membrane filter and injected into the HPLC column. The amount of ondansetron hydrochloride was estimated by HPLC method as described above.
Preparation of Rat Epidermis
Male albino rats (150 to 200 g) were obtained from M/s Gosh enterprises, Kolkata, India. All the experiments involving the rats were conducted in accordance with institutional guidelines and approved prior by the Institutional Ethical Committee. The animals could have a free access to food and water until used for the study. The rats were euthanized using carbon dioxide asphyxiation before the experiments. The dorsal hair was removed with a clipper and full thickness skin was surgically removed from each rat. The epidermis was prepared by a heat separation technique (Takahashi et al. Citation1991). The entire abdominal skin was soaked in water at 60°C for 60 sec, followed by careful removal of the epidermis. The epidermis was washed with water and used in the in vitro permeation studies.
In Vitro Transdermal Permeation Studies
Modified Keshary-Chien diffusion cells (Keshary et al. Citation1984) were used in the in vitro permeation studies. The rat abdominal skin was mounted between the compartments of the diffusion cell with stratum corneum facing the donor compartment. The effective diffusion area was 6.6 cm2 and the volume of the receiver compartment was 35 mL. Two grams of the drug reservoir formulation or 2 ml of drug solution containing 30 mg of ondansetron hydrochloride, was placed in the donor cell. The drug-free solvent system (60% v/v ethanol-water) was added to the receiver cell to maintain sink conditions. This selection of receptor solution for the in vitro transdermal permeation study was based on the results of the solubility study that showed highest solubility of ondansetron hydrochloride in 60% v/v ethanol-water. The cells were maintained at 37 ± 0.5°C by placing on a magnetic stirrer with heater (M/s Remi Motors, India). The contents in the receiver compartment were stirred with the help of a magnetic bar rotating at 500 rpm. The permeate samples were withdrawn from the receiver compartment at different time intervals upto 24 h, and an equivalent volume of drug-free solvent (60:40 v/v ethanol-water) was added to the receiver compartment to maintain a constant volume. The skin permeate samples were assayed for ondansetron hydrochloride by HPLC method as described above.
After 24 h of the sampling, the skin sample was removed from the cells, washed briefly in methanol (25 mL) for 15 sec (Michniak et al. Citation1994) to remove the adhering HPC gel drug reservoir. Following drying at room temperature for 10 min, the skin was cut into pieces and then homogenized in 4 mL methanol. The samples were centrifuged, the supernatant layer was filtered through 0.2 μ m membrane filter and analyzed for the drug content by HPLC method as described above.
Permeation Data Analysis
The ondansetron hydrochloride concentration in the skin permeates was corrected for sampling effects according to the following equation described by Hayton and Chen (Citation1982).
where ‘C1n’ is the corrected concentration of the nth sample, ‘Cn’ is the measured concentration of ondansetron hydrochloride in the nth sample, ‘Cn − 1’ is the measured concentration of the ondansetron hydrochloride in the (n−1)th sample, ‘VT’ is the total volume of the receiver fluid, and ‘VS’ is the volume of the sample drawn.
The flux (μ g/cm2.h) of ondansetron hydrochloride was calculated from the slope of the plot of the cumulative amount of ondansetron hydrochloride permeated per cm2 of rat epidermis at steady state against the time using linear regression analysis (Julraht et al. Citation1995; Takahashi et al. Citation1991). The steady state permeability coefficient (kp) of the drug through rat epidermis was calculated by using the following equation (Yamane et al. Citation1995): kp = J/C, where ‘J’ is the drug flux and ‘C’ is the initial concentration of ondansetron hydrochloride in donor cell. The penetration enhancing effect of menthol was calculated in terms of enhancement ratio (ER), and was calculated by using the following equation (Williams and Barry Citation1991): ER = kp with penetration enhancer/ kp without penetration enhancer.
Statistical Analysis
The difference observed in the permeation parameters on treatment with selected concentrations of ethanol-water solvent systems or ethanolic solutions of selected concentrations of menthol was tested by using analysis of variance (ANOVA) with a post hoc test such as Bonferroni test for multiple comparison using SPSS© computer program (PC Version 14.0, SPSS Inc., 1989–2005). A value of p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Optimization of Ethanol-Water Solvent System
The transdermal delivery of ondansetron hydrochloride depends on its permeation across stratum corneum which in turn depends on the development of an optimal solvent system. The solubility study was carried out with an intention of choosing an optimal vehicle for use in the receptor compartment of the diffusion cell. There was no further increase in the solubility of ondansetron hydrochloride after 24 hours of study. The results indicate that the equilibrium was achieved at 24 h for optimal solubility of ondansetron hydrochloride. The solubility of ondansetron hydrochloride in various ethanol-water cosolvent systems and ethanol or water alone is shown in . The solubility of ondansetron hydrochloride in water and ethanol was 343.7 ± 0.9 and 507.4 ± 1.2 mg/mL respectively indicating a higher solubility of the drug in ethanol than that in water. The solubility of the drug in 20:80 v/v, 40:60 v/v and 60:40 v/v ethanol-water cosolvent system was 586.2 ± 0.5, 867.9 ± 1.0 and 924.5 ± 1.2 mg/mL, respectively. The solubility of ondansetron hydrochloride in ethanol-water solvent systems was higher than that in water or ethanol alone, which may be due to cosolvency effect. The highest solubility was observed in ethanol-water solvent system in the ratio of 60:40 v/v, which was approximately 1.8 and 2.7 times more than that in water alone and ethanol alone. The stability of ondansetron in various solvent systems was assessed by HPLC method. The HPLC chromatograms showed no additional peaks without a change in the retention time of ondansetron hydrochloride, indicating that the drug was stable in the reservoir system.
TABLE 2 Mean (+SD) amount permeated (Q24), flux (J), permeability coefficient (kp) and enhancement ratio (ER) of ondansetron hydrochloride from various solvent systems across rat epidermis (n = 3)
The cumulative amount of ondansetron hydrochloride permeated across the rat epidermis from water, ethanol and ethanol-water cosolvent systems is shown in . The total drug used in study was accounted (mean total recovery, 93.65%) when the drug content in the skin, donor compartment and receptor compartment was summed up. This indicates that there was a mass balance of the drug used in the study. There was a lag period of about 2 h for obtaining steady-state flux of ondansetron hydrochloride through rat abdominal skin from all solvent systems used in the study. This may be because of the time required for the skin to get saturated with the drug. There was an increase in the amount of drug permeated (Q24), flux and permeability coefficient of the drug from ethanol alone when compared with those values obtained from water alone. However, there was a further increase in the permeation of ondansetron with an increase in the concentration of ethanol in water (). The drug flux from water alone was 2.47 ± 0.13 μ g/cm2.h and on adding ethanol to water, it increased to 8.91 ± 0.27 μ g/cm2.h with 60% v/v ethanol-water. This increase in the permeation of the drug was progressive when adding ethanol to water to a level of 60% v/v in the ethanol-water solvent system. Thus, the Q24, flux and permeability coefficient from 60% v/v ethanol-water system were 205.24 ± 6.80 μ g/cm2, 8.91 ± 0.27 μ g/cm2.h and 0.59 ± 0.02 cm/h X10− 3 respectively. The mean enhancement ratio (ER) in the permeation of the drug with ethanol, 20% v/v ethanol-water, 40% v/v ethanol-water and 60% v/v ethanol-water when compared to water was 3.07, 1.42, 2.21, and 3.61, respectively. However, from ethanol alone, the value of Q24, flux and permeability coefficient were only 174.76 ± 6.40 μ g/cm2, 7.58 ± 0.23 μ g/cm2.h and 0.51 ± 0.02 cm/h X10− 3 respectively indicating that 40% v/v of water is necessary in ethanol to provide maximum permeation of the drug through rat epidermis.
FIG. 1 Mean (± SD) amount of ondansetron hydrochloride permeated across rat epidermis from water alone, ethanol alone, 20:80 v/v, 40:60 v/v or 60:40 v/v of ethanol-water cosolvent systems.
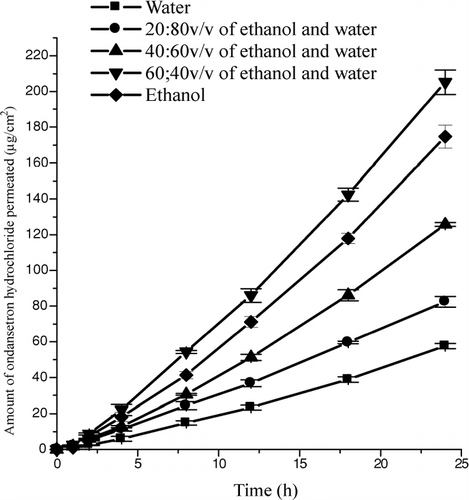
On adding ethanol to water, the flux of ondansetron hydrochloride increased to a level of 60% v/v of ethanol in water. But the flux declined in ethanol alone. Similar relationship was observed in solubility pattern wherein the solubility of the drug increased with an increase in the amount of alcohol in the cosolvent upto a level of 60% v/v of ethanol in water. Still the amount permeated (Q24), flux, and permeability coefficient from ethanol alone was significantly (p < 0.001) higher than that obtained with water indicating that ethanol is showing profound effect on the transdermal permeation of ondansetron hydrochloride. But the flux of the drug from ‘ethanol alone’ was higher when compared with that obtained from 20% v/v or 40% v/v of ethanol in water. This is in contrast to the solubility of the drug wherein the solubility in ‘ethanol alone’ was lower than that in 20% v/v or 40 %v/v of ethanol-water vehicle () indicating that the increased permeation of ondansetron hydrochloride is not due to the increased solubility of the drug in ethanol-water cosolvent system. Thus, the proportion of ethanol in ethanol-water solvent system required to provide optimal transdermal permeation may be varying with the nature of the drug.
Ethanol is commonly used in many transdermal formulations, and is often the solvent of choice for use in patches (Gao and Singh Citation1998). It is also employed as a cosolvent with water for ensuring sink conditions during in vitro permeation experiments. As with water, ethanol permeates rapidly through human skin with a steady state flux of approximately 1 mg/cm2.h (Berner et al. Citation1989). Ethanol exerts its permeation enhancing activity by increasing the solubility of poorly soluble drug in the donor phase (Pershing et al. Citation1990). Furthermore, permeation of ethanol into the stratum corneum can alter the solubility properties of the tissue with a consequent improvement for drug partitioning into the membrane (Megrab et al. Citation1995). Ethanol-water vehicles have also been shown to alter the lipoidal pathway of stratum corneum, and thereby enhanced the transdermal permeation of lipophilic permeants such as estrone, ß-estradiol, and hydrocortisone (Ghanem et al. Citation1992).
The flux of ondansetron hydrochloride with 60% v/v of ethanol-water was 8.91± 0.27 μ g/cm2.h, whereas that obtained with water was 2.47 ± 0.13 μ g/cm2.h. Thus, there was about 3.61 times increase in the flux of ondansetron with ethanol-water vehicle. This means that 60% v/v ethanol-water is acting as a penetration enhancer mostly by increasing the drug solubility in the vehicle (Pershing et al. Citation1990) as well as by improving the drug partitioning into the skin membrane (Megrab et al. Citation1995) as a consequence of its ability to permeate through the skin (Berner et al. Citation1989). It is evident from the results that 60% ethanol-water is an effective vehicle for formulating a suitable drug reservoir system for use in membrane-moderated TTS of ondansetron hydrochloride. However, the flux obtained with 60% v/v ethanol-water vehicle (8.91 ± 0.27 μg/cm2.h) is not sufficient to provide the required flux (84 μ g/cm2.h) for formulating the drug reservoir system. The required flux (J) was calculated based on the mean pharmacokinetic parameters of ondansetron hydrochloride (Clearance, CL = 21,240 ml/h) so as to obtain a mean steady-state plasma drug concentration (Css) of 26.2 ng/ml in humans (Markham and Sorkin Citation1993; Bozigian et al. Citation1994) using the equation: J = [(Css X CL)/ Area of the membrane]. The area of membrane exposed to drug permeation during in vitro permeation study was 6.6 cm2. Menthol was chosen as penetration enhancer so as to increase the flux of ondansetron hydrochloride to the required extent using 60% v/v ethanol-water as a vehicle. In vitro permeation studies were done to investigate the penetration enhancing effect of selected concentrations of menthol dissolved or dispersed in 2% w/w HPC gel drug reservoir prepared with 60% v/v ethanol-water.
Penetration-Enhancing Effect of Menthol
Menthol, used in the present study, was l-menthol that occurs most widely in nature. The penetration enhancing effect of menthol on the permeation of ondansetron hydrochloride across rat epidermis from the drug reservoir (2% w/w HPC gel with selected concentrations of menthol) was investigated so as to optimize its concentration for use in membrane-moderated TTS. The HPC was added to 60% v/v ethanol-water solvent system to impart desired viscosity that prevents the possible leakage of the drug solution from TTS. Furthermore, the presence of a hydrophilic polymer (HPC) prevents crystallization of ondansetron hydrochloride and thereby improves the stability of the drug reservoir (Raghavan et al. Citation2000). The HPC gel drug reservoir formulations were found to contain 98.20 to 99.01% of ondansetron hydrochloride. The stability of ondansetron hydrochloride in the drug reservoir containing menthol (2% w/w, 4% w/w, 8% w/w or 10% w/w) was assessed by HPLC method. The HPLC chromatograms showed no additional peaks without a change in the retention time of ondansetron hydrochloride, indicating that the drug was stable in the reservoir system.
The amount of ondansetron permeated across rat epidermis from HPC gel drug reservoir containing various selected concentrations of menthol is shown in . The total drug used in study was accounted (mean total recovery 94.9%) when the drug content in the skin, donor compartment and receptor compartment was summed up. This indicates that there was a mass balance of the drug used in the study. The maximum amount of ondansetron hydrochloride permeated during the 24 h of the study (Q24) from HPC gel drug reservoir without menthol was 162.16± 4.57 μ g/cm2 and the corresponding flux of the drug was 5.96 ± 0.08 μ g/cm2.h. A marked effect of menthol on ondansetron hydrochloride permeation was observed when it was incorporated in drug reservoir in varying quantity. The value of Q24 increased from 162.16 ± 4.57 to 1894.38 ± 62.48 μ g/cm2 from HPC gels containing 0% w/w to 10% w/w of menthol respectively. The corresponding flux and permeability coefficient were ranging from 5.96 ± 0.08 to 77.81 ± 2.31 μ g/cm2.h and 0.39 ± 0.01 to 5.19 ± 0.15 cm/h X 10− 3, respectively. However, there was a lag period of about 1 to 2 h to produce steady-state permeation of drug across rat epidermis.
FIG. 2 Mean (±SD) amount of ondansetron hydrochloride permeated across rat epidermis from 2% w/w of HPC gel containing selected concentrations of menthol as a penetration enhancer.
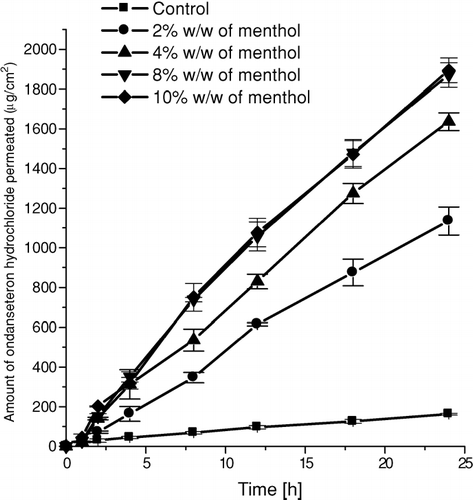
It may be observed from the results () that there was a constant increase in the flux of the drug upto 8% w/w of menthol in HPC gel, and such increase in the flux of the drug, and permeability coefficient () was significant (p < 0.001) when compared with control (without menthol). But with 10% w/w of menthol, the increase in the flux (77.81 ± 2.31 μ g/cm2.h) and permeability coefficient (5.19 ± 0.15 cm/h X 10− 3) was insignificant (p > 0.05) when compared with that obtained with 8% w/w of menthol (J = 77.85 ± 2.85 μ g/cm2.h, kp = 5.19 ± 0.19 cm/h X 10− 3). A plateau effect was observed beyond 8% w/w of menthol in the drug reservoir. We observed an approx 13.06-fold increase in the permeability of the drug from the HPC gel containing 8% w/w of menthol. The results of the study indicate that menthol, at a concentration more than 8% w/w in HPC gel, showed a plateau effect on the permeation of ondansetron hydrochloride across the rat epidermis. The HPC gel reservoir containing higher amount of menthol (10% w/w) showed a fine layer of precipitated menthol during the permeation studies. This might be because of the saturation solubility of menthol in ethanol-water (Zhao et al. Citation1999; Takahashi et al. Citation1991).
TABLE 3 Effect of menthol on the in vitro permeation parameters of ondansetron hydrochloride from HPC gel reservoir system across rat epidermis (n = 3)
There was an increase in the amount of drug retained in the rat epidermis (DRE) with an increase in the quantity of menthol incorporated in HPC gel drug reservoir. Such an increase was statistically significant (p < 0.001) when compared with that observed without menthol in HPC gel reservoir system (). The increased DRE upto 8% w/w of menthol in the drug reservoir indicates that the drug might be occupying the lipid bilayers of the skin and increased the transdermal permeation of ondansetron hydrochloride. Also there was no further increase in the value of DRE beyond 8% w/w of menthol in the HPC gel drug reservoir. In contrast, there was no significant increase in the solubility of ondansetron hydrochloride with increasing concentrations of menthol in the drug reservoir. The solubility of ondansetron hydrochloride in the solvent system (60% v/v ethanol-water) was 924.5 mg/mL, whereas the solubility in ethanolic solution of 8% w/w of menthol was 921.01 mg/mL, which did not differ significantly (p > 0.05) from each other. Thus, the results of DRE and solubility studies indicate that the enhanced flux of the drug with menthol is not due to the increased solubility of the drug in the menthol-containing drug reservoir, but due to partitioning of the drug into the stratum corneum.
Terpenes act as penetration enhancers due to their ability to modify the solvent nature of the stratum corneum and thereby improve drug partitioning into the tissue (Williams and Barry Citation2004). Mostly for this reason, the quantity of drug retained in the rat epidermis (DRE) at the end of in vitro permeation study increased with an increase in the concentration of menthol in the HPC gel drug reservoir (). Many terpenes permeate human skin well, and large amounts of terpenes (upto 1.5 μ g/cm2) have been found in the epidermis after application from a matrix type patch (Williams and Barry Citation1991). With loss of terpenes, which are generally good solvents, from a formulation there could be an alteration to the thermodynamic activity of the permeant in the formulation. Terpenes may also modify drug diffusion through the membrane (Williams and Barry Citation2004). This was evident from the enhanced flux of ondansetron hydrochloride when menthol was incorporated in HPC gel drug reservoir system prepared with 60% v/v of ethanol-water. L-menthol is a cyclic terpene alcohol with a log P value of 3.23 (Suzuki et al. Citation2000). Thus, the highly lipophilic l-menthol might have enhanced the transdermal permeation of weakly lipophilic ondansetron (log P = 1.94) across the rat epidermis (Ruell et al. Citation2004).
Several studies have indicated that terpenes partially extract the stratum corneum lipids and thereby enhance transdermal drug permeation (Cornwell et al. Citation1994; Zhao and Singh Citation1998; Williams and Barry Citation1991). Our earlier report also showed that menthol, at a concentration of 5% w/w, partially extracted the stratum corneum lipids and thereby enhanced the transdermal permeation of nicardipine hydrochloride (Krishnaiah et al. Citation2002). As discussed above, ethanol-water vehicle also disrupts the intercellular lipids of stratum corneum (Krishnaiah et al. Citation2002) and thus, it appears that the enhanced transdermal permeation of ondansetron hydrochloride is due to the combined effect of ethanol (60% v/v) and menthol (Hadgraft 1999). Furthermore, in vivo bioavailability study in human volunteers showed no signs of damage or irritation when a transdermal patch, containing 5% w/w of menthol, was applied to the skin (Krishnaiah et al. Citation2003). In the present study, the optimal concentration of menthol providing the required flux was 8% w/v which is not much higher than that used in our earlier study (Krishnaiah et al. Citation2003). Hence, it is unlikely to cause adverse effects when used in transdermal formulations. On the basis of the in vitro permeation studies, it appears that menthol at a concentration of 8% w/w in 2%w/w of HPC gel containing 60:40 v/v of ethanol-water as a solvent system was effective for enhancing the transdermal permeation of ondansetron hydrochloride. Though menthol showed enhanced permeation, the transdermal components of the proposed membrane-moderated TTS such as rate-controlling membrane (e.g., EVA2825) and adhesive coat may exhibit their own resistance to the permeation of ondansetron hydrochloride across them. This has to be taken into account before fabricating the proposed membrane-moderated TTS of ondansetron hydrochloride.
CONCLUSION
The results of the in vitro permeation studies across rat epidermis (a skin model) with selected concentrations of ethanol-water solvent systems showed that 60% v/v ethanol produces optimal transdermal permeation of ondansetron hydrochloride. When the HPC gel drug reservoir (prepared with 60% v/v ethanol-water) containing selected concentrations of menthol was subjected to in vitro permeation study, 8% w/w of menthol showed highest penetration enhancing activity indicating a synergistic penetration enhancing effect on transdermal permeation of ondansetron hydrochloride.
ACKNOWLEDGMENTS
The authors gratefully acknowledge M/s Natco Fine Chemicals Pvt. Ltd., Hyderabad, India for providing a gift sample of ondansetron hydrochloride.
REFERENCES
- Berner B., Otte J. H., Mazzenga G. C., Steffens R. J., Ebert C. D. Ethanol: water mutually enhanced transdermal therapeutic system I: Nitroglycerin solution properties and membrane transport. J. Pharm. Sci. 1989; 78: 314–318
- Bozigian H. P., Pritchard J. F., Gooding A. E., Pakes G. E. Ondansetron absorption in adults: effect of dosage form, food, and antacids. J. Pharm. Sci. 1994; 83: 1011–1013
- Cornwell P. A., Barry B. W., Stoddart C. P., Bouwstra J. A. Wide-angle X-ray diffraction of human stratum corneum: effects of hydration and terpene enhancer treatment. J. Pharm. Pharmacol. 1994; 46: 938–950
- Dimas D. A., Dallas P. P., Rekkas D. M. Ion pair formation as a possible mechanism for the enhancement effect of lauric acid on the transdermal permeation of ondansetron. Pharm. Dev. Technol. 2004; 9: 311–320
- Dimas D. A., Dallas P. P., Rekkas D. M. Use of an 8(1)3(2) asymmetrical factorial design for the in vitro evaluation of ondansetron permeation through human epidermis. Pharm. Dev. Technol. 2004a; 9: 39–48
- Elias P. M. The stratum corneum revisited. J. Dermatol. 1996; 23: 756–758
- Gao S., Singh J. In vitro percutaneous absorption enhancement of lipophilic drug tamoxifen by terpenes. J. Control. Release 1998; 51: 193–199
- Ghanem A. H., Mahmoud H., Higuchi W. I., Liu P., Good W. R. The effects of ethanol on the transport of lipophilic and polar permeants across hairless mouse skin: Methods/validation of a novel approach. Int. J. Pharm. 1992; 78: 137–156
- Gwak H. S., Oh I. S., Chun I. K. Transdermal delivery of ondansetron hydrochloride: effects of vehicles and penetration enhancers. Drug Dev. Ind. Pharm. 2004; 30: 187–194
- Gwak H. S., Oh I. S., Chun I. K. In vitro percutaneous absorption of ondansetron hydrochloride from pressure-sensitive adhesive matrices through hairless mouse skin. Arch. Pharm. Res. 2003; 26: 644–648
- Hayton W. L., Chen T. Correction of perfusate concentration samples removal. J. Pharm. Sci. 1982; 71: 820–821
- Ho H. O., Chen L. C., Lin H. M., Sheu M. T. Penetration enhancement by menthol combined with a solubulization effect in a solvent system. J. Control. Release 1998; 51: 301–311
- Hori M., Satoch H., Miabach H. I., Guy R. H. Enhancement effect of propranolol hydrochloride and diazepam skin absorption in vitro: effect of enhancer lipophilicity. J. Pharm. Sci. 1991; 80: 32–35
- Hsyu P. H., Pritchard J. F., Bozigian H. P., Lloyd T. D., Griffin R. H., Shamburek R., Krishna G., Barr W. H. Comparison of the pharmacokinetics of an ondansetron solution (8 mg) when administered intravenously, orally, to the colon, and to the rectum. Pharm. Res. 1994; 11: 156–159
- Hussain A. A., Dakkuri A., Itoh S. Nasal absorption of ondansetron in rats: an alternative route of drug delivery. Cancer Chemother. Pharmacol. 2000; 45: 432–434
- Julraht K., Keith A. P., James A. W. Development of a transdermal delivery device for melatoin in vitro studies. Drug Dev. Ind. Pharm. 1995; 21: 1377–1387
- Katayama K., Takahashi O., Matasui R., Morigaki S., Aiba T., Koizumi T. Effect of L-menthol on the permeation of indomethacin, mannitol and cortisone through excised hairless mouse skin. Chem. Pharm. Bull. 1992; 40: 3097–3099
- Keshary P. R., Chien Y. W. Mechanism of transdermal controlled nitroglycerin administration. Part 2. Assessment of rate controlling steps. Drug Dev. Ind. Pharm. 1984; 10: 1663–1699
- Kobayashi D., Matsuzawa T., Sugibayashi K., Morimoto Y., Kobayashi M., Kimura M. Feasibility of use of several cardiovascular agents in transdermal therapeutic Systems with l-menthol-ethanol system on hairless rat and human skin. Biol. Pharm. Bull. 1993; 16: 254–258
- Krishnaiah Y. S. R., Satyanarayana V., Bhaskar P. Influence of menthol and pressure sensitive adhesives on the in vivo performance of membrane-moderated transdermal therapeutic system of nicardipine hydrochloride in human volunteers. Eur. J. Pharm. Biopharm. 2003; 55: 329–337
- Krishnaiah Y. S. R., Satyanarayana V., Karthikeyan R. S. Penetration enhancing effect of menthol on the percutaneous flux of nicardipine hydrochloride from HPC gel through excised rat epidermis. Pharm. Dev. Technol. 2002; 7: 305–315
- Mao-Qiang M., Feingold K. R., Elias P. M. Inhibition of cholesterol and spingolipid synthesis causes paradoxical effects on permeability barrier homeostasis. J. Invest. Dermatol. 1993; 1011: 85–90
- Markham A., Sorkin E. M. Ondansetron: an update of its therapeutic use in chemotherapy-induced and postoperative nausea and vomiting. Drugs 1993; 45: 931–952
- Megrab N. A., Williams A. C., Barry B. W. Estradiol permeation across human skin, silastic and snake skin membranes: Effects of ethanol/water co-solvent systems. Int. J. Pharm. 1995; 116: 101–112
- Michniak B., Player M. R., Chapman J. M., Sowell J. W. Azone analogues as penetration enhancers: effect of different vehicles on hydrocortisone acetate skin permeation and retention. J. Control. Release 1994; 32: 147–154
- Obata Y., Takayama K., Maitani Y., Machida Y., Nagai T. Effect of ethanol on skin permeation of nonionised diclofenac. Int. J. Pharm. 1993; 89: 191–198
- Okabe H., Obata Y., Takayama K., Nagai T. Percutaneous absorption enhancing effect and skin irritation of monocyclic monoterpenes. Drug Des. Deliv. 1990; 6: 229–238
- Pershing L. K., Lambert L. D., Knutson K. Mechanism of ethanol-enhanced estradiol permeation across human skin in vivo. Pharm. Res. 1990; 7: 170–175
- Raghavan S. I., Trividic A., Davis A. F., Hadgraft J. Effect of cellulose polymers on supersaturation and in vitro membrane transport of hydrocortisone acetate. Int. J. Pharm. 2000; 193: 231–237
- Ruell J. A., Tsinman O., Avdeef A. Acid-base cosolvent method for determining aqueous permeability of amiodarone, itraconazole, tamoxifen, terfenadine and other very insoluble molecules. Chem. Pharm. Bull. 2004; 52: 561–565
- Suzuki T., Ishida M., Fabian W. M. F. Classical QSAR and comparative molecular field analyses of the host-guest interaction of organic molecules with cyclodextrins. J. Comput. Aid. Mol. Des. 2000; 14: 669–678
- Takahashi K., Rytting J. H. Novel approach to improve permeation of ondansetron across shed snake skin as a model membrane. J. Pharm. Pharmacol. 2001; 53: 789–794
- Takahashi K., Tamagawa S., Kattagi T., Yoshitomi H., Kamada A., Rytting J., Nishihata T., Mizuno N. In vitro transport of sodium diclofenac across rat abdominal skin: Effect of selection of oleaginous component and the addition of alcohols to the vehicle. Chem.Pharm. Bull. 1991; 39: 154–158
- Walker R. B., Smith E. W. The role of percutaneous penetration enhancers. Adv. Drug Deliv. Rev. 1996; 18: 295–301
- Weiss I., Wolf H. M., Cordes G., Cawello W. Transdermal delivery of Bupranolol. Transdermal Controlled Systemic Medications, Y. W. Chien. Marcel Dekker, New York 1989; 333–348
- Williams A. C., Barry B. W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004; 56: 603–618
- Williams A. C., Barry B. W. Terpenes and the lipid protein-partitioning theory of skin penetration enhancement. Pharm. Res. 1991; 8: 17–24
- Yamane M. A., Williams A. C., Barry B. W. Terpene penetration enhancers in propylene glycol/water co-solvent systems: effectiveness and mechanism of action. J. Pharm. Pharmacol. 1995; 47: 978–989
- Zettersten E. M., Ghadially K. R., Feingold D., Crumrine A., Elias P. M. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J. Am. Acad. Dermatol. 1997; 37: 403–40
- Zhao K., Singh J. In vitro percutaneous absorption of propranolol hydrochloride through porcine epidermis by terpenes/ethanol. J. Control. Release 1999; 62: 359–366
- Zhao K., Singh J. Mechanisms of percutaneous absorption of tamoxifen by terpenes: eugenol, D-limonene and menthone. J. Control. Release 1998; 55: 253–260