Abstract
The purpose of this study was to investigate the influence of different types of chitosan and of the preparation technique of the drug–polymer combination in improving the dissolution and permeation abilities of naproxen, a very poorly water-soluble anti-inflammatory drug. Drug–chitosan systems were prepared by simple physical mixing, kneading, cogrinding, or coevaporation using five types of chitosan (base and glutamate or hydrochloride salts, both at two different molecular weights). The products were tested for drug-dissolution behavior and for permeation properties through both Caco-2 cell monolayers and artificial lipophilic membranes. All combinations with chitosan base were significantly (p < .01) more effective in enhancing drug-dissolution rate than those with both its salts, probably in virtue of its higher amorphizing effect toward the drug, as observed in solid-state studies. A different rank order was found in permeation experiments in which chitosan glutamate was the most powerful partner in improving the drug-apparent permeability (p < .01), followed by the hydrochloride salt (p < .05), whereas no significant effect was obtained with chitosan base. Cogrinding was the most powerful technique in promoting both dissolution and permeation properties of the drug, thus pointing out the importance of the preparation method in obtaining efficacious drug-carrier systems. Finally, the good correspondence between permeation experiments with Caco-2 cells and those with the artificial lipophilic membrane indicated the suitability of this latter in preformulation studies for a rapid screening of the best carrier and the most efficient drug-carrier preparation method for improving the biopharmaceutical properties of drugs.
Adequate biopharmaceutical and pharmacokinetic characteristics are essential for the clinical success of drug candidates. Unfortunately, a high percentage of drugs endowed with a good efficacy/toxicity ratio are ultimately destined to fail in preclinical and clinical phases due to inadequate absorption properties (CitationKansy et al. 2001; CitationGombar et al. 2003). Dissolution rate in gastrointestinal fluids and permeability through biological membranes are considered as the most important factors in determining the bioavailability of orally administered drugs (CitationAmidon et al. 1995; CitationLobenberg and Amidon 2000). Due to the frequent difficulties encountered in the last few years in the area of drug discovery, related to the increasing number of drug candidates with low bioavailability, the search for new, more effective delivery systems, able to overcome these problems is a current challenge for the pharmaceutical research. Moreover, an analogous search should also be successfully extended to the improvement of the biopharmaceutical properties, and thus the therapeutic effectiveness, of already well-known active principles.
Naproxen (NAP) is a potent nonsteroidal anti-inflammatory agent very poorly water-soluble (25 mg/l at 25°C). According to the biopharmaceutic classification system (CitationAmidon et al. 1995), its low solubility and dissolution rate are the limiting steps for its absorption (CitationZecchi et al. 1984), and they can give rise to problems of variable bioavailability and bioinequivalence among commercial formulations (CitationMishra and Pandit 1988). Therefore, an improvement of NAP dissolution properties is highly desirable in order to overcome these inconveniences and enable drug promotion to the biopharmaceutic classification system class I (CitationAmidon et al. 1995). Chitosan [(1->4)-2-amino-2-deoxy-β-d-glucan] is a linear cationic copolymer obtained from N-deacetylation of chitin, a polysaccharide widely distributed in nature. It has been extensively studied recently in the pharmaceutical field for its potential in the development of several drug delivery systems, in virtue of its high biocompatibility, bioadhesiveness, dissolution, and permeation enhancer properties, in addition to its biodegradability and nontoxicity (CitationFelt et al. 1998; CitationIllum 1998; CitationPortero et al. 1998; CitationBaldrick 2000; CitationPaul and Sharma 2000) and wide availability in nature. Moreover, its antiacid and antiulcer action can be utilized to decrease gastric irritation caused by some drugs such as nonsteroidal anti-inflammatory drugs (CitationAçikgoz et al. 1995).
The ability of chitosan and its glutamate and hydrochloride salts to improve the dissolution properties of NAP has already been demonstrated (CitationMura et al. 2003b; CitationMaestrelli et al. 2004; CitationZerrouk et al. 2004). In particular, coground systems were more efficacious than simple physical mixtures in increasing the dissolution properties of NAP (CitationMura et al. 2003b), and chitosan base showed higher solubilizing power than its salts, in spite of its lower water-solubility (CitationMaestrelli et al. 2004). Moreover, unexpectedly, transport studies through Caco-2 cell monolayers showed that among the examined drug–chitosan combinations, only coground mixtures with chitosan glutamate 113 significantly improved NAP permeability with respect to the drug alone (CitationMaestrelli et al. 2004).
Taking into account all these results, we considered it worthy of interest to obtain more insight about the mechanisms responsible for these particular findings. Therefore, in the present work we investigated in depth the influence of some critical formulation and process variables, such as the type of chitosan and the method used to disperse the drug within the polymer, in order to evaluate the role of these factors on the improvement of NAP dissolution and permeation abilities. These studies should allow a proper selection of the best partner and the most effective preparation technique for optimizing the drug biopharmaceutical properties. With this aim, we prepared a series of naproxen–chitosan binary systems by keeping the drug-to-carrier ratio constant at 30:70 w/w and varying the type of chitosan (base, glutamate, or hydrochloride salts, both at two different molecular weights) as well as the preparation technique (simple physical mixing, kneading, cogrinding in a high-energy micromill, or coevaporation). All these products were then tested for NAP dissolution properties, according to the dispersed amount method, and for permeation properties using the Caco-2 cells technique. Such colon adenocarcinoma cell line of human origin has been selected for these studies since it is considered as the most advanced in vitro model able to mime most transport pathways in the gastrointestinal tract (CitationYamashita et al. 2000; CitationArtursson et al. 2001), and its effectiveness in evaluating the transport enhancer properties of chitosan has been demonstrated (CitationKotzè et al. 1997; CitationDodane et al. 1999).
However, permeation studies with Caco-2 cells present some disadvantages due to long cell growth cycles, risks of microbial contamination, high costs, and rather elevated interexperimental and interlaboratory variability. Therefore, as a further objective of this study, we also evaluated the possibility of employing a new alternative technique, based on the use of a suitable artificial lipophilic membrane, recently proposed by some of us as an in vitro model for studying drug permeability (CitationCorti et al. 2006a). Previous studies performed to validate this method showed a very good correlation between apparent permeability values obtained by in vitro experiments with such an artificial membrane and the corresponding absorbed human fraction for a series of drugs (including naproxen) covering the whole range of fraction absorbed in humans and comprising most of the drugs belonging to the Food and Drug Administration (FDA) list for validation of in vitro permeation methods (CitationCorti et al. 2006b).
MATERIALS AND METHODS
Materials
NAP (pKa 4.5) and chitosan base (CS) (Mw 150 KDa) were supplied by Sigma (St. Louis, MO, USA). Chitosan hydrochloride (CSCl, Seacure® Cl113 (Mw 110 KDa) and Cl213 (Mw 272 KDa)) and glutamate (CSG, Seacure® G113 (Mw 128 KDa) and G213 (Mw 260 KDa) were from Pronova Biopolymer (Drammen, Norway). The degree of deacetylation was 75–85% for CS, 87% for CSCl113, 84% for CSCl213, 85% for CSG113, and 86% for CSG213. Solvents used in the high-performance liquid chromatography (HPLC) procedure were of HPLC grade. All other reagents were of analytical grade.
Preparation of Solid Systems
NAP–polymer binary systems (30:70 w/w) were prepared by (a) physical mixing for 15 min in a turbula mixer (PM) of the powders previously sieved (75–150 μ m) and weighed; (b) cogrinding physical mixtures for 60 min at 24 Hz in a high-energy vibrational micromill (GR); (c) kneading physical mixtures with the minimum amount of ethanol–water 6:1 (v/v) (KN); and (d) coevaporation of NAP–polymer (30:70 w/w) ethanol–water 5:5 (v/v) solutions (COE).
Differential Scanning Calorimetry (DSC)
The DSC analysis was performed with a Mettler TA4000 apparatus equipped with DSC 25 cell on 5- to 10-mg samples (Mettler M3 balance) scanned in pierced Al pans at 10°C/min between 30 and 200°C under static air. Calibration of temperature and heat flow was performed with standard Indium samples. The relative degree of crystallinity of NAP in the different binary systems, expressed as a percentage of the NAP mass fraction in the starting sample, NAPRDC%, was calculated according to the following equation (CitationMura et al. 2003a):
where Δ Hmix and Δ Hst are the heats of fusion of NAP measured in the different mixed systems with the polymers and in the starting pure NAP sample, respectively. Heat of fusion measurements were carried out in duplicate, and the relative standard deviation of crystallinity data was ± 5%.
Scanning Electron Microscopy (SEM)
SEM analysis was carried out using a Philips XL-30 SEM. Prior to examination, samples were gold-sputter coated to render them electrically conductive.
Dissolution Rate Studies
Dissolution rate studies of pure NAP and of the NAP–polymer systems obtained with different techniques were performed according to the dispersed amount method (CitationNogami et al. 1969). Freshly sieved (75–150 μ m) solid systems, all containing 30 mg of drug, were added to 75 ml of water at 37 ± 0.5°C and stirred at 100 rpm. At time intervals, samples were withdrawn with a syringe-filter (pore size 0.45 μ m) and spectrometrically assayed at 272 nm (UV/VIS 1601 Shimadzu) for drug content. A correction was made for the cumulative dilution due to sample replacement with an equal volume of original medium. Each test was repeated four times (coefficient of variation CV < 1.5%). Dissolution efficiency (DE) was calculated from the area under the dissolution curve at time t and expressed as a percentage of the area of the rectangle described by 100% dissolution in the same time (CitationKhan 1975).
Artificial Membrane Preparation
A porous support of acetate–nitrate cellulose esters (0.025-μ m pore size, 70% porosity, 105-μ m thickness, 47-mm diameter) kindly supplied by Millipore (Millipore Italia S.p.A., Milan, Italy) was impregnated with a lipidic mixture consisting of Lipoid® E80 (1.70%), (kindly donated by Lipoid, Ludwigshafen, Germany), n-octanol (96.2%), and cholesterol (2.10%) (Sigma-Aldrich, Milan, Italy) according to Corti et al. (Citation2006a and Citationb). The percent of lipidic mixture impregnated, calculated by weighing the membrane before and after the soaking step, was ranged between 95 and 105%.
Permeation Studies Through Artificial Membrane
Permeation studies were performed using a Sartorius Model SM 16750 absorption simulator (Sartorius Membranfilter GmbH, Gottingen, Germany) according to the previously developed method (CitationCorti et al. 2006a). Briefly, the artificial membrane (see the “Artificial Membrane Preparation” section) was inserted in the diffusion cell connected to the donor and receptor compartments, both constituted by 100 ml of pH 7.4 phosphate buffer solutions thermostated at 37 ± 0.5°C and continuously circulated by means of two peristaltic pumps. NAP, alone or in the different binary systems with the examined polymers, was added in the donor compartment at a constant drug concentration of 12.4 mg/100 ml (i.e., about 50 times lower than its saturation solubility) to assure maintenance of sink conditions during the entire diffusion experiment. At fixed intervals, samples were withdrawn from the receptor compartment, spectrometrically assayed for drug content as described above (see the “Dissolution Rate Studies” section), and immediately put back in the medium. The components of the membrane did not interfere with the assay of the drug during diffusion experiments. The cumulative amount of drug permeated was plotted as a function of time. Each experiment was performed at least three times and the results were averaged.
Cell Cultures
The Caco-2 cell line (kindly donated by Dr. Zweibaum and Dr. Rousset, (INSERM U170, Villejuif, France) was grown routinely in T-flasks at 37°C in an atmosphere of 10% CO2/90% air with 95% relative humidity. The culture medium (Dulbecco's modified Eagle's medium, pH 7.4, containing 10% fetal bovine serum and 1% nonessential amino acids [Life Technologies Eragny, France]) was changed every day. All cells used in these studies were received at passage 8 and used at passages 80–85. Cells were seeded at a density of 102 cells/cm2on tissue culture-treated polycarbonate filters (area 1.13 cm2) in Costar Snapwell six-well plates (Costar Europe Ltd, Badhoevedorp, The Netherlands). Filters were used for transport studies 21–28 days after seeding (CitationKotzé et al. 1997).
Permeation Studies Through Caco-2 Cells
The polycarbonate filters (see the “Cell Cultures” section) were placed into Grass-Swettana chambers, thermostated at 37°C in the presence of 1.5 ml pH 7.4 buffered Hanks' balanced salt solution (HBSS) on both the sides of the chambers in an atmosphere of 95% air and 5% CO2. Transport studies were run from the apical (AP) to the basolateral (BL) direction. After equilibration, 1.5 ml of test solution (prepared by dissolving in 100 ml of HBSS 2.5 mg of NAP, as such or as binary combination with the different polymers) was added to the AP side (the donor compartment) of cells (total volume 3 ml). At established intervals, aliquots were taken from the BL side (the receptor compartment), replaced with an equal volume of fresh HBSS solution, and assayed for drug content by HPLC as described below (see the “High-Performance Liquid Chromatography Assay” section). Results were corrected for dilution and expressed as cumulative transport as a function of time. Each experiment was performed on six filters contemporaneously.
The hydrophilic fluorescent dye Lucifer Yellow (St. Quentin Fallavier, France) was used as a paracellular marker to check the integrity of the Caco-2 cell monolayer during the experiments. Three hundred microliters of an HBSS solution of Lucifer Yellow (3 mg/100 ml) were added to the AP side, and samples were withdrawn at time intervals from the BL side and assayed using a fluorescence 96-well plate reader Cytofluor™ 4000 (Perkin Elmer). The integrity of the cell monolayer was further verified by measuring the transepithelial electrical resistance (TEER), that is, the potential difference between the two sides of the monolayer, using a Millicell® Electric Resistance System (ERS)meter (Millipore, Bedford, MA, USA) connected to a pair of chopstick electrodes. Measurements were taken on the AP side of the cells, before starting and at the end of the experiment. Average TEER values for untreated cell monolayers were over 350 Ω /cm2.
High Performance Liquid Chromatography Assay
HPLC analyses were carried out by means of a Shimadzu SPD-6A apparatus endowed with an injector valve with a 20-μ l sample loop (Mod. Rheodyne), using a Luna C18 column (5 μ m 150 × 4.6 mm ID Phenomenex®). The mobile phase was a mixture of 0.02M phosphate buffer (adjusted at pH 3 with 96% phosphoric acid), acetonitrile, and methanol (40:10:50 v/v). The flow rate was 1 ml/min, and NAP was detected spectrometrically (Shimadsu LC-6A) at 280 nm. Linearity (r2 > 0.995) was checked in the 0.3- to 18-μ g/ml concentration range. No interference was found for the marker Lucifer Yellow.
Apparent Permeability Coefficient Calculation
The apparent permeability coefficient of the drug was calculated according to the following equation (CitationMainprize and Grady 1998):
where Papp is the apparent permeability coefficient (cm · s− 1), dQ/dt (μ g · s− 1) the amount of drug permeated per unit of time, A the effective surface area of the artificial membrane (9.6 cm2) or of the cell monolayers (1.13 cm2) exposed to the medium, and C0 (μ g · ml− 1) the initial drug concentration in the donor compartment.
Statistical Analysis
The results of the dissolution and permeation experiments were statistically analyzed by one-way analysis of variance followed by the Student-Neuwman Keuls multiple comparison posttest (GraphPad Prism, version 4). The differences were considered statistically significant when p < .05.
RESULTS AND DISCUSSION
Solid-State Studies
The thermal curves of pure components and the 30/70 (w/w) drug–polymer binary systems obtained with different techniques are shown in , whereas the main thermal parameters related to the drug-melting peak in such systems, together with its relative degree of crystallinity, are collected in . The DSC curve of NAP was typical of a pure crystalline anhydrous substance, exhibiting only a sharp melting peak with Tonset = 153.4 ± 0.3°C, Tpeak = 156.7 ± 0.4°C, and fusion enthalpy 140 ± 6 J · g− 1 (mean of four runs). The DSC profiles of CS and both its salts, typical of amorphous hydrated compounds, exhibited a broad endothermal effect between 50 and 130°C due to their dehydration process. The CSG showed an additional broad endothermal effect between 150 and 180°C, typical of this polymer, as also observed by other authors (CitationPortero et al. 1998; CitationGenta et al. 2003). The characteristic thermal profile of the drug appeared almost unchanged in all the physical mixtures with both CS base and its salts. A progressive reduction of intensity of the drug-melting peak, index of an increasing loss of crystallinity, was instead observed when passing from coevaporated, to kneaded, and even more to coground products, as a consequence of the stronger drug-carrier solid-state interactions obtained with the different preparation methods, even though complete disappearance of the NAP-melting peak was never achieved. The most effective technique in promoting drug amorphization was in all cases the cogrinding one, probably due to the greater particle size reduction and the more intimate and homogeneous dispersion of the drug into the amorphous polymeric matrix obtained through the high-energy mechanical treatment. As for the influence of the type of polymer, even if all the carriers were intrinsically amorphous, CS was clearly more efficacious as amorphizing agent toward NAP than both its salts. In fact, binary systems with CS base showed a more marked reduction of NAP fusion enthalpy, accompanied by a concomitant broadening of the endothermal peak. The NAP-CS coground product showed only a 3% residual drug crystallinity, in comparison with the 26 and 28% residual values still present in the corresponding products with CSG113 and CSCl113, respectively. On the contrary, no relevant influence was observed in the thermal behavior of the systems prepared with the two kinds of salts, nor by varying their molecular weight, that is, passing from 113-series products to the corresponding 213 ones (DSC curves not shown).
FIG. 1. Differential scanning calorimetry (DSC) curves of 70:30 w/w binary systems of naproxen (NAP) with (A) chitosan (CS), (B) chitosan glutamate 113 (CSG), and (C) chitosan hydrochloride 113 (CSCl). Key: PM = physical mixture; COE = coevaporated; KN = kneaded; GR = coground.
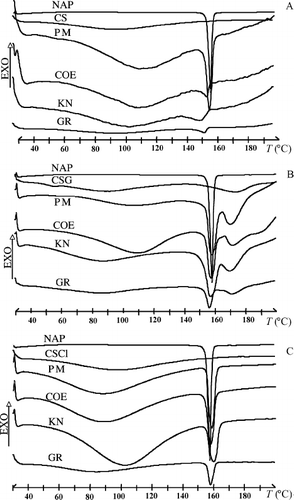
TABLE 1 Thermal parameters of naproxen-melting peak in its 30/70 w/w physical mixtures (PM), coground (GR), kneaded (KN), and coevaporated (COE) products with chitosan (CS), chitosan glutamate 113 (CSG113) and chitosan hydrochloride 113 (CSCl113), and % of relative degree of crystallinity (RDC) with respect to the pure drug
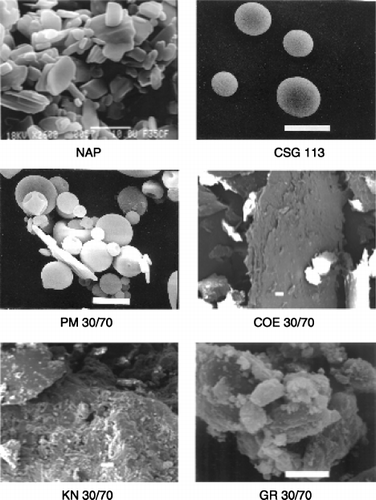
The changes in morphological features undergone by various drug-carrier systems as a function of their preparation method were investigated by SEM analysis. shows the SEM photographs of the series of NAP-CSG113 30:70 w/w systems, obtained with the different techniques, together with those of pure components. NAP powder appeared as small plate-like crystal of variable dimensions, whereas CSG113 consisted of homogeneous spherical particles. Both the components maintained their features almost unchanged and were clearly detectable in their physical mixture. On the contrary, the original morphology of both drug and carrier disappeared in coevaporated, kneaded, and coground products, in which it was no longer possible to differentiate the two components. All these products appeared as particles of irregular shape and more or less variable dimensions, characterized by a rough and porous surface, thus revealing their substantially amorphous nature. The only detectable difference among these samples was the better homogeneity and the greater particle size reduction obtained in the coground system. An analogous behavior was observed also for the other series of drug–polymer combinations.
Dissolution Studies
The drug dissolution profiles from the most representative drug–polymer binary systems investigated are shown in together with that of pure drug for comparison purposes. The main dissolution parameters of all the examined systems are summarized in in terms of percent dissolved at 10 and 30 min, DE at 60 min, relative dissolution rate at 5 min, and time to dissolve 10% drug. All these parameters were analyzed by one-way analysis of variance followed by the Student-Neuwman Keuls multiple comparison posttest to evaluate the actual statistical significance of the observed differences. The unsatisfactory dissolution properties of pure NAP can be attributed to its hydrophobic nature, poor wettability, and tendency toward particle agglomeration. The drug dissolution rate clearly increased in its different combinations with each examined hydrophilic polymer, and the observed improvement clearly depended on both the nature of the polymer and the binary system preparation method. In particular, all the binary combinations with CS base were significantly (p < .01) more effective in enhancing drug dissolution properties than the corresponding ones with both its salts, in spite of the lower water solubility of the unsalted form. This finding could be reasonably attributed to the higher amorphizing effect of CS toward the drug, as observed in solid-state studies. On the other hand, with regard to the influence of the preparation method, coground products were always the most effective for each series of drug–polymer systems. Also, such a result can be considered in agreement with those of solid-state studies that pointed out the greater degree of drug amorphization obtained with the cogrinding technique, compared with the kneading or coevaporation ones (see ). Finally, as regards the effect of the type of salt, all products with glutamate salt were significantly more efficacious (p < .01) than the corresponding ones with the hydrochloride salt, in spite of the similar amorphizing properties exhibited by both polymers toward NAP. On the contrary, the polymer molecular weight (i.e., 113-series vs. 213-series) did not have a significant influence on the dissolution performance of the products (p > .05), even though a slight decrease in drug dissolution parameters was observed for its combinations with the polymers at higher molecular weight (213-series). These findings could be attributed to the slightly greater viscosity of their aqueous solutions, which reduces the drug-diffusion rate.
FIG. 3. Dissolution profiles of naproxen (NAP) alone and from its 30/70 w/w binary systems with (A) chitosan, (B) chitosan glutamate 113, and (C) chitosan hydrochloride 113 (n = 4, CV < 1.5%). Key: (▪) NAP; (ˆ) physical mixture; (•) coground; (□) kneaded; and (▴) coevaporated products.
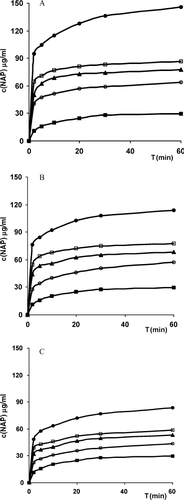
TABLE 2 Percent dissolved (PD) at 10 and 30 min, dissolution efficiency (DE) at 60 min, relative dissolution rate (RDR) at 5 min in comparison with drug alone and time to dissolve 10% of naproxen (NAP) from 30/70 w/w physical mixtures (PM), coground (GR), kneaded (KN), or coevaporated (COE) systems with chitosan (CS), chitosan glutamate (CSG113 or 213), or chitosan hydrochloride (CSCl13 or 213)
Permeation Studies
Permeation profiles through the artificial membrane and Caco-2 cell monolayers of drug alone and from some representative solid binary systems with the three types of chitosan examined are shown in and , whereas the apparent drug permeability coefficients, calculated from the slopes of the permeation profiles of both these series of experiments, are collected in and , respectively. All the apparent permeability values were statistically analyzed to assess the actual significance of the differences found among the various samples. As for the transport experiments through the Caco-2 monolayer, both the results of Lucifer Yellow transport and the TEER values, which were never below 200 Ω × cm2, indicated that the monolayer's integrity was maintained and no cellular damage occurred during the experiments (CitationPhillips and Arena 2003).
FIG. 4. Permeation profiles through artificial membrane (A) or Caco-2 cell monolayer (B) of naproxen alone (▴) and from its 30/70 w/w coground with chitosan (CS) (□) or coground (▪), kneaded, and coevaporated (ˆ) products with chitosan glutamate (CSG113). Each point represents the mean of six experiments.
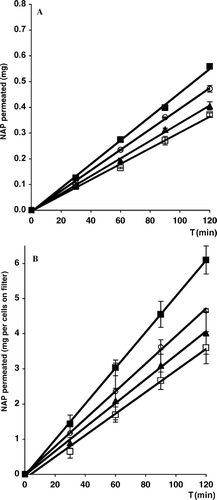
TABLE 3 Effects of chitosan base (CS) or its glutamate (CSG) or hydrochloride (CSCl) salts on naproxen (NAP) apparent permeability (Papp) across artificial lipophilic membrane
TABLE 4 Effects of chitosan base (CS) or its glutamate (CSG) or hydrochloride (CSCl) salts on naproxen (NAP) apparent permeability (Papp) across Caco-2 cells
Interestingly, a different rank order in the patterns of permeation was observed with respect to the in vitro dissolution behavior of the three different kinds of NAP–chitosan combinations examined. In fact, no significant increase in NAP permeability (p > .05) was obtained for any of the systems with CS, whereas it was the most effective carrier in enhancing drug dissolution; on the contrary, a positive influence was observed in the case of CS salts. In particular, the combinations with glutamate salt were more effective than the corresponding ones with the hydrochloride salt, all (except for the physical mixture) giving drug permeability apparent values significantly higher than that of NAP alone (p > .01), whereas in the case of systems with chitosan hydrochloride such an effect was obtained only with the coground product (p > .05). These results suggest that, even though the solubilizing effect of the carrier surely plays a role in allowing the drug to quickly dissolve into the aqueous medium (thus making it available to permeation), it was not the only factor responsible for the improved NAP permeation. Therefore, there must be an additional function of the carrier in enabling the solubilized drug to permeate quickly. On the other hand, it has been proved that a possible enhancer effect due to opening of the tight junctions can be excluded, being chitosan base more effective than its glutamate salt in this regard (CitationMaestrelli et al. 2004). Moreover, it should also be taken into account that the preparation technique had an important part in determining the performance of the final products, since none of the examined drug-carrier simple physical mixtures showed permeability values significantly different (p > .05) from NAP alone. Thus, it is possible to hypothesize the formation, during sample preparation, of specific drug-carrier interactions depending on both the characteristic of the carrier and the type of sample treatment. In particular, for each examined carrier, coground products emerged as the most efficacious in both dissolution and permeation tests. These findings seem to indicate the high-energy cogrinding as the most powerful technique in promoting the establishment of effective drug-carrier interactions, thus giving rise to an activated, more soluble and/or permeable form of the drug.
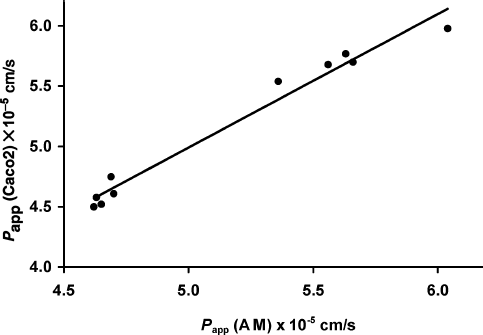
The NAP apparent permeability values obtained by using the artificial membrane (see ) were then plotted versus the corresponding ones obtained by experiments with Caco-2 cells (see ) to investigate the relationship between the two series of data (). As can be observed, an excellent linear correlation (R2 = 0.975) was obtained in the whole range of experiments, thus confirming the good predictive ability for drug-absorption properties of the proposed in vitro method (CitationCorti et al. 2006a and b), also in the presence of possible permeation enhancers such as the examined chitosan polymers.
CONCLUSIONS
This study allowed the selection of CSG113 as the most effective partner and of the cogrinding technique as the most effective preparation method for improving the biopharmaceutical properties of NAP. Administration of the drug as coground product with CSG113 should enable a reduction of the daily-drug dosage and, consequently, of the frequency of appearance of its typical side effects, also considering the antiacid and antiulcer effect of chitosan polymers (CitationAçikgoz et al. 1995).
Moreover, the good correspondence found between permeability experiments performed with Caco-2 cells and those with the proposed artificial membrane method, indicated the possibility of extending the application of this in vitro method, at the preformulation study level, to select the most suitable carrier for improving the performance of a given drug and evaluate the influence of the drug-carrier preparation method and/or the effect of added formulation excipients on the drug permeation properties, so as to optimize the effectiveness of the final product.
Financial support from MIUR is gratefully acknowledged.
REFERENCES
- M. Açikgoz, H. S. Kas, Z. Hasçelik, U. Milli, and A. A. Hincal. (1995). Chitosan microspheres of diclophenac sodium, II: in vitro and in vivo evaluation. Pharmazie 50:275–277.
- G. L. Amidon, H. Lennernäs, V. P. Shah, and J. R. Crison. (1995). A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420.
- P. Artursson, K. Palm, and K. Luthman. (2001). Caco-2 monolayers in experimental and theoretical prediction of drug transport. Adv. Drug. Del. Rev. 46:27–43.
- P. Baldrick. (2000). Pharmaceutical excipient development: the need for pre-clinical guidance. Regul. Toxicol. Pharmacol. 32:210–218.
- G. Corti, F. Maestrelli, M. Cirri, S. Furlanetto, and P. Mura. (2006a). Development and evaluation of an in vitro method for prediction of human drug absorption. I. Assessment of artificial membrane composition. Eur. J. Pharm. Sci. 27:346–353.
- G. Corti, F. Maestrelli, M. Cirri, N. Zerrouk, and P. Mura. (2006b). Development and evaluation of an in vitro method for prediction of human drug absorption. II. Demonstration of the method suitability. Eur. J. Pharm. Sci. 27:354–362.
- V. Dodane, M. Amin Khan, and J. R. Merwin. (1999). Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 182:21–32.
- O. Felt, P. Buri, and R. Gurny. (1998). Chitosan: a unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 24:979–993.
- I. Genta, P. Perugini, T. Modena, F. Pavanetto, F. Castelli, R. A. A. Muzzarelli, C. Muzzarelli, and B. Conti. (2003). Miconazole-loaded 6-oxychitin-chitosan microcapsules. Carbohydr. Polym. 52:11–18.
- V. K. Gombar, I. S. Silver, and Z. Zhao. (2003). Role of ADME characteristics in drug discovery and their in silico evaluation: in silico screening of chemicals for their metabolic stability. Curr. Top. Med. Chem. 3:1205–1225.
- L. Illum. (1998). Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 15:1326–1331.
- M. Kansy, H. Fisher, K. Kratzat, F. Senner, B. Wagner, and I. Parrilla. (2001). High-throughput artificial membrane permeability studies in early lead discovery and development: in Pharmacokinetic Optimization in Drug Research, eds. B. Testa, H. Van de Waterbeend, G. Folkers, and R. Guy., 447–464. Verlag Helvetica Chimica Acta, Weinheim, Germany, Wiley-VCH.
- K. A. Khan. (1975). The concept of dissolution efficiency. J. Pharm. Pharmac. 27:48–49.
- A. F. Kotzé, B. J. de Leeuw, H. L. Lueßen, A. G. de Boer, J. C. Verhoef, and H. E. Junginger. (1997). Chitosans for enhanced delivery of therapeutics peptides across intestinal epithelia: in vitro evaluation in Caco-2 cell monolayers. Int. J. Pharm. 159:243–253.
- R. Lobenberg, and G. L. Amidon. (2000). Modern bioavailability, bioequivalence and biopharmaceutics classification system, new scientific approaches to international regulatory standards. Eur. J. Pharm. Sci. 50:3–12.
- F. Maestrelli, N. Zerrouk, C. Chemtob, and P. Mura. (2004). Influence of chitosan and its glutamate and hydrochloride salts on naproxen dissolution rate and permeation across Caco-2 Cells. Int. J. Pharm. 271:257–267.
- T. Mainprize, and L. T. Grady. (1998). Standardization of an in vitro method of drug absorption. Pharm. Forum 24:6015–6023.
- B. Mishra, and J. K. Pandit. (1988). Comparative bioavailability of four naproxen tablets. Indian J. Pharm. Sci. 50:44–47.
- P. Mura, F. Maestrelli, M. Cirri, S. Furlanetto, and S. Pinzauti. (2003a). Differential scanning calorimetry as an analytical tool in the study of drug-cyclodextrin interactions. J. Therm. Anal. Calorim. 73:635–646.
- P. Mura, N. Zerrouk, N. Mennini, F. Maestrelli, and C. Chemtob. (2003b). Development and characterization of naproxen-chitosan solid systems with improved drug dissolution properties. Eur. J. Pharm. Sci. 19:67–75.
- H. Nogami, T. Nagai, and I. Yotsuyanagi. (1969). Dissolution phenomena of organic medicinals involving simultaneous phase changes. Chem. Pharm. Bull. 17:499–509.
- W. Paul, and C. P. Sharma. (2000). Chitosan, a drug carrier for the 21th century. S.T.P. Pharm. Sci. 10:5–22.
- J. Phillips, and A. Arena. (2003). Optimization of Caco-2 Cell Growth and Differentiation for Drug Transport Studies, Millipore Corporation Protocol Note PC1060EN00: Danvers, MA (USA).
- A. Portero, C. Remuñan-Lopez, and J. L. Vila-Jato. (1998). Effect of chitosan and chitosan glutamate enhancing the dissolution properties of the poorly water soluble drug nifedipine. Int. J. Pharm. 175:75–84.
- S. Yamashita, T. Furubayashi, M. Kataoka, T. Sakane, H. Sezaki, H. Sakane, and H. Tokuda. (2000). Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 10:195–204.
- V. Zecchi, L. Rodriguez, A. Tartarini, A. Chiarini, and P. Valenti. (1984). In vitro absorption studies on naproxen and its sodium and piperazine salts. Pharm. Acta Helv. 59:91–94.
- N. Zerrouk, N. Mennini, F. Maestrelli, C. Chemtob, and P. Mura. (2004). Comparison of the effect of chitosan and polyvynilpyrrolidone on dissolution properties and analgesic effect of naproxen. Eur. J. Pharm. Biopharm. 57:93–99.