Abstract
The skin permeation enhancement of many kinds of drugs and cosmetic substances by microemulsions has been widely known; however, the correlations between microemulsion microstructures and the efficiency of skin permeation are not fully elucidated. Therefore, the aim of our study was to investigate the influence of microemulsion types on in vitro skin permeation of model hydrophobic drugs and their hydrophilic salts. The microemulsion systems were composed of isopropyl palmitate (IPP), water, a 2:1 w/w mixture of Aerosol OT (AOT) and 1-butanol, and a model drug. The concentrations of surfactant mixture and model drug were maintained at 45% and 1% w/w, respectively. The concentrations of IPP and water were 15% and 39% w/w, respectively, for oil-in-water (o/w) type and vice versa for water-in-oil (w/o) type. The samples were prepared by simple mixing and characterized by visual appearance, pH, refractive index, electrical conductivity, viscosity, and determination of the state of water and IPP in the formulations using differential scanning calorimetry. Transdermal flux of lidocaine, tetracaine, dibucaine, and their respective hydrochloride salts from the drug-loaded AOT-based microemulsions through heat-separated human epidermis was investigated in vitro using modified Franz diffusion cells. The o/w microemulsions resulted in the highest fluxes of the model drugs in base form as compared with the other formulations within the same group of drugs. Moreover, the skin permeation of drug from microemulsions depended on drug molecular structure and interaction between drug and surfactant.
Microemulsions are thermodynamically stable, transparent, low-viscosity dispersions of oil and water stabilized by an interfacial film of a surfactant and usually combined with a cosurfactant such as a medium-chain alcohol. They can be used to deliver drugs via several routes including topical route (CitationBhargava et al. 1987; CitationLawrence and Rees 2000). Many advantages of microemulsions on transdermal delivery are generally known, including possibilities of incorporating large amount of drug due to the high solubility power, favoring drug partition into skin by modification of the thermodynamic activity of the drug, and reducing diffusion barrier stratum corneum (CitationDelgado-Charro et al. 1997). Moreover, the microemulsions are aesthetic with optical transparence, and thermodynamic stability, and they are easy to prepare.
During the recent decades, microemulsions have been developed and used as topical vehicles for many drugs and cosmetic substances (CitationLinn et al. 1990; CitationMalcolmson and Lawrence 1993; CitationGarcia-Celma et al. 1994; CitationSchmalfuβ et al. 1997; CitationBolzinger et al. 1998; CitationBaroli et al. 2000; CitationChangez and Varshney 2000; CitationKreilgaard et al. 2000; CitationRhee et al. 2001; CitationPeltola et al. 2003; CitationBoonme 2007). Although the skin permeation enhancement by microemulsions has been widely studied as mentioned, the correlations between microemulsion microstructures and the efficiency of skin permeation are not fully elucidated (CitationKreilgaard 2002).
Aerosol OT (AOT) or bis(2-ethylhexyl)sulfosuccinate sodium has been extensively used to prepare microemulsions for transdermal drug delivery (CitationOsborn et al. 1988; CitationTrotta et al. 1989; CitationOsborn et al. 1991; CitationLiu et al. 2006) and one reported that AOT microemulsion acts as a safe transdermal carrier (CitationChangez and Varshney 2000). Therefore, AOT was selected in this study to develop microemulsion of water and isopropyl palmitate (IPP) by using 1-butanol as a cosurfactant to increase the flexibility of the surfactant film around the microemulsion droplet (CitationAlany et al. 2000). The ratio of AOT and 1-butanol was selected at 2:1 since the largest microemulsion region was obtained at this ratio compared with other ratios (CitationJunyaprasert et al. 2007).
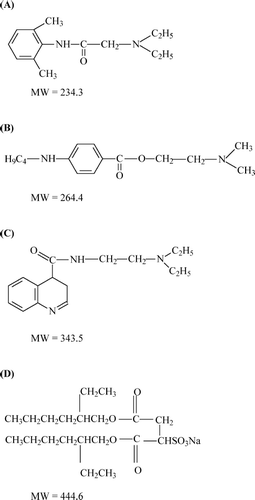
The influence of the microemulsion types, oil-in-water (o/w) or water-in-oil (w/o), on their permeation enhancement of model hydrophobic and hydrophilic drugs was studied to investigate the influence of microemulsion types on the skin permeation of drugs having different hydrophilicity. The model drugs used in this study were local anesthetics, i.e., the lidocaine group, the tetracaine group, and the dibucaine group. For topical administration, local anesthetics are used to alleviate such unpleasant sensations as pain, itching, and burning associated with laser pulses; minor surgical operations; injections; and dermatological disorders. The drugs produce reversible loss of those sensation by preventing or diminishing the conduction of sensory nerve impulses near to the site of their application (CitationReynolds 1996; CitationRang et al. 1999; CitationFriedman et al. 2001). The molecular structures of the model drugs and AOT are illustrated in .
FIG. 1. Molecular structures of (A) lidocaine, (B) tetracaine, (C) dibucaine, and (D) Aerosol OT. The molecular weight (MW) of a hydrochloride salt form is 36.4 higher than its base form.
In a previous study by our group (CitationBoonme et al. 2006), the colloidal structures of IPP/water/AOT:1-butanol (2:1) systems were investigated and characterized. We found that the transition point from water-in-oil (w/o) to oil-in-water (o/w) microemulsions occurred at a water concentration of 30% to 35% w/w when the concentration of the surfactant mixture (2:1 AOT:1-butanol) was maintained at 45% w/w. Therefore, we expected that formulations containing less than 30% and more than 35% w/w water would be w/o and o/w microemulsions, respectively. However, addition of the drug into the microemulsion formulation might affect the microstructure of the system. Thus, the drug loaded microemulsions should be characterized and compared with their blank counterparts to ascertain the microemulsion type before further investigation of the skin permeation.
In this article, we report on the characterization and skin permeation of AOT microemulsions carrying local anesthetics. We attempted to understand the relationship between microstructures of microemulsions and their efficiency of skin permeation enhancement of the drugs with different hydrophilicity.
MATERIALS AND METHODS
AOT was purchased from Fluka (Buche, Switzerland); IPP was obtained from Uniquema (DE, USA); 1-butanol was obtained from BDH Chemicals (Poole, UK). Local anesthetic drugs, i.e., lidocaine (L), tetracaine (T), and dibucaine (D) and their respective hydrochloride salts (L-H, T-H and D-H) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Isotonic phosphate buffer pH 7.4 (IPB), composed of disodium hydrogen phosphate (Carlo Erba, Rodano, Italy), sodium dihydrogen orthophosphate (BDH Chemicals Poole, UK), and sodium chloride (Carlo Erba, Rodano, Italy). Glacial acetic acid and methanol, which were used in high performance liquid chromatography (HPLC) assay, were supplied by Lab-Scan Analytical Science, (Bangkok, Thailand). Distilled water was used throughout the experiments. All chemicals were pharmaceutical grade and used without further modification.
Microemulsion Preparation
The microemulsion region of the IPP/water/AOT:1-butanol (2:1) system was determined by construction of a pseudoternary phase diagram using the water titration method as described in a previous study (CitationBoonme et al. 2006). Typical formulations of o/w and w/o microemulsions were selected from the obtained microemulsion region at 45% w/w AOT:1-butanol (2:1), 15%/39% w/w internal/external pseudophase ratio, and 1% w/w model drug. The o/w and w/o microemulsion formulations were designated as ME1 and ME2, respectively. The model drugs used in this study were lidocaine (L), tetracaine (T), and dibucaine (D) as model hydrophobic drugs and their respective hydrochloride salts (L-H, T-H, and D-H) as model hydrophilic drugs.
Therefore, 12 drug-loaded AOT-based microemulsions were prepared according to the typical formulations as presented in . The preparation was performed by incorporating model hydrophobic drug in oil phase or model hydrophilic drug in water phase, adding the mixture of AOT and 1-butanol, and mixing with the other phase (water for model hydrophobic drug or IPP for model hydrophilic drug). All components were mixed with a vortex mixer until the uniform mixture was obtained. The obtained microemulsions were characterized by several experimental techniques to determine their type before further skin permeation studies.
TABLE 1 Microemulsion formulations (% w/w)
Microemulsion Characterization
Observation of Appearance
Samples were visually observed for their physical appearance, i.e. clearness, color, and homogeneity. Their microscopic appearance was investigated using cross-polarized light microscopy (Nikon Optiphot PFX microscope, Tokyo, Japan) at a magnification of ×40. The visual appearance of microemulsions can be quite similar to that of liquid crystals, especially lamellar phases as the latter often have only slightly increased viscosities. Cubic phases, on the other hand, are highly viscous and can thus not be confused with a microemulsion. Cross-polarized light microscopy is the best way to distinguish lamellar liquid crystals from microemulsions and should always be a standard tool in the investigation of microemulsions (CitationFriberg 1990).
pH and Conductivity Measurements
The apparent pH of the formulations was measured by a pH meter (Accument AB15, Fisher Scientific, USA) in triplicate at 25°C. Electrical conductivity of the samples was measured using a Riac CM/100 conductivity meter fitted with an YSI 3418 electrode (Yellow Springs Instruments, USA), having a cell constant of 0.11 cm− 1. The measurements were carried out in triplicate at 25°C.
Refractive Index and Viscosity Measurements
The refractive indices of the formulations were determined using a refractometer (Fisher Scientific, Japan) in triplicate at 25°C. Viscosity of the samples was measured using a Brookfield DV-III programmable cone and plate rheometer (Brookfield Engineering Laboratories, USA) fitted with a CP-42 cone spindle. Brookfield Rheocalc operating software was used to control the rheometer. The jacketed sample cup was connected to a circulating water bath operating at 25°C. A sample volume of 1 ml was used for viscosity measurements and the experiment was repeated in triplicate.
Differential Scanning Calorimetry (DSC)
DSC measurements were performed with a differential scanning calorimeter TA Q100 equipped with a refrigerated cooling system (TA Instruments, USA). Nitrogen with a flow rate of 50 ml/min was used as a purge gas. Approximately 4 to 13 mg of sample were weighed precisely into hermetic aluminum pans. An empty hermetically sealed pan was used as a reference. Samples were cooled from 25°C to −50°C at a cooling rate of 5°C/min, held for 3 min at −50°C and then heated to 25°C at a heating rate of 10°C/min. All measurements were preformed at least in three experiments.
In Vitro Skin Permeation Studies
In vitro skin permeation studies were performed using modified Franz diffusion cells. The diffusion area of the cells was 1.77 cm2 and the receptor compartment volume was 12 cm3. The diffusion cells were connected with a circulating water bath and the temperature was controlled at 37°C. IPB was used as a receptor fluid and stirred by an externally driven Teflon-coated magnetic bar. The heat-separated human epidermis was placed on the receptor compartment with the stratum corneum facing upward and then the donor compartment was connected with a clamp. The human epidermis membranes were prepared from human skin tissues, obtained from abdominoplasty surgeries from Asian healthy women ranging in age from 26 to 57 years and provided by Yanhee General Hospital, Bangkok, Thailand, using a heat separation technique (CitationHaigh and Smith 1994).
Briefly, the skin was separated of subcutaneous fat and then immersed into warm water at 60°C for 2 min. Consequently, the epidermal layer was carefully separated from the dermis using blunt forceps. The obtained epidermis was wrapped with aluminum foil and stored at −20°C for no longer than 1 month. This has been reported to be a satisfactory method of storage for human skin (CitationHarrison et al. 1984). The stored epidermis was allowed to thaw, cut into 4.5 × 4.5 cm2 pieces, and hydrated by placing in IPB overnight before use (CitationSongkro et al. 2003). The necessary information, patient donor age and nationality, were recorded on receipt of skin. The study was carried out with the approval of the Committee on Human Rights Related to Human Experimentation, Mahidol University, Bangkok, Thailand.
First, 1 of microemulsion formulation was applied onto the skin surface of each donor compartment. Second, at suitable time intervals (0, 1, 2, 3, 4, 6, 8, 10, 12, 24 hr), an accurate amount of each sample was withdrawn from the center of the receptor compartment with a syringe connected with a needle. Third, an equal volume of fresh IPB was replenished immediately. The amount of the drug in the receptor fluid samples was assayed by the HPLC method as described below.
The flux of drug from each microemulsion was calculated and compared with that from the other microemulsions. The cumulative drug permeation (Qt) was calculated from equation 1:
where Ct is the drug concentration of the receptor fluid at each sampling time, Ci is the drug concentration of the ith sample, and Vr and Vs are the volumes of the receptor fluid and the sample, respectively. Data were expressed as the cumulative drug permeation per unit of skin surface area, Qt/S (S = 1.77 cm2). The steady-state fluxes (JSS(4-12 h)) were calculated by linear regression interpolation of the experimental data at steady state (between 4 and 12 h, Δ T) as shown in equation 2:
HPLC Assay
The concentrations of model drugs permeated to the receptor fluid were quantitatively analyzed by HPLC. The HPLC system (Shimadzu, Japan) connected with a HPLC column, 5 μ m particle size, 250 × 4.6 mm (Chrompack Inertsil ODS-3, The Netherlands). A mixture of 10% acetic acid in water and methanol (75:25 v/v, 65:35 v/v, and 50:50 v/v for L/L-H, T/T-H, and D/D-H, respectively) was used as a mobile phase. The injection volume was 20 μ l. The samples were detected at 254 nm and integrated with the RF 10A (version 1.1) LC software program. The calibration curve was constructed by running standard solutions of each model drug in IPB for every series of samples. Validation of the method was performed to ensure that the calibration curve between 1 and 50 μ g/ml of all model drugs was in the linearity range and coefficients of variation were less than 5%, both intraday and interday.
Statistical Analysis
The skin permeation data were expressed as the means of at least 5 experiments ± S.E.M. One-way analysis of variance (ANOVA) and Tukey's multiple comparison test were used to compare fluxes of different formulations and a p value of 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
Characterization of Microemulsions
All 12 drug-loaded AOT-based microemulsions were clear colorless liquids and no birefringence was found under cross-polarized light microscopy. The drug-loaded AOT-based microemulsions were characterized and determined for their type as illustrated in . The results show that the incorporated drug slightly changed the apparent pH of each formulation depending on the acid-base properties of each drug, i.e., the free bases provided higher pH values whereas the hydrochloride salts provided lower pH values. The o/w microemulsions showed much higher conductivity values than w/o microemulsions due to the conductivity of the aqueous external pseudophase. Incorporation of the model drugs in salt form resulted in reduced conductivity compared with the base form since dissociated ions of the salt form could interact with anionic ions of AOT.
TABLE 2 The pH, conductivity, refractive index (RI), viscosity, and correlation coefficients (Rxy) for Newtonian flow behavior of drug-loaded AOT-based microemulsions
Furthermore, the refractive indices of all drug-loaded AOT-based microemulsions remained unchanged. However, the refractive indices of o/w microemulsions were slightly lower than those of w/o type due to the lower refractive index of water external pseudophase (1.3336 ± 0.0004) compared with that of IPP external pseudophase (1.4378 ± 0.0003). All samples possessed a low viscosity and showed Newtonian flow behavior, which are the characteristics of microemulsions (CitationAlany et al. 2001).
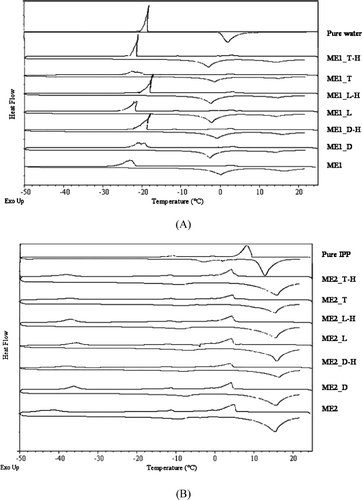
DSC curves of the drug-loaded AOT-based microemulsions are shown in . For the o/w microemulsions (ME1 group), the onsets of freezing and melting peaks of water (−21 to −18°C and −4 to −1°C, respectively) were observed in , indicating free water external pseudophase. In contrast, the onsets of freezing and melting peaks of IPP (5 to 6°C and 11 to 12°C, respectively) were observed for the w/o microemulsions (ME2 group) as illustrated in . The DSC curves of drug-loaded microemulsions and their blank counterparts were generally similar; however, slight shifts in the thermal events were found. This may be because drugs have some effects on a surfactant film or on interaction between surfactant and water molecules (CitationPodlogar et al. 2004; CitationBoonme et al. 2006). From all characterization results, we concluded that incorporation 1% w/w of the model drugs into AOT-based microemulsions did not change the type of all microemulsions.
In Vitro Skin Permeation
The in vitro permeation profiles of the model drugs through heat-separated human epidermis from the AOT-based microemulsion vehicles are presented in . A steady increase of the amount of permeated drug in the receptor compartments with time was observed. Lidocaine and dibucaine exhibited an initial lag time ∼3–4 hr and reached a plateau state after 12 hr. Therefore, the flux values of all drug permeation between 4 and 12 hr (Jss(4-12 h)) were calculated for comparison. The calculated flux values are shown in .
FIG. 3. Permeation profiles of the model drugs through human epidermis from the o/w (ME1) and w/o (ME2) AOT-based microemulsions containing: (A) L and L-H, (B) T and T-H, and (C) D and D-H. Each data point represents the mean ± S.E.M. (5 ≤ n ≤ 6).
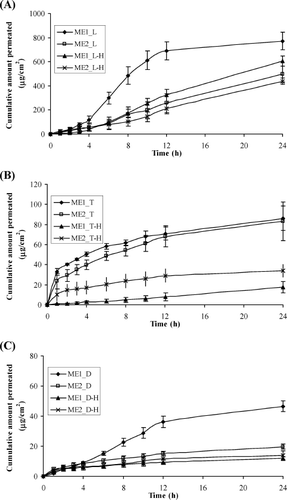
TABLE 3 Skin permeation fluxes between 4 and 12 h (Jss(4-12 h)) of the model drugs from aerosol OT-based microemulsions
From and , we can see that lidocaine and dibucaine groups provided similar patterns of skin permeation profiles. The o/w microemulsions of lidocaine and dibucaine in base forms (ME1_L and ME1_D) resulted in the highest amount of permeated drug. As seen in , the flux values of the o/w microemulsions of lidocaine and dibucaine in base forms provided significantly highest flux (p < 0.05) when compared within the same group of drugs, whereas the fluxes of the other three formulations were insignificantly different (p > 0.05). This finding could be explained by the oil droplets in o/w microemulsions acting as a drug carrier (CitationPeltola et al. 2003; CitationSintov and Botner 2006). Owing to lipophilicity of the base forms, they accumulated in higher concentration in the oil droplets. As a result, the o/w microemulsions of lidocaine and dibucaine bases provided the highest skin permeation enhancement.
In contrast, the pattern of skin permeation profiles of the tetracaine group differed from those of the lidocaine and dibucaine groups. As shown in , both o/w and w/o microemulsions of tetracaine base (ME1_T and ME2_T) provided higher cumulative amount of permeated drug than those of tetracaine salt. The mechanism of drug permeation of tetracaine by the AOT-based microemulsions is unclear; however, the difference in skin permeation profile may be due to the differences in molecular structure of tetracaine as compared with molecular structures of lidocaine and dibucaine as shown in . Therefore, different interaction with AOT might occur and subsequently result in different skin permeation profiles. Tetracaine-base processes butyl-side chain linked to the aromatic ring that might have high affinity with the two-tail hydrocarbon structure of AOT resulting in participation in the interfacial film with AOT around the microemulsion droplets. As the droplets act as drug carrier, the skin permeation of tetracaine base from both o/w and w/o microemulsions was not significantly different. For tetracaine hydrochloride, it can dissociate in water and the ionic charges hamper the participation in the interfacial film, resulting in lower skin permeation.
We tried to measure the particle size of the studied microemulsions using a photon correlation spectroscopy technique; however, phase separation was found while diluting the microemulsions to obtain low disperse-phase volume fraction to avoid inter-droplet interaction. Estimating from molecular structures of tetracaine group and AOT, it was highly possible that an association between the drug and AOT film resulted in large particle size of microemulsion droplets for the permeation into the skin.
Comparing the drug release from all three groups of the model drugs, we found that the microemulsions in o/w type of hydrophobic form provided the highest fluxes (). This phenomenon might be owing to the lipophilic nature of the stratum corneum. The oil can enter the hydrophobic tail areas of the stratum corneum bilayer, perturb it by creating separate domains, and induce highly permeable pathways in the stratum corneum (CitationTanojo et al. 1997).
Comparing the microemulsions of the three drugs, our present study showed that lidocaine-loaded AOT-based microemulsions provided very high fluxes () since the lidocaine group has the smallest molecule (). In general, drugs with lower molecular weight have higher drug mobility or diffusion coefficient and subsequently higher permeation through intact epidermis (CitationBarry 2001).
Tetracaine-loaded and dibucaine-loaded AOT-based microemulsions provided very low fluxes. Although the tetracaine group has smaller molecule than the dibucaine group (), their flux values in were not significantly different (p > 0.05). The skin permeation of these drugs might be influenced by other factors. For the tetracaine group, a possible reason involved the interaction between tetracaine and AOT moieties. For the dibucaine group, the large molecules of dibucaine and dibucaine hydrochloride provided lower diffusion coefficients. Thus, there was a limitation in permeation enhancement of these drugs.
CONCLUSIONS
From in vitro skin permeation studies, the results show that the nature of the microemulsion, intrinsic properties of the drug, and interaction between drug and surfactant affected the skin permeation of the model drugs from the microemulsion formulations of the IPP/water/AOT:1-butanol (2:1) system. The oil droplets in o/w microemulsions of the hydrophobic base form of the drugs enhanced skin permeation higher than the other formulations. The lidocaine group delivered from AOT-based microemulsions through heat-separated epidermis showed the highest flux as compared with the tetracaine group and the dibucaine group due to its smallest molecule. The skin permeation of the last two drugs from AOT-based microemulsions was hampered by interaction between drug and surfactant and by intrinsically large molecule, respectively.
The authors thank the financial support received from the Thailand Research Fund through the Royal Golden Jubilee PhD Program (PHD/0146/2544). We acknowledge Yanhee General Hospital, Bangkok, Thailand, for supporting the skin tissues used in the permeation studies.
REFERENCES
- R. G. Alany, T. Rades, S. Agatonovic-Kustrin, N. M. Davies, and I. G. Tucker. (2000). Effects of alcohols and diols on the phase behaviour of quaternary systems. Int. J. Pharm. 196:141–145.
- R. G. Alany, I. G. Tucker, N. M. Davies, and T. Rades. (2001). Characterizing colloidal structures of pseudoternary phase diagrams formed by oil/water/amphiphile systems. Drug Dev. Ind. Pharm. 27:31–38.
- B. Baroli, M. A. Lopez-Quintela, M. B. Delgado-Charro, A. M. Fadda, and J. Blanco-Mendez. (2000). Microemulsions for topical delivery of 8-methoxsalen. J. Control. Rel. 69:209–218.
- B. W. Barry. (2001). Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 14:101–114.
- H. N. Bhargava, A. Narurkar, and L. M. Lieb. (1987). Using microemulsions for drug delivery. Pharm. Technol. 11:46–50.
- M. A. Bolzinger, T. C. Carduner, and M. C. Poelman. (1998). Bicontinuous sucrose ester microemulsion: a new vehicle for topical delivery of niflumic acid. Int. J. Pharm. 176:39–45.
- P Boonme. (2007). Applications of microemulsions in cosmetics. J. Cosmet. Dermat. 6:223–228.
- P. Boonme, K. Krauel, A. Graf, T. Rades, and V. B. Junyaprasert. (2006). Characterisation of microstructures formed in isopropyl palmitate/water/Aerosol OT:1-butanol (2:1) system. Pharmazie 61:927–932.
- M. Changez, and M. Varshney. (2000). Aerosol-OT microemulsions as transdermal carriers of tetracaine hydrochloride. Drug Dev. Ind. Pharm. 26:507–512.
- M. B. Delgado-Charro, G. Iglesias-Vilas, J. Blanco-Mendez, M. A. Lopez-Quintela, J. Marty, and R. H. Guy. (1997). Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur. J. Pharm. Biopharm. 43:37–42.
- S. E. Friberg. (1990). Micelles, microemulsions, liquid crystals, and the structure of stratum corneum lipids. J. Soc. Cosmet. Chem. 41:155–71.
- P. M. Friedman, E. A. Mafong, E. S. Friedman, and R. G. Geronemus. (2001). Topical anesthetics update: EMLA and beyond. Dermatol. Surg. 27:1019–1026.
- M. J. Garcia-Celma, N. Azemar, M. A. Pes, and C. Solans. (1994). Solubilization of antifungal drugs in water/POE(20) sorbitan monooleate/oil systems. Int. J. Pharm. 105:77–81.
- J. M. Haigh, and E. W. Smith. (1994). The selection and use of natural and synthetic membranes for in vitro diffusion experiments. Eur. J. Pharm. Sci. 2:311–330.
- S. M. Harrison, B. W. Barry, and P. H. Dugard. (1984). Effects of freezing on human skin permeability. J. Pharm. Pharmacol. 36:261–262.
- V. B. Junyaprasert, P. Boonsaner, S. Leatwimonlak, and P. Boonme. (2007). Enhancement of the skin permeation of clindamycin phosphate by Aerosol OT/1-butanol microemulsions. Drug Dev. Ind. Pharm. 33:874–880.
- M. Kreilgaard. (2002). Influence of microemulsions on cutaneous drug delivery. Adv. Drug Del. Rev. 54 (suppl. 1):S77–S98.
- M. Kreilgaard, E. J. Pedersen, and J. W. Jaroszewski. (2000). NMR characterisation and transdermal drug delivery potential of microemulsion systems. J. Control. Rel. 69:421–433.
- M. J. Lawrence, and G. D. Rees. (2000). Microemulsion-based media as novel drug delivery systems. Adv. Drug Del. Rev. 45:89–121.
- E. E. Linn, R. C. Pohland, and T. K. Byrd. (1990). Microemulsion for intradermal delivery of cetyl alcohol and octyl dimethyl PABA. Drug Dev. Ind. Pharm. 16:899–920.
- H. Liu, S. Li, Y. Wang, F. Han, and D. Yang. (2006). Bicontinuous water-AOT/Tween 85-isopropyl myristate microemulsion: a new vehicle for transdermal delivery of cyclosporin A. Drug Dev. Ind. Pharm. 32:549–557.
- C. Malcolmson, and M. J. Lawrence. (1993). A comparison of the incorporation of model steroids into non-ionic micellar and microemulsion system. J. Pharm. Pharmacol. 45:141–143.
- D. Osborn, A. Ward, and K. O'Neil. (1988). Microemulsions as topical drug delivery vehicles: characterization of a model system. Drug Dev. Ind. Pharm. 14:1203–1219.
- D. Osborn, A. Ward, and K. O'Neil. (1991). Microemulsions as topical delivery vehicles: in vitro transdermal studies of a model hydrophilic drug. J. Pharm. Pharmacol. 43:451–454.
- S. Peltola, P. Saarinen-Savolainen, J. Kiesvaara, T. M. Suhonen, and A. Urtti. (2003). Microemulsions for topical delivery of estradiol. Int. J. Pharm. 254:99–107.
- F. Podlogar, M. Gasperlin, M. Tomsic, A. Jamnik, and M. B. Rogac. (2004). Structural characterisation of water-Tween 40/Imwitor 308-isopropyl myristate microemulsions using different experimental methods. Int. J. Pharm. 276:115–128.
- H. P. Rang, M. M. Dale, and J. M. Ritter. ( eds.) (1999). Pharmacology, 4th ed, 634–645. London: Churchill Livingstone.
- J. F.E. Reynolds. (1996). Martindale: The Extra Pharmacopoiea. 31st ed, 1317–1340. London: Royal Pharmaceutical Society.
- Y. Rhee, J. Choi, E. Park, and S. Chi. (2001). Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 228:161–170.
- U. Schmalfuβ, R. Neubert, and W. Wohlrab. (1997). Modification of drug penetration into human skin using microemulsions. J. Control. Rel. 46:279–285.
- A. C. Sintov, and S. Botner. (2006). Transdermal drug delivery using microemulsion and aqueous systems: influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int. J. Pharm. 311:55–62.
- S. Songkro, Y. Purwo, G. Becket, and T. Rades. (2003). Investigation of newborn pig skin as an in vitro animal model for transdermal drug delivery. STP Pharma Sci. 13:133–139.
- H. Tanojo, A. B. Geest, J. A. Bouwstra, H. E. Junginger, and H. E. Bodde. (1997). In vitro human skin barrier perturbation by oleic acid: thermal analysis and freeze fracture electron microscopy studies. Thermochim. Acta 293:77–85.
- M. Trotta, M. R. Gasco, and S. Morel. (1989). Release of drugs from oil-water microemulsions. J. Control. Rel. 10:237–243.