Abstract
The reduction of particle size to nanometers has been an important tool used for efficient drug delivery. Solid drug nanoparticles can be conveniently prepared by nanocomminution. This process relies on mechanical energy and the selection of a proper polymeric stabilizer. The long chains of polymers provide steric stabilization for drug nanoparticles. In this research, itraconazole and hydroxypropyl cellulose were used to study the effect of the molecular weight of a polymer on particle size reduction. In principle, an increase in molecular weight produces two counteracting effects: a decrease in the diffusion rate of chains and an increase in the physical adsorption of a polymer. The effects of particle size reduction are more pronounced in systems involving smaller molecular weights, and the effects of changing molecular weights disappear with time. Systems of higher molecular weight show larger aggregates in their redispersion after drying. Based on the results of our research, it appears that polymers of smaller molecular weight are more suitable than larger polymers for efficient nanocomminution. This indicates that the kinetic aspects of molecular weight are important.
Recent advances in the preparation technology of solid pharmaceutical nanoparticles have increased their maximal achievable bioavailability (Liu 2000; CitationLee 2003; CitationLiversidge and Conzentino 1995; CitationSerajuddin 1999; CitationTakano et al. 2006; CitationYamada et al. 1999). By reducing particle size by orders of magnitude, surface area increases dramatically and leads to increased rates of absorption and dissolution. This direct relationship between surface area and dissolution rate is demonstrated in the modified Noyes-Whitney equation (CitationSix et al. 2004; CitationCraig 2002; CitationLeuner and Dressman 2000; CitationRasenack and Muller 2002). In addition to increasing maximal achievable bioavailability, the development of the nanoparticle technology also has increased the effectiveness of drug discovery, since many drug candidates in the developmental stages are insoluble.
Other advantages of using nanoparticles include both a significant reduction in fed/fasted variability and the achievement higher doses in a smaller dose volume. Additionally, the technique can be combined with intelligent drug delivery systems to achieve a synergistic performance (CitationTorchilin 2000). Finally, by adding specific excipients to drug nanoparticles during preparation, additional advantages, such as taste-masking or longer retention time in blood and tumors, can be obtained.
A decrease in particle size leads to an increase in Gibbs free energy by increasing the extra ‘subdivision potential’ term (CitationHill 2001). This extra term is mainly related to the increase in surface energy. Determining how to compensate for this term is the key to both the preparation and subsequent unit operation of nanoparticles (CitationMorrison and Ross 2002; CitationLee 2004).
To compensate for the extra Gibbs energy related to particle size reduction, a surface stabilizing material and an external energy source are often used in combination (Kumar 2006). For example, liquid-based methods use surface stabilizers and high pressure homogenization. Although this method is not in a true equilibrium state, it significantly relies on nanostructures in thermodynamic equilibrium, such as emulsions and lipids. Nanocommunition uses mechanical energy with the aid of one or more stabilizer.
Nanocomminution is an effective process that successfully launched commercial solid dosage forms such as Rapamune® and Emend® (CitationLiversidge and Conzentino 1995; CitationLiversidge and Cundy 1995; CitationMerisko-Liversidge et al. 1996; CitationGrau et al. 2000; CitationZheng and Bosch 1997). Since the crystallinity of drugs is not significantly destroyed during the process, the stability of drugs can be better maintained during unit operations. Furthermore, no organic solvent or harsh environments are needed, which is a distinct advantage over other preparation methods. In the nanocomminution, water is used as a medium, and a water soluble polymer is employed as a surface stabilizer.
In previous investigations (CitationLiversidge and Cundy 1995; CitationMerisko-Liversidge et al. 1996; CitationLee et al. 2005), the critical parameter in determining the degree of particle size reduction was the selection of a proper polymeric stabilizer. Among the generally recognized as safe (GRAS) polymers, hydroxypropyl cellulose (HPC), polyvinyl pyrrolidone, poly(ethylene glycol-co-propylene glycol), polyvinyl pyrrolidone, and other amphiphilic polymers are known to be successful stabilizers. For a specific drug, only a few polymers can reduce the particle size down to nanometers. Selecting a proper stabilizer is a complex subject that cannot be easily generalized (CitationLee et al. 2005). When amino acid copolymers were used, the hydrophobicity of polymers was more critical than the morphology of polymer chains (CitationLee et al. 2005).
The steric stabilization of polymers is the most widely used method for handling and preparing nanoparticles. A critical property of polymers is their molecular weight, which determines both the solution properties of polymer chains and the capability for steric stabilization (CitationAdamson and Gast 1997; CitationPloehn and Russel 1990). Therefore, the molecular weight of polymers should be tailored to achieve the best performance of a polymeric stabilizer in nano-communition. In this research, the effect of molecular weight on the nanocomminution of itraconazole particles is investigated. After stabilizing the itraconazole surface with HPC, the steady state particle sizes (the smallest sizes achieved by nanocomminution) are examined as a function of molecular weight. While nanocomminution has much significance in the industrial fields, very little research has been done concerning this phenomenon.
MATERIALS AND METHODS
Itraconazole (Choongwae Pharm, GMP0004, South Korea) was used without preparation. Its particle size as received was 9.25 (± 8.43) μ m (measurement described below). HPCs of different molecular weights were obtained from Fluka (USA, gel permeation chromatography standard series).
Preparation of Nanosuspensions
Drug particles (8 wt%) were mixed with polymer (1.33 wt%) in distilled water. The mixture slurry of 7.5 g was put into a 30 ml vial with yttria-stabilized zirconia beads (1 mm, 50 v/v%, SamWha Ceramics, South Korea), and the nanocomminution process of drug particles progressed for 4 days by ball milling at 100 rpm at room temperature. Temperature elevation was measured by inserting a thermocouple into the slurry, which was +4°C. Compared with the melting temperature of itraconazole (166.2°C), the processing heat seemed to be negligible. The resulting suspension after filtering out zirconia beads was stored at 5°C for further characterizations.
Characterizations after Nanocomminution
Particle size measurement was performed using a Horiba laser light scattering particle size analyzer LA-910 (relative refractive index = 1.06, ultrasonic chamber power = 40W and 39 kHz, 340 mL/min stirring flow [level 3], 95–100 mL water medium, detection size range 0.02–1000 μ m). The drug concentration in the particle size analyzer chamber was 0.02 wt%, and repeated measurements of at least three times produced the error ranges of volume-averaged mean sizes. Particle morphology was investigated using a Hitachi (Japan) scanning electron microscope S-4700 at 4 kV and 0.5 Hz. Samples were prepared by drying suspension drops on SEM sample stages previously cleaned, and they were coated with Pt-Pd at a coating speed of 6.7 nm/min for 2 min.
As stated in our early report (CitationLee 2003), thermal gravimetric analysis (TA 2050 TA Instrument, USA.) was accurate enough to measure the amount of polymer in the supernatant that remained after centrifuging at 15 k rpm and 4°C for 400 min (Micro 17R plus centrifuge, Hanil, South Korea). By comparing the polymer concentrations of supernatants and those of mother liquids, the amounts of polymer adsorbed on drug surfaces could be calculated. Samples of 150 mg were heated from 20 to 140°C at 10°C/min and held at 140°C for 100 min.
For the redispersion tests of dried powders, nanosuspensions of 3.75 g were first centrifuged at 4°C and 15 k rpm for 400 min. After the supernatant was removed, the remaining particles were fully dried under vacuum for 24 hrs at room temperature. Dried particles were grinded by pestle and mortar for 5 min, and redispersed in distilled water (10 wt%) under stirring for 10 min. The mean sizes of dispersed particles were measured following the same method described above after sonication for 3 min.
RESULTS
Particle Size Reduction
Itraconazole can benefit from particle size reduction since it is a class II drug according to the BCS systems (CitationSix 2004; CitationAmidon et al. 1995). shows the chemical structures of itraconazole and HPC. Ether functional groups are present in both structures, which might partially explain why HPC can stabilize itraconazole. The polymers used in this study have different molecular weights as shown in . The polydispersity (Mw/Mn) had a relatively narrow range of 1.6 to 1.9. Their designations follow the gel permeation molecular weights (![]() p), which are used in our discussion.
p), which are used in our discussion.
TABLE 1 Molecular weight of hydroxypropyl cellulose (from the manufacturer)
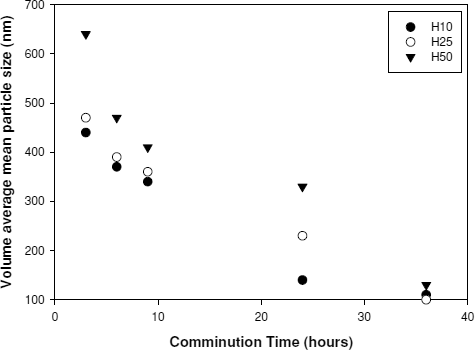
and show the effect of molecular weight on particle size reduction as a function of time. The results from 3 hrs to 4 days were obtained during nanocomminution, and the results from 30 to 60 days were obtained during storage at 5°C. demonstrates that the initial size reduction was rapid; in the first 3 hrs, the mean particle size decreased to 0.4–0.6 micron.
TABLE 2 Volume-averaged mean particle sizes (μ m) of drugs as a function of nanocomminution (3–4 days) and their changes after comminution (30–60 days) in the presence of HPCs having different molecular weights.
Our main focus was on the overall trends of the particle size data in . We concluded that particle size reduction was more pronounced in lower molecular weight cases than the higher molecular weight cases. Additionally, the size difference caused by the difference in molecular weight became negligible after 3 days and disappeared after 60 days. The smallest particle size (0.12 μ m) attained in the H50 system was only slightly larger than that attained in other systems. After 60 days, all the steady state particle sizes of itraconazole appeared to be 100–120 nm.
Polymers of higher molecular weights were not effective in the initial particle size reduction and appeared to require a longer time to achieve steady-state particle sizes. A processing time longer than 1 day was needed for H10–H30, while more than 2 days were required for H35 and H50.
Redispersion after Drying
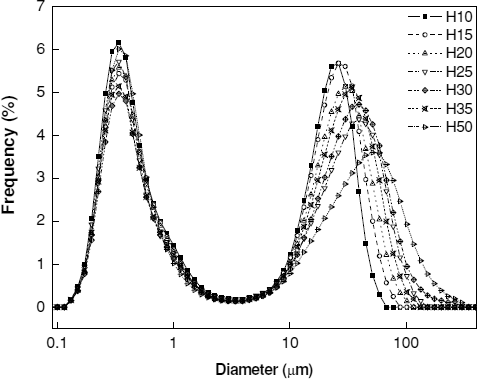
Successfully processed itraconazole nanosuspensions having HPCs of different molecular weights were dried into powders. To observe their dissolution behavior, the powders were redispersed into water. Ideally, the dry powder would instantaneously return to the original nanoparticle suspensions of 100–120 nm with the aid of a proper cryoprotectant. However, no cryoprotectant was used in this experiment, and the original dispersion state was difficult to attain. Instead, bimodal distribution of particle size was observed.
shows typical bimodal results of the redispersion experiment. The peak below 1 micron corresponds to the primary particles (or the aggregates of a few primary particles), and the peak between 10 and 500 microns corresponds to aggregates. The mean particle size of polymers generally increases with an increase in the molecular weight. The particle size distribution curves show that the increase mainly comes from the increase and broadening of larger aggregate sizes of 10–500 μ m. Interestingly, the peak below 1 micron is independent of the molecular weight of HPC. Meanwhile, longer polymer chains have stronger chain entanglements that resist disentanglement as well as the subsequent disintegration of aggregates in the redispersion experiment. Therefore, molecular weight has a more pronounced effect in the peaks of 10–500 μ m.
Degree of Polymer Adsorption
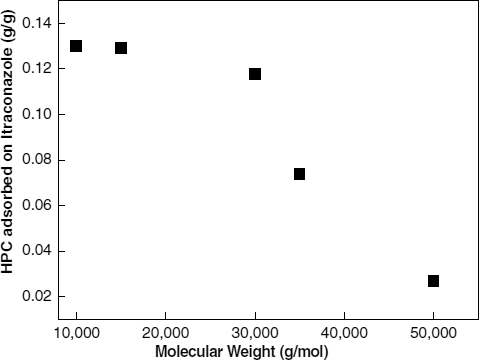
An indirect method of assessing the degree of stabilization achieved by the physical adsorption of polymers is measuring the amount of adsorbed polymers. shows the results of nanosuspensions processed for 1 day. The amount of adsorbed polymer divided by the amount of drug is between 0.02 and 0.14 g/g, which is similar to values we have reported previously. The thickness of the layers was calculated between 1.5 and 16 nm (CitationLee 2003). Using the same calculation, the adsorbed polymer layer appears to be close to a single molecular layer.
FIG. 4 Amount of polymer (HPC) adsorbed on the surface of itraconazole particles obtained from thermal gravimetric analysis.
Thermodynamic principles related to polymer adsorption predict a higher driving force of physical adsorption associated with higher molecular weights (CitationPloehn and Russel 1990). , however, shows the opposite result: the amount of adsorbed polymer decreases with an increase in the molecular weight of polymer. Particularly, the decrease is rapid with molecular weights above 30,000 g/mol. These findings are consistent with the results in , which show that particle size reduction is more distinct in the cases of smaller molecular weights.
Particle Morphology
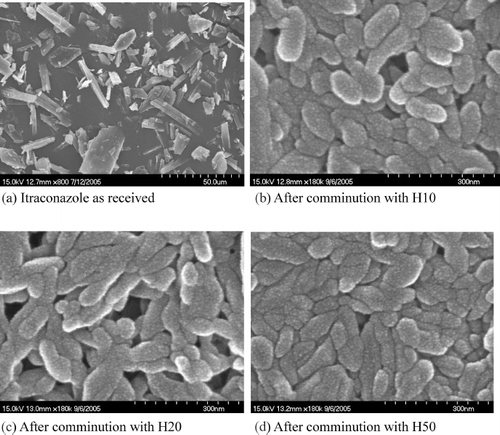
The morphology of particles is given in . In this figure, particle size reduction of several orders of magnitude is apparent. shows itraconazole particles that are plate- and rod-type crystals. The comminution process rounded up the sharp edges of the crystals and produced oval shaped nanoparticles with an aspect ratio between 1 and 3. There was not a distinct difference among the three processed nanoparticles, as shown in and .
DISCUSSION
Nanocomminution reduces the size of drug particles via fracture, but common parameters related to fracture, such as stress states, fracture resistance, micromechanical deformations, energy input, transfer, and dissipation, cannot solely explain the process. The nanocommunition is indeed a complex process involving several intertwined mechanisms (CitationLee et al. 2005; CitationChoi et al. 2005). During this process, several mechanisms occur concurrently and in close connection. These include, but are not limited to, the adsorption and desorption of polymers on drug surfaces, the fracture of drug crystals, the dissolution and precipitation of drug, the aggregation and segregation of particles, and the micelle formation of polymers. Therefore, a systematic change in the properties of a polymer can affect the various mechanisms involved in nanocomminution.
In our previous studies (CitationLee 2003), the rate of particle size reduction depended on time and mechanical energy input, but the particle size eventually reached its steady-state value, which was unique for each polymer. The critical role of polymeric stabilizers in nanocomminution originates from their steric stabilization. Effective steric stabilization requires firm physical anchoring of a polymer on a drug surface.
The molecular weight of a polymer influences the thermodynamic driving force of physical adsorption. Physical adsorption involves favorable polymer-surface interactions, dewetting of a polymer chain from the drug surface, and the loss of freedom of motion (CitationMorrison and Ross 2002; CitationPloehn and Russel 1990). Differences in adsorption strength and the thickness of adsorption layers can result from different molecular weights. Polymer chain configurations, such as train, loop, and tails, also depend on molecular weight. In particular, polymers of higher molecular weights have less entropy loss related to their freedom of motion, which results in a higher affinity to the drug surface (stronger adsorption and slower desorption) (CitationMorrison and Ross 2002; CitationPloehn and Russel 1990). Therefore, according to the thermodynamic prediction, polymers of higher molecular weights should provide better stabilization. However, , , , , and present contrary results.
FIG. 2 Volume-averaged mean sizes of itraconazole particles milled in presence of HPCs having different molecular weights.
Kinetic aspects of adsorption explain this contradiction. Adsorption occurs over a long period of time due to the slow diffusion rate of polymer chains. The higher the molecular weight of a polymeric stabilizer, the slower the rate of adsorption. Viscosity also increases with an increase in the molecular weight. For example, an increase in the molecular weight of HPC from 96 k to 140 k g/mol results in an increase in viscosity from 75–150 to 150–400 mPa.s (CitationRowe et al. 2003). Since HPC is known as a semiflexible polymer (CitationSiddiquee and Egmond 2002), this kinetic aspect of adsorption seems to play an important role as shown in and . For a short processing time, kinetic aspects seem to be more pronounced. For fast and effective processing, smaller molecular weight polymers such as H10 and H15 appear to be superior. To provide enough time for adsorption, different processing times must be used for polymers of different molecular weights.
Interestingly, the effect of kinetics appears to exist for a relatively long period of time. Even performing nanocomminution for 4 days does not completely guarantee that full equilibrium adsorption will be reached. Subsequently storing the particles for 45 and 60 days allowed the H35 and H50 systems to further equilibrate. After 60 days, the particle size of H50 neared that of H10, although H50 still was slightly larger. This relatively long equilibration time cannot be entirely explained by the slow adsorption of higher molecular weight chains. Compared with the previous experimental and theoretical studies on polymer adsorption (CitationBerglund et al. 2003a, Citation2003b; CitationEvertsson and Nilsson, 1997), our drug surfaces that are continually dissolving and precipitating are “non-ideal.” Furthermore, during nanocomminution, the situation is complex since the fracturing process continually generates fresh surfaces and the aggregation process continually destroys these surfaces. Therefore, it is more difficult to achieve an equilibrium of physical adsorption through nanocomminution than through an ‘ideal’ adsorption experiment using nondissolving surfaces (CitationBerglund et al. 2003a, Citation2003b; CitationEvertsson and Nilsson 1997).
Once the hydrophobic surface of a drug is covered by polymers, particle fusion is less likely to occur. Consequently, the entanglement between polymers on two drug nanoparticles is the major obstacle in utilizing the full surface area for the dissolution of dry particles. Polymers of relatively low molecular weights have a redispersion advantage if a solid dosage form is targeted, as shown in . Drying nanosuspensions makes steric stabilization inactive, and polymer chains become entangled. Entanglement increases with an increase in molecular weight. Additionally, the dependence of the number of entanglements on the molecular weight differs when the molecular weight is above the characteristic molecular weight of a polymer (CitationAdamson and Gast 1997; CitationMorrison and Ross 2002).
Changes in the molecular weight of HPC could influence mechanisms other than polymer adsorption, including drug particle fracture. For instance, higher molecular weight HPC results in a slurry of higher viscosity. This increase in viscosity can lead to more effective energy transfer in medium and a higher shear stress experienced by the drug particles. This series of events allows nanocomminution to reduce particle size more rapidly when higher molecular weight polymers are used. However, data in contradict this conjecture. The effect of energy transfer efficiency appears to be counterbalanced by the effect of polymer chain mobility.
HPC does not form micelles because of its uniform chain morphology, and its influence on the colligative properties is negligible due to its macromolecular properties. Thus, the dissolution and precipitation behavior of drugs may not depend on molecular weight.
One of the most critical tuning points in nanocomminution is the selection of a proper stabilizer. By designing the proper polymer for the nanocomminution of a drug, we can control its steric stabilization and effectively develop novel nanocomminution methods. Moreover, the basic understanding we have obtained will help other nanotechnologies because the steric stabilization of a polymer is the key mechanism in various applications for dealing with nanoparticles.
CONCLUSIONS
The effect of the molecular weight of a polymer on the nanocommunition of a drug was systematically investigated. Initially, when reducing particle size, HPC of a lower molecular weight was more effective among the studied polymers (![]() p 11,200–49,000 g/mol). After 60 days, however, the effects of molecular weight almost disappeared and steady state particle sizes of itraconazole attained by HPC were 0.10–0.12 micron. An increase in molecular weight did not cause any significant differences in the oval particle morphology observed by SEM, but instead led to a decrease in the amount of adsorbed polymer. Redispersion after drying showed higher particle sizes in the cases of higher molecular weights. In the bimodal size distribution curves of redispersion, the use of higher molecular weight polymers resulted in the broadening of aggregate peaks (> 1 micron) to several hundred microns. On the other hand, the peak below 1 micron did not reflect the changes in molecular weight. The relatively slow diffusion of longer chains seemed to counteract their superior driving force of physical adsorption. Overall, the kinetic properties of polymer chains were more important in actual processing possibly because nanocomminution is complex of a process to rapidly reach an equilibrium state.
p 11,200–49,000 g/mol). After 60 days, however, the effects of molecular weight almost disappeared and steady state particle sizes of itraconazole attained by HPC were 0.10–0.12 micron. An increase in molecular weight did not cause any significant differences in the oval particle morphology observed by SEM, but instead led to a decrease in the amount of adsorbed polymer. Redispersion after drying showed higher particle sizes in the cases of higher molecular weights. In the bimodal size distribution curves of redispersion, the use of higher molecular weight polymers resulted in the broadening of aggregate peaks (> 1 micron) to several hundred microns. On the other hand, the peak below 1 micron did not reflect the changes in molecular weight. The relatively slow diffusion of longer chains seemed to counteract their superior driving force of physical adsorption. Overall, the kinetic properties of polymer chains were more important in actual processing possibly because nanocomminution is complex of a process to rapidly reach an equilibrium state.
Financial support from the Korea Institute of Industrial Technology Evaluation and Planning (project number 10011410) is gratefully acknowledged. C.H. Park gives thanks to Seoul Science and BK21 Fellowship.
REFERENCES
- A. W. Adamson, and A. P. Gast. (1997). Physical Chemistry of Surfaces, 348, 506. New York: John Wiley & Sons.
- G. L. Amidon, H. Leenernäs, V. P. Shah, and J. R. Crison. (1995). Theoretical basis for a biopharmaceutical drug classification: the correlation of in vivo drug product dissolution and in vivo bioavailability. Pharm. Res 12 (3):413–420.
- K. D. Berglund, T. M. Przybycien, and R. D. Tilton. (2003a). Coadsorption of sodium dodecyl sulfate with hydrophobically modified nonionic cellulose polymers. 1. Role of polymer hydrophobic modification. Langmuir 19:2705–2713.
- K. D. Berglund, T. M. Przybycien, and R. D. Tilton. (2003b). Coadsorption of sodium dodecyl sulfate with hydrophobically modified nonionic cellulose polymers. 2. Role of surface selectivity in adsorption hysteresis. Langmuir 19:2714–2721.
- J.-Y. Choi, J. Y. Yoo, H.-S. Kwak, B. U. Nam, and J. Lee. (2005). Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Physics 5:472–474.
- D. Q.M. Craig. (2002). The mechanisms of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm. 231:131–144.
- H. Evertsson, and S. Nilsson. (1997). Microviscosity in clusters of ethyl hydroxyethyl cellulose and sodium dodecyl sulfate formed in dilute aqueous solutions as determined with fluorescence probe techniques. Macromolecules 30:2377–2385.
- M. J. Grau, O. Kayser, and R. H. Müller. (2000). Nanosuspensions of poorly soluble drugs—reproducibility of small scale production. Int. J. Pharm. 196:155–157.
- T. L. Hill. (2001). A different approach to nanothermodynamics. Nano Lett. 1:273–275.
- C.S.S.R. Kumar. (ed.) (2006). Preparation methods of drug nanoparticles. In Biological and Pharmaceutical Nanomaterials (Nanotechnologies for the Lifesciences, vol. 2). Weinheim: Wiley Science, 225.
- J. Lee. (2003). Drug nano- and microparticles processed into solid dosage forms: physical properties. J. Pharm. Sci 92 (10):2057–2068.
- J. Lee. (2004). Intrinsic adhesion forces of lubricants to steel surface. J. Pharm. Sci 93 (9):2310–2318.
- J. Lee, S.-J. Lee, J.-Y. Choi, J. Y. Yoo, and C.-H. Ahn. (2005). Amphiphilic amino acid copolymers as stabilizers for the preparation of nanocrystal dispersion. Eur. J. Pharm. Sci. 24:441–449.
- C. Leuner, and J. Dressman. (2000). Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 50:47–60.
- R. Liu. (ed). (2000). Particle size reduction, in water-insoluble drug formation. IL: Buffalo Grove, Interpharm Press, 455–492.
- G. G. Liversidge, and P. Conzentino. (1995). Drug particle size reduction for decreasing gastic irritancy and enhancing absorption of naproxen in rats. Int. J. Pharm. 125:309–313.
- G. G. Liversidge, and K. Cundy. (1995). Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm 125:91–97.
- E. Merisko-Liversidge, P. Sarpotdar, J. Bruno, S. Hajj, L. Wei, N. Peltier, J. Rake, J. M. Shaw, S. Pugh, L. Pollin, J. Jones, T. Corbett, E. Cooper, and G. G. Liversidge. (1996). Formulation and antitumor activity evaluation of nanocrystalline suspensions of poorly soluble anticancer drugs. Pharm. Res. 13:272–278.
- I. D. Morrison, and S. Ross. (2002). Colloidal Dispersions, New York: Wiley-Interscience, 225.
- H. J. Ploehn, and W. B. Russel. (1990). Interactions between colloidal particles and soluble polymers. Adv. Chemi Eng 15:137–228.
- N. Rasenack, and B. W. Muller. (2002). Dissolution rate enhancement by in situ micronization of poorly water-soluble drugs. Pharm. Res. 19 (12):1894–1900.
- R. C. Rowe, P. J. Sheskey, and P. J. Weller. (2003). Handbook of Pharmaceutical Excipients, 4th ed. Pharmaceutical Press.
- A. T.M. Serajuddin. (1999). Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 88 (10):1058–1066.
- S. K. Siddiquee, and J. W. Egmond. (2002). Molecular weight dependence of shear-induced structures in nematic semiflexible polymer solutions. J. Rheol. 46 (2):367–381.
- K. Six, G. Verreck, J. Peeters, M. Brewster, and G. van den Mooter. (2004). Increased physical stability and improved dissolution properties of itraconazole, a class II drug, by solid dispersions that combine fast- and slow-dissolving polymers. J. Pharm. Sci. 93 (1):124–131.
- R. Takano, K. Sugano, A. Higashida, Y. Hayashi, M. Machida, Y. Aso, and S. Yamashita. (2006). Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm. Res. 23 (6):1144–1156.
- V. Torchilin. (2000). Drug targeting. Eur. J. Pharm. Sci. 11 (suppl 2):S81.
- T. Yamada, N. Saito, and T. Imai. (1999). Effect of grinding with hydroxypropyl cellulose on the dissolution and particle size of a poorly water-soluble drug. Chem. Pharm. Bull. 47 (9):1311–1313.
- J. Y. Zheng, and H. W. Bosch. (1997). Sterile filtration of nanocrystal drug formulation. Drug Devel Ind Pharm 23 (11):1087–1093.