Abstract
Biodegradable polyetherester copolymer (PCL/PEG/PCL, PCEC) was synthesized by ring-opening polymerization of ε-caprolactone initiated by poly(ethylene glycol) (PEG). The PCEC nanoparticles were prepared by solvent diffusion method or w/o/w double emulsion method. The obtained particles' morphology was observed on scanning electron microscopy, and the particle size distribution was determined using Malvern laser particle sizer. Bovine serum albumin was used as the model water-soluble protein drug, which was successfully encapsulated in PCEC nanoparticles, the drug release behavior was studied in detail. The hydrolytic degradation behavior of the PCEC nanoparticles was also studied.
Aliphatic polyesters, in particular FDA-approved bioresorbable poly(DL-lactide) (PLA) and poly(ε-caprolactone) (PCL), constitute a class of biomedical materials of growing interest in the field of temporary therapeutic applications in surgery, sustained drug delivery, and tissue engineering (CitationBirnbaum, Kosmala, and Peppas 2000; CitationKwon et al. 2001; CitationMolpeceres, Aberturas, and Guzman 2000; CitationChen et al. 2002; CitationZhang and Zhou 2005).
PCL is a kind of biocompatible and biodegradable polyester, which has attracted great attention in controlled drug delivery system due to its nontoxicity and great penetrability. However, its further application in drug delivery system has been restricted by its some shortcomings, such as slow degradation rate due to its relatively high crystallinity and great hydrophobicity. So, hydrophilic poly(ethylene glycol) (PEG) was incorporated into or blended with PCL to increase the hydrophilicity and the biodegradation rate. PEG is a nontoxic and nonimmunogenic water soluble polymer, and it has a great effect on preventing protein, adsorption and escaping capture of the reticuloendothelial system (CitationRyu et al. 2001; CitationZhang and Zhuo 2005). So, in this study, we prepared PCL/PEG/PCL (PCEC) triblock copolymers by ring-opening polymerization of ε-caprolactone initiated by PEG. Due to the hydrophilicity of PEG and hydrophobicity of PCL, the obtained PCEC copolymer is amphiphilic.
Nanoparticles are colloidal polymeric drug carriers, and their potential uptake as well as their stability in the gastrointestinal tract indicates that nanoparticles are expected to be promising carriers for delivery of therapeutic drugs (CitationBirnbaum et al. 2000; CitationMolpeceres et al. 2000; CitationKwon et al. 2001; CitationRyu et al. 2001; CitationChen et al. 2002; CitationUbrich et al. 2004; CitationZhang and Zhuo 2005). So, nanoparticles have been widely employed for drug delivery systems and many other biomedical applications recently. Since nanoparticle drug delivery systems can provide intravenous injection of drugs for site-specific drug delivery, this local drug delivery rather than systemic administration might be a more effective way to obtain higher tissue drug levels and decrease potential adverse systemic side effects (CitationKwon et al. 2001).
Proteins and peptides are new kinds of bioactive drugs developed more recently due to great development in biotechnology, and they have the advantage of curing many diseases over traditional drugs. But unfortunately, it is very difficult to keep bioactivity of such bioactive protein or peptide drugs. So, it is very important to avoid their denaturation of protein or peptide drugs before delivery to the target organs (CitationZhou, Deng, and Li 2001). In our study, bovine serum albumin (BSA) was employed as a model water soluble protein to be encapsulated in PCEC nanoparticles by solvent diffusion method or w/o/w double emulsion methods, and in vitro release behavior was also studied in our work.
EXPERIMENTAL
Materials
Sn(Oct)2 and ε-caprolactone (CL) were obtained from Aldrich Company (USA). BSA was BR grade and purchased from Shanghai Biotechnology Company (P.R. China) Poly (ethylene glycol) (PEG, Mn = 4000) and poly(vinyl alcohol) (PVA, Mn = 8000–12000, 88% hydrolyzed) were purchased from Sigma (USA). All the other materials were obtained from Chengdu KeLong Chemicals (China). In this study all the materials except BSA were analytical grade and used as received without any purification.
Synthesis of Triblock PCEC Copolymers
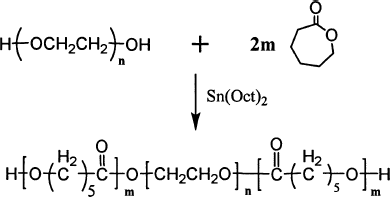
Triblock PCEC copolymer was synthesized from ε-caprolactone by ring-opening method initiated by PEG, (Diagram 1) (CitationRyu et al. 2001). The typical PCEC100000 copolymer was prepared as follows: ε-CL (24 g), PEG4000 (1 g), and Sn(Oct)2 (0.05 g) were added into reaction vessel under nitrogen atmosphere. The mixture was first kept at 110°C for 1 hr under nitrogen atmosphere. Later, the temperature was gradually elevated to 160°C over 30 min. Then, the mixture was rapidly heated to 180°C under vacuum for another 7 hr. At the end, the resultant melt was poured onto a steel plate under nitrogen atmosphere, thus PCEC100000 copolymer was obtained.
Purification of PCEC Copolymers
The resultant PCEC copolymers were first dissolved in AR grade trichloromethane (TCM) and reprecipitated from the filtrate using AR grade n-hexane. Then, this mixture was filtered and vacuum dried to constant weight. The purified PCEC copolymers were kept in air-tight bags in desiccators before use.
Fourier Transform Infrared Spectroscopy (FTIR) and 1H Nuclear Magnetic Resonance
FTIR (KBr) spectrum of the copolymers was taken in NICOLET 200SXV560 infrared spectrophotometer (Nicolet, USA). 1H-NMR spectrum (in CDCl3) was recorded on Varian 400 spectrometer (Varian, USA) at 400 MHz using tetramethylsilane as internal reference standard.
Preparation of PCEC Nanoparticles
In this work, PCEC100000 copolymer was used to prepare PCEC polyetherester nanoparticles. The solvent displacement (diffusion) method and solvent evaporation method were both used. PCEC100000 was first dissolved in 5 ml of organic solvents (DMSO, DMSO/CH2Cl2, or CH2Cl2). The resultant polymer solution was then added into 100 ml of aqueous polyvinyl alcohol (PVA) solution with continuous stirring at predetermined rate. Then, PCEC organic solution was immediately dispersed into drops by extensive stirring with a four-blade stirrer at a predetermined rate. For the diffusion or evaporation of organic solvent, the drops became smaller and smaller, and PCEC nanoparticles formed after 2 hr. The resultant PCEC nanoparticles suspension was ultracentrifuged for 20 min at 30000 rpm. The obtained nanoparticles were washed with distilled water and then ultracentrifuged three times. Then, the suspension was obtained by addition of distilled water before test. The preparing parameters of different PCEC nanoparticles are shown in .
TABLE 1 Preparing parameters for PCEC nanoparticles
Determination of Particle Size Distribution
PCEC nanoparticles were first dispersed in distilled water using ultrasound dispersion, and the particle size distribution was determined on Malvern Instrument (NanoZS90, Malvern Instruments, UK).
Drug Loading Determination
BSA content of the BSA/PCEC nanoparticles was determined using alkaline digestion method, which was shown as follows: ∼20 mg of BSA-loaded PCEC nanoparticles was suspended in 2 ml of 0.01 M sodium hydroxide, and the suspension was stirred at room temperature for 24 hr, until complete hydrolysis of the PCEC copolymers. The resultant transparent hydrolysate was then spectrophotometrically measured at 278 nm (UV spectrophotometer, Analytik Jena Specord 200, Germany), and the BSA concentration in hydrolysate was calculated according to the standard curve of BSA. Each sample was assayed in triplicate. In this study, drug loading (DL) and entrapment efficiency (EE) were calculated according to Equations 1 and 2 (CitationJia et al. 2007);
In Vitro Degradation Study
The PCEC100000 nanoparticles were incubated in 0.01 mol/L alkaline NaOH solution (pH = 12) at 37°C. The samples were removed from the bottles at predetermined time. Then the surface morphology of PCEC nanoparticle was observed on SEM (Amray, USA) after sputtered with gold.
In Vitro Drug Release Studies
First, 100 mg of PCEC nanoparticles were immersed in 25 ml of PBS solution (0.01M, pH 7.4) in Eppendorf test tube shaken at 60 rpm at 37°C. Second, predetermined intervals, the Eppendorf test tube was ultracentrifuged at 30000 rpm for 20 min, and 5.0 ml PBS solution was removed from the supernatant and replaced by fresh release medium. BSA concentration was determined by UV method as already mentioned. The accumulative release weight of BSA was calculated according to equation 3 (CitationJia et al. 2007):
where Q is accumulative release weight, Cn is measured concentration of the drug, Vt is volume of the medium, and Vs is volume of solution removed from supernatant. The results shown in this work are the mean of three parallel experiments.
Polyacrylamide Gel Electrophoresis (PAGE)
The structural integrity of BSA extracted from nanoparticles or released in vitro was detected by SDS-PAGE, compared with native BSA and reference markers. Protein samples were diluted with Tris-buffer (pH 6.8) with 2% SDS. Electrophoresis of samples was performed at a constant voltage of 200V in a Tris/glycine/SDS buffer using a BioRad Mini-Protein II electrophoresis system. After migration, the gel was stained with coomassie bright blue in methanol-acetic-water (2.5:1:6.4) to reveal protein, then destained and dried.
Statistical Data Analysis
Multiple experiment data were obtained and presented as mean values. Statistical comparisons were done at a significant level of P < 0.05 using Student's t-test.
RESULTS AND DISCUSSION
Polymer Characterization
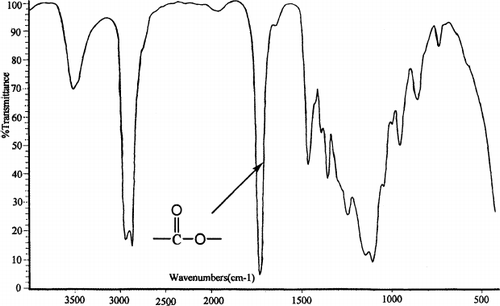
The biodegradable PCEC triblock copolymer was successfully prepared by ring opening copolymerization of ε-caprolactone initiated by PEG. FTIR and 1H-NMR were used to characterize the chemical structure of PCEC copolymer. The FTIR spectrum is shown in . The absorption band at 1733 cm−1 was attributed to the C═ O stretching vibrations of ester carbonyl group. The absorption bands at 1142 cm−1 and 1243 cm−1 were attributed to the characteristic C─ O─ C stretching vibrations of the repeated OCH2CH2 units of PEG and the ─ COO/bond bands stretching vibrations, respectively. It is obvious that the PCEC copolymer exhibits characteristic peaks of both PEG and PCL.
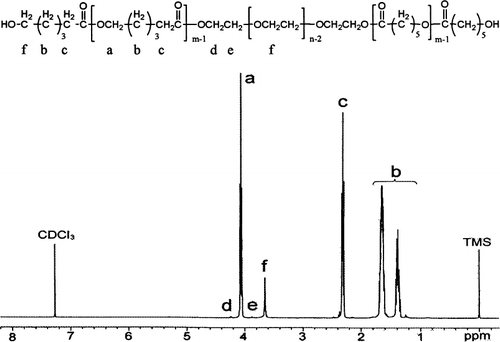
To furtherly confirm the formation of PCEC copolymer, 1H-NMR spectrum was also recorded and is shown in . The characteristic absorption peaks were also indicated in this figure. Peaks at 1.40, 1.65, 2.32, and 4.06 ppm were assigned to methylene protons of ─ (CH2)3─, ─ OCCH2─, and ─ CH2OOC─ in PCL chains, respectively. The sharp peak at 3.65 ppm is attributed to the methylene protons of PEG segments. The very weak peaks at 4.23 ppm and 3.82 ppm were attributed, respectively, to the methylene protons of ─ O─ CH2─ CH2─ in PEG end unit. All the FTIR and 1H-NMR results indicated that the PCEC copolymer formed successfully.
Preparation of PCEC Nanoparticles
In this study, several kinds of PCEC nanoparticles were prepared successfully, and the detailed results are shown in . The particle size was greatly affected by inner aqueous phase, organic solvent, and drug loading, which is discussed in detail as follows.
TABLE 2 Basic data of PCEC nanoparticles prepared in mean values (n = 3)
Effect of Volume of Inner Aqueous Phase (w1)
shows the basic data of nanoparticles. Samples N1, N2, and N3 have the same parameter except for the volume of inner aqueous phase. The average diameter of N1, N2, and N3 are 598.18, 512.37, and 431.58 nm, respectively. With the increase in volume of inner aqueous phase, the particle size of PCEC nanoparticles increased accordingly. This increase in particle size might be due to a coarse and less stable W1/O emulsion, which favored the coalescence of suspended droplets. At the same time, the larger the volume of inner aqueous phase, the higher the entrapment efficiency, because the larger nanoparticles have higher drug-loading potential. And the PDI (polydispersity index) of PCEC nanoparticles are all less than or nearly 0.1, so these kinds of nanoparticles can be considered a monodispersed system (CitationLamprecht, Ubrich, and Perez 1999).
Effect of Organic Phase
DMSO is water soluble, and it could be diffused into water very quickly. CH2Cl2 cannot be dissolved in water, but it could evaporate from water. When DMSO is substituted or partly substituted by CH2Cl2, the particle size of the obtained PCEC nanoparticles will change, greatly.
The partly substitution of DMSO by CH2Cl2 significantly makes the size of the nanoparticle larger, and even the size of R3 (CH2Cl2 only) is more than 5000 nm. This phenomenon might be due to the first W1/O emulsion becoming coarse and less stable, which favored the coalescence of suspended droplets, as already mentioned in the section discussing the effect of the volume of inner aqueous phase. When the DMSO was partly substituted by CH2Cl2, the entrapment efficiency of the nanoparticles decreased accordingly, which might be due to slower solidification speed evoked by participation of CH2Cl2, and partial drug diffusion into the outer aqueous phase. Thus, the more the CH2Cl2 in the solvent, the lower the drug entrapment efficiency. All the results are shown in .
Effect of Nominal Drug Loading
In this study, the nominal drug loading is from 5% to 15%. In this region, the higher the nominal drug loading, the higher the actual drug loading. But the entrapment efficiency of Z2 and Z3 are 42.99% and 35.21%, respectively, which decreased accordingly compared with N1 whose entrapment efficiency is 49.5%.
In Vitro Degradation Study
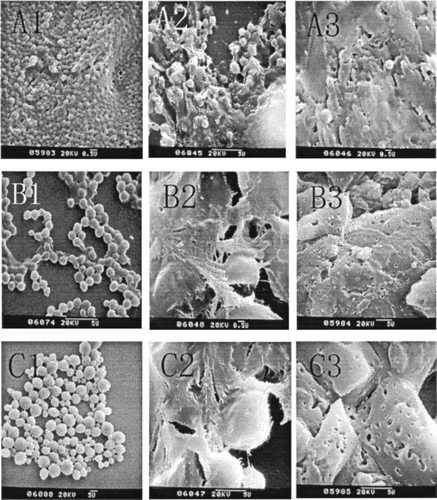
shows the surface morphology of PCEC nanoparticles during degradation, According to , we found that the original PCEC particles had a spherical shape and the particle size ranged from 400 to 5000 nm with change in organic phase. The particles prepared by DMSO had a much smaller size and great uniformity, but the particles were easy to conglutinate with each other; on the contrary, when the organic phase was substituted or partly substituted by CH2Cl2, the size of the particles became larger, and the uniformity decreased, but the particles became less conglutinated. All of these results are consistent with the basic data discussed above. When degraded for 3 days, the particles became syncretic and the surface of the particles started flaking off, so the boundary of the particles was blurry and the surface obviously became coarse. When degraded for 5 days, the particles degraded into pieces, and many holes appeard on the fragment after degradation.
FIG. 3. SEM morphology of various nanoparticles prepared by different organic solvents (with different mixing ratio of DMSO and CH2Cl2). (A) Organic phase is DMSO, degradation Time T = 0d (A1), T = 3 d (A2), and T = 5 d (A3). (B) Organic phase is DMSO + CH2Cl2, degradation Time T = 0d (B1), T = 3d (B2), and T = 5d (B3). (C) Organic phase is CH2Cl2 only, degradation Time T = 0d (C1), T = 3d(C2), and T = 5d (C3).
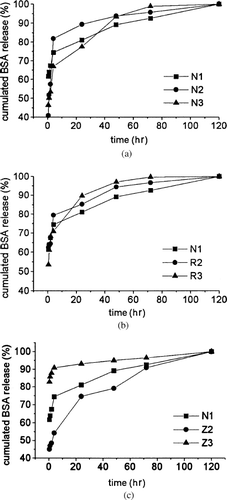
In Vitro Drug Release Studies
illustrates the in vitro release profiles of BSA loaded nanoparticles in 120 hr, by representing the percentage of BSA release with respect to the total encapsulated BSA. For all kinds of nanoparticles, there were two stages for BSA release. Stage I: a first initial burst release, the BSA, adsorbed on the surface of nanoparticles, was immediately released. Stage II: after initial burst, the drug release displayed a plateau release profile, resulting from diffusion of BSA from polymer matrix after erosion of PCEC polymers.
FIG. 4. Release profiles of BSA nanoparticles in phosphate buffer at 37°C and pH 7.4 in 120 hr. (a) Effect of inner aqueous phase on BSA release profiles of PCEC nanoparticles: release profiles of N1, N2, and N3. (b) Effect of organic solvent on BSA release profiles of PCEC nanoparticles: release profiles of N1, R2, and R3. (c) Effect of drug loading on BSA release profiles of PCEC nanoparticles: release profiles of N1, Z2, and Z3.
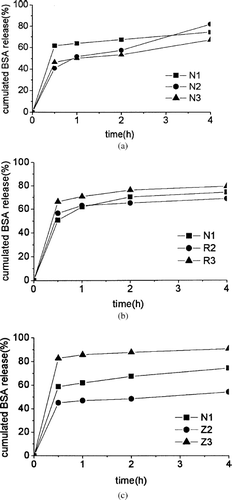
shows the release profiles in the first 4 hr. Burst effect is defined here as the percentage released in the first 0.5 hr, compared to the total released BSA, because all samples of nanoparticles showed obvious BSA release in the first 0.5 hr. All the samples had burst release effect because of the surface absorption of the nanoparticles. The burst effect of Z2, N1, and Z3 were 45.1, 61.7, and 87.8%, respectively, which ordinarily increased with increase in nominal drug loading. Obviously, higher nominal drug loading leads to the tremendous burst effect. As a matter of fact, an increase in nominal drug loading to very high levels is not always desirable, because the amount of polymer could be insufficient to completely entrap the drug, and a sustained release is expected (CitationBilati, Allemann, and Doelker 2005).
FIG. 5. Release profiles of BSA nanoparticles in phosphate buffer at 37°C and pH 7.4 in 4 hr. (a) Effect of inner aqueous phase on BSA release profiles of PCEC nanoparticles: release profiles of N1, N2, and N3. (b) Effect of organic solvent on BSA release profiles of PCEC nanoparticles: Release profiles of N1, R2 and R3. (c) Release profiles of N1, Z2, and Z3.
PAGE Behavior of BSA in Vitro Release
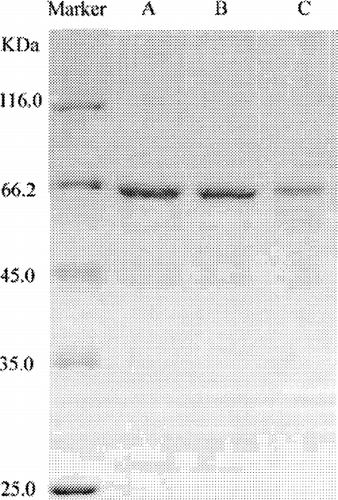
shows the SDS-PAGE results of original BSA solution (band A) and BSA released from N1 (band B), and R2 (band C). It we see that the samples—BSA before encapsulation, and BSA released from N1 and R2—showed no additional peak of higher or lower molecular BSA. This suggests that no chemical polymerization, noncovalent aggregation, or molecular hydrolysis occurred during the preparation and release processes. From the above discussion, we conclude that biodegradable (PCEC) nanoparticles have a great potential application in protein delivery system.
CONCLUSION
The PCEC triblock copolymer was synthesized by ring-opening of ε-caprolactone with PEG and characterized by 1H-NMR and FT-IR. The PCEC nanoparticles were prepared by solvent diffusion method or w/o/w double emulsion method. The particles were characterized by size, SEM. Also, the effect of the experiment parameter on the particles, and the degradation, and drug release in vitro were all studied in detail. The model drug BSA can be efficiently entrapped in PCEC nanoparticles, and it can be released extendedly for 5 days without denaturation.
REFERENCES
- U. Bilati, E. Allemann, and E. Doelker. (2005). Poly(D, L-lactide-co-glycolide) protein-loaded nanoparticles prepared by the double emulsion method--processing and formulation issues for enhanced entrapment efficiency. J Microencapsulation 22:205–214.
- D. T. Birnbaum, J. D. Kosmala, and L. B. Peppas. (2000). Optimization of preparation techniques for poly(lactic acid-co-glycolic acid) nanoparticles. J Nanoparticle Res. 2:173–181.
- J. L. Chen, C. H. Chiang, and M. K. Yeh. (2002). The mechanism of PLA microparticle formation by water-in-oil-in-water solvent evaporation method. J Microencapsulation 19 (3):333–346.
- W. J. Jia, J. G. Liu, Y. D. Zhang, J. W. Wang, and J. Wang. (2007). Preparation, characterization, and optimization of pancreas-targeted 5-Fu loaded magnetic bovine serum albumin microspheres. J Drug Target. 15:140–155.
- H. Y. Kwon, J. Y. Lee, S. W. Choi, Y. S. Jang, and J. H. Kim. (2001). Preparation of PLGA nanoparticles containing estrogen by emulsification-diffusion method. Colloid Surface A: Physicohem Eng Aspects 182:123–130.
- A. Lamprecht, N. Ubrich, and M. H. Perez. (1999). Biodegradable monodiapersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm. 184:97–105.
- J. Molpeceres, M. R. Aberturas, and M. Guzman. (2000). Biodegradable nanoparticles as a delivery system for cyclosporine: preparation and characterization. J Microencapsulation. 17 (5):599–614.
- J. G. Ryu, Y. I. Jeong, Y. H. Kim, I. S. Kim, D. H. Kim, and S. H. Kim. (2001). Clonazepam release from core-shell type nanoparticles of poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) triblock copolymers. Bull. Korean Chem. Soc. 22 (5):467–475.
- N. Ubrich, P. Bouillot, C. Pellerin, M. Hoffman, and P. Maincent. (2004). Preparation and characterization of propranolol hydrochloride nanoparticles: a comparative study. J Control Rel. 97:291–300.
- Y. Zhang, and R. Zhuo. (2005). Synthesis and in vitro drug release behavior of amphiphilic triblock copolymer nanoparticles based on poly (ethylene glycol) and polycaprolactone. Biomaterials. 26:6736–6742.
- S. B. Zhou, X. M. Deng, and X. H. Li. (2001). Investigation on a novel core-coated microspheres protein delivery system. J Control Rel. 75:27–36.