Abstract
We prepared a nanoemulsion system with benzyl alcohol/ ethanol/Solutol/smash® HS 15 /water. Ketoprofen was used as a model drug in this study. The nanoemulsions of this system evidenced a high degree of stability. The droplet diameter did not change over a period of at least 3 months. The nanoemulsion containing 4% benzyl alcohol evidenced a permeation rate higher than was observed with the 1% and 2% nanoemulsions. Also the nanoemulsion containing 1% Solutol® HS 15 provided a permeation rate higher than was seen with the 2% and 4% nanoemulsions. All ketoprofen-loaded nanoemulsions enhanced the in vitro permeation rate through mouse skins as compared to the control.
INTRODUCTION
Ketoprofen [(RS)-2-(3-benzoylphenyl)-propionic acid], a potent nonsteroidal anti-inflammatory drug (NSAID), has been utilized extensively in the treatment of rheumatoid arthritis and associated diseases (CitationKantor 1986). However, ketoprofen is associated with certain adverse side effects when administered orally, including gastrointestinal irritation. As ketoprofen is normally administered to patients over an extended period of time, many efforts have been made to ameliorate its adverse side effects. One promising method for this involves the administration of the drug via the skin. Its gastrointestinal side effects could be avoided by using transdermal forms. Ketoprofen is an excellent candidate for transdermal delivery among the variety of known NSAIDs (CitationLiu et al. 1997; CitationTakanashi et al. 1999; CitationFujii et al. 2000; CitationPaolino et al. 2002). It has an appropriate partition coefficient and adequate aqueous solubility as compared to other NSAIDs. It would be desirable to deliver ketoprofen to deeper skin layers, thus enabling it to function at the inflammation site. Thus, transdermal ketoprofen delivery may provide better patient compliance than can be achieved via oral administration. A variety of transdermal dosage forms containing ketoprofen have been previously reported (CitationYim et al. 1994; CitationSingh et al. 1996; CitationValenta and Kerner 1996), including patches, gels (CitationChi and Jun 1990), and creams (Itoh et al. 1985). In an attempt to improve the skin permeation characteristics of ketoprofen, several physicochemical methods have been assessed with an effort to develop appropriate vehicles (CitationRhee et al. 1999; CitationValenta, Wanka, and Heidlas 2000).
Nanoemulsion is defined as a dispersion consisting of oil, surfactants, cosurfactants, and an aqueous phase, which is a single optically isotropic and thermodynamically stable liquid solution, usually with a droplet diameter within the range of 10–100 nm (Tenjarla 1999). Nanoemulsions have several advantages, including enhanced drug solubility, good thermodynamic stability, and an enhancing effect on transdermal ability, as compared to conventional formulations (CitationLawrence and Rees 2000; CitationZhao et al. 2006). Thus far, a great deal of attention has been focused on the topical delivery of drugs using nanoemulsions (CitationPeltola et al. 2003; CitationSintov and Shapiro 2004; CitationYuan et al. 2006). The permeation rate of emulsion droplets for a drug can be altered by their impact on particle size, as well as a variety of environmental factors, including oils, enhancers, and surfactants.
Thus, the principal objective of our study was to formulate a new nanoemulsion system with the modified nonionic surfactant, Solutol HS® 15. Stable nanoemulsion systems consisting of benzyl alcohol (BA), SLT. ethanol, and water were prepared. The percutaneous permeation profiles of ketoprofen-loaded nanoemulsions through excised mouse skin are also discussed herein.
MATERIALS AND METHODS
Materials
Ketoprofen, benzyl alcohol (BA), and ethyl alcohol were purchased from Sigma (St. Louis, USA). Polyethylene glycol 660 12-hydroxystearate, Solutol® HS 15 (SLT) was acquired from BASF (Ludwigshafen, Germany). Other chemicals were of HPLC or analytical grade.
Formulation of Ketoprofen Nanoemulsion
Pseudoternary phase diagrams were constructed to obtain the components and their concentration ranges, which can result in large areas of the nanoemulsions in which the drug does or does not exist (CitationPark et al. 2005).
The ketoprofen-containing BA was utilized as the oil phase, and SLT and ethanol were utilized as the surfactant and cosurfactant. A ketoprofen-containing BA and ethanol mixture (1:1 and 1:2) was used as the oil phase, and the surfactant content was changed. After the microemulsion regions in the phase diagrams had been identified, the nanoemulsion formulations were selected at different component ratios, as is described in . After the ketoprofen was dissolved in the BA and ethanol mixture, the surfactant was melted with heating and stirring. To formulate the nanoemulsions, appropriated water was added in a dropwise fashion with gentle agitation.
TABLE 1 Composition of the selected nanoemulsion formulation
Determination of Droplet Size in Nanoemulsions
The particle sizes of the nanoemulsion were assessed via light-scattering techniques. The dynamic light scattering (DLS) of samples was measured at a scattering angle of 90° using a DLS-8000 system (Otsuka Electronics, Japan). Each sample was measured in triplicate, and the average particle size was expressed as the mean diameter.
Measurement of Skin Permeation Rate of Ketoprofen
For this investigation, static Franz glass diffusion cells were used. These cells comprise donor and receptor chambers between which a membrane is positioned (CitationFranz 1975). Skins were obtained from male mouse weighing 25 ± 2 g. After the hair was carefully removed, a 2.5 × 2.5 cm patch of skin was excised from the dorsal region of each sacrificed mouse, and the subcutaneous fat and other extraneous tissues were trimmed. The excised mouse skins were maintained at a temperature of −20° and used within 1 week after skin harvesting.
The extent and efficacy of ketoprofen skin permeation from the prepared nanoemulsions were determined using Franz diffusion cells fitted with excised mouse skins. The effective diffusional area was 1.77 cm2. The receptor compartment was filled with 11.5 mL of phosphate buffer at a pH of 7.4 (0.01 M), and its temperature was maintained at 37 ± 0.5°C and stirred at 600 rpm throughout the experiment. After 2 mL of the nanoemulsion was applied to the epidermal skin surface, 0.2 mL of the receptor medium was withdrawn every hour for up to 24 hr after application, and immediately replaced with an equal volume of fresh phosphate buffer equilibrated at 37 ± 0.5°C.
HPLC Analysis of Ketoprofen
The ketoprofen concentration in the receiver sample was determined by a slightly modified version of the HPLC method, as previously described (CitationSatterwhite and Boudinot 1988). The conditions for the ketoprofen determination were as follows: an ODS column (Tosoh, Tskgel ODS-80TS, 4.6 mm i.d × 150 mm) was utilized. The mobile phase was a mixture of acetonitrile and pH 7.4 phosphate buffer (78:22 v/v). The flow rate of the mobile phase was 1 mL/min, and the detection wavelength was set at 258 nm.
Data Analysis
The cumulative quantity of ketoprofen permeating through the excised mouse skin was plotted as a function of time. The slope and intercept of the linear portion of the plot was derived via regression. The permeation rate at steady state (Js, μg/cm2/hr) was calculated as the slope divided by the surface area of the skin.
RESULTS AND DISCUSSION
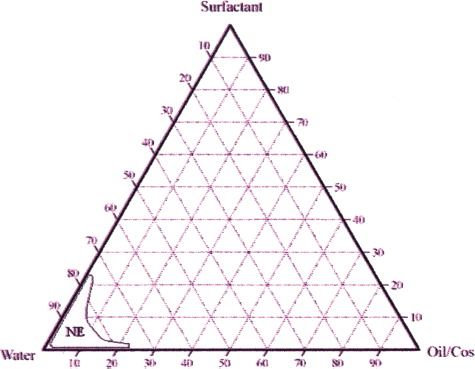
The studied systems were composed of safe constituents, including oil, surfactant, cosurfactant, and water. The construction of phase diagrams makes it easy to determine the concentration range of the components in the existence range of the nanoemulsions. The pseudoternary phase diagram with various weight ratios of BA to the ethanol area is described in . The translucent nanoemulsion region is shown in the phase diagram. The conversion from the water-in-oil to oil-in-water emulsion was observed in the water addition step. The area of the nanoemulsion isotropic region was slightly altered in size with increasing surfactant-to-cosurfactant ratios.
FIG. 1 Pseudoternary phase diagrams of emulsion composed of Oil/CoS (BA/ethanol). surfactant (SLT), and water. (NE represents nanoemulsion region).
Nanoemulsions were prepared in the water/SLT/BA/ethanol system via the addition of water to oil/cosurfactant/surfactant mixtures. Translucent liquid dispersions appeared after the addition of water. In this experiment, we determined the appropriate ketoprofen weight to be 0.8%. When the ketoprofen percentage was in excess of 0.8%, the emulsion did not form.
The droplet sizes of the nanoemulsion are shown in . The nanoemulsion I containing 2% oil. 2% cosurfactant, and 4% surfactant evidenced the lowest average droplet size. Otherwise, nanoemulsion V containing 2% surfactant resulted in an average droplet size of 226.3 ± 12.7 nm.
TABLE 2 Mean particle size of nanoemulsion
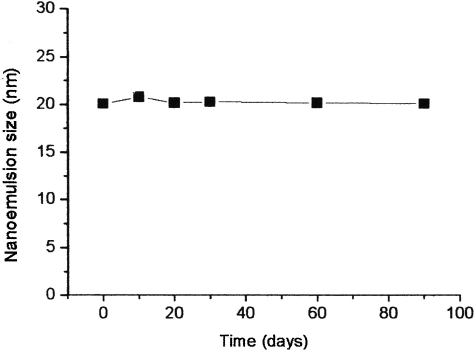
Nanoemulsion stability was determined via measurements of droplet size as a function of time. All nanoemulsion formulations were found to be stable at 37°C in the presence or absence of ketoprofen. The changes in particle size, phase separation, and degradation were not observed over a period of 1 month. Also, the nanoemulsions stored at 4°C evidenced no change in droplet size over a period of 3 months. shows the size of a emulsion, which is composed of 0.8% ketoprofen, 4% BA, 2% ethanol, and 2% SLT, over a period of 3 months.
FIG. 2 The change of mean particle size of ketoprofen-loaded nanoemulsion for 90 days. Average particle size of the NE2. The sample was stored at 4°C.
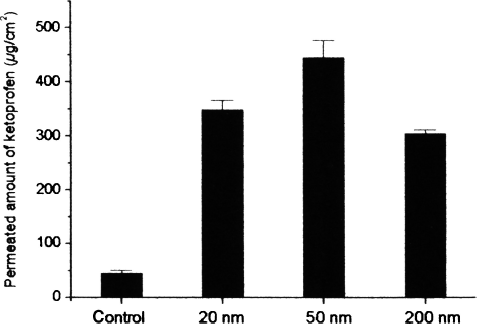
The permeation ability of the various nanoemulsions was evaluated via in vitro permeation experiments. The profiles of ketoprofen permeation through mouse skin from various size emulsions and the controls are shown in . The majority of the nanoemulsions provided fluxes higher than those of the controls, by at least 6 fold (ethanol solution containing 0.8% ketoprofen). The emulsion of 200 nm size had a slightly lower permeation rate as compared to other emulsions. However, when the emulsions of 20 and 50 nm were compared, the 50 nm emulsion evidenced a permeation rate higher than that of the 20 nm emulsion. Previous studies have demonstrated that the small size of the emulsion is responsible for the observed effects on skin permeation. This may be due to the small droplet diameter of the nanoemulsions, and the likely mechanism may also involve the permeation of ketoprofen directly from the droplets into the stratum corneum without the fusion of the emulsion to the stratum corneum and subsequent permeation (CitationChen et al. 2006). However according to our results, a size range of 20–200 nm evidenced relatively less significant effects on the ketoprofen permeation rate.
FIG. 3 Permeation profiles of ketoprofen through excised mouse skins from emulsion of different compositions and control. (Control: ketoprofen contained ethanol solution, permeation time = 24 hr) (mean ± SD, n = 3).
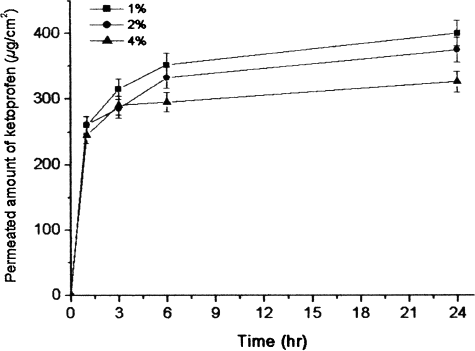
The effect of surfactant dosage in nanoemulsions on the permeation rate is shown in . The emulsion containing 1% SLT provided a higher flux than was seen in the 2% and 4% SLT nanoemulsions, respectively. The surfactant content in the nanoemulsions affected the skin permeation flux of the ketoprofen. This may be the result of an increased thermodynamic activity of the drug in emulsions at lower surfactant concentrations (CitationRhee et al. 2001), as ketoprofen is not particularly water soluble. The thermodynamic activity of the drug in the formulation is a significant driving force for the release and penetration of the drug into the skin (CitationHilton et al. 1994). The thermodynamic driving force for release is reflective of the relative activities of the drug in different phases. As the drug can be released from the internal phase to the external phase and then from the external phase to the skin, the relative activities may be utilized to monitor the skin permeation rate (CitationLee, Langer, and Shastri 2003; CitationChen et al. 2004).
FIG. 4 Effects of SLT content in emulsion on permeation of ketoprofen through mice skin (mean ± SD, n = 3).
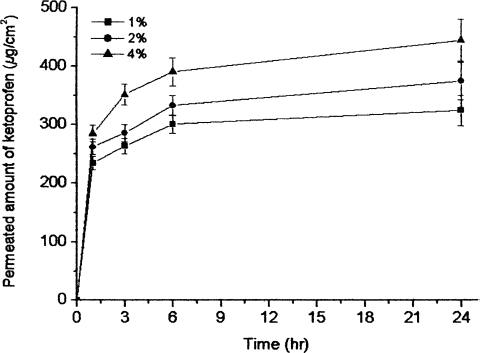
In addition, the oil phase content affected the rate of ketoprofen permeation. The effects of oil content in nanoemulsions on the permeation rate is shown in . The emulsion containing 4% of BA evidenced a higher flux than was seen with the 1% and 2% BA emulsions. It has been concluded that oil phase BA also functions as an enhancer. BA, a widely used, low-risk preservative and fragrance additive in pharmaceutical and cosmetic systems was selected on the premise that it is capable of solvating strongly hydrophobic compounds via the formation of micelles while, due to its moderate hydrophilicity, it maintains contact with the aqueous solution, thus rendering it ideal for penetration through the stratum corneum (CitationMikulak, Vangsness, and Nimni 1998). BA induced significant reductions in the skin's water holding capacity and hygroscopicity, with the effect on the former being substantial (CitationSaitoh, Ikeda, and Takagishi 1995) The BA also effectively loosens the lamellar structure while maintaining the overall lipid organization. The inclusion of BA in topical formulations has been demonstrated to significantly increase the drug retention and accumulation within the skin (CitationMikulak, Vangsness, and Nimni 1998).
CONCLUSION
An oil-in water nanoemulsion containing ketoprofen with SLT was formulated for transdermal application in our study. Also, BA was used as the oily phase of emulsion due to its solubilization and permeation-enhancing effects for ketoprofen. The components and their concentration ranges for the formation of nanoemulsions were obtained via the construction of a phase diagram. The in vitro permeation studies demonstrated that nanoemulsions could increase topical ketoprofen delivery when compared with the controls. Nanoemulsions with lower surfactant contents and small droplet size might, then, augment transdermal ability.
These results suggest that with poorly water-soluble drugs such as ketoprofen, topical delivery using nanoemulsion systems may hold a great deal of promise for transdermal drug delivery systems.
This work was supported by the Oriental Medicine R & D Project, Ministry of Health & Welfare, Republic of Korea (0405-0M00-0815- 0001).
REFERENCES
- H. Chen, X. Chang, D. Du, J. Li, H. Xu, and X. Yang. (2006). Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int. J. Pharm. 315:52–58.
- H. Chen, X. Chang, T. Weng, X. Zhao, Z. Gao, Y. Yang, H. Xu, and X. Yang. (2004). A study of microemulsion systems for transdermal delivery of triptolide. J. Control. Release 98:427–436.
- S. C. Chi, and H. W. Jun. (1990). Anti-inflammatory activity of ketoprofen gel on carrageenan-induced paw edema in rats. J. Pharm. Sci. 79:974–977.
- T. J. Franz. (1975). Percutaneous absorption on the relevance of in vitro data. J. Invest. Dermatol. 64:190–195.
- M. Fujii, N. Hori, K. Shiozawa, K. Wakabayashi, E. Kawahara, and M. Matsumoto. (2000). Effect of fatty acid Esters on permeation of ketoprofen through hairless rat skin. Int. J. Pharm. 205:117–125.
- J. Hilton, B. H. Woollen, R. C. Scott, T. R. Auton, K. L. Trebilcock, and M. F. Wilks. (1994). Vehicle effects on in vitro percutaneous absorption through rat and human skin. Pharm. Res. 11:1396–1400.
- T. G. Kantor. (1986). Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy. 6:93–103.
- M. J. Lawrence, and G. D. Rees. (2000). Microemulsion-based media as novel drug delivery systems. Adv. Drug. Deliv. Rev. 45:89–121.
- P. J. Lee, R. Langer, and V. P. Shastri. (2003). Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm. Res. 20:264–269.
- K. Liu, Y. Y. Chia, Y. Lo, Y. Y. Kan, J. J. Wang, and S. T. Ho. (1997). Effect of preoperative transdermal ketoprofen on post-hysterectomy pain. Chin. Med. J. (Taipei) 60:290–295.
- S. A. Mikulak, C. T. Vangsness, and M. E. Nimni. (1998). Transdermal delivery and accumulation of indomethacin in subcutaneous tissues in rats. J. Pharm. Pharmacol. 50:153–158.
- D. Paolino, C. A. Ventura, S. Nistico, G. Puglisi, and M. Fresta. (2002). Lecithin microemulsions for the topical administration of ketoprofen: percutaneous adsorption through human skin and in vivo human skin tolerability. Int. J. Pharm. 244:21–31.
- J. H. Park, K. Y. Kyong, G. W. Lee, and U. K. Jee. (2005). Formulation design and evaluation of ursolic acid microemulsion delivery system for topical formulation. J. Kor. Pharm. Sci. 35:233–341.
- S. Peltola, P. Saarinen-Savolainen, J. Kiesvaara, T. M. Suhonen, and A. Urtti. (2003). Microemulsions for topical delivery of estradiol. Int. J. Pharm. 254:99–107.
- G. J. Rhee, J. S. Woo, S. J. Hwang, Y. W. Lee, and C. H. Lee. (1999). Topical oleo-hydrogel preparation of ketoprofen with enhanced skin permeability. Drug. Dev. Ind. Pharm. 25:717–726.
- Y. S. Rhee, J. G. Choi, E. S. Park, and S. C. Chi. (2001). Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 228:161–170.
- I. Saitoh, K. Ikeda, and Y. Takagishi. (1995). Effect of benzyl alcohol on rat skin as a solvent of liquid droplet dispersion ointment. Biol. Pharm. Bull. 18:321–325.
- J. H. Satterwhite, and F. D. Boudinot. (1988). High-performance liquid chromatographic determination of ketoprofen and naproxen in rat plasma. J. Chromatogr. 431:444–449.
- S. K. Singh, M. J. Durrani, I. K. Reddy, and M. A. Khan. (1996). Effect of permeation enhancers on the release of ketoprofen through transdermal drug delivery systems. Pharmazie 51:741–744.
- A. C. Sintov, and L. Shapiro. (2004). New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug Bioavailabillty in vivo. J. Control. Release 95:173–183.
- Y. Takanashi, K. Higashiyama, H. Komiya, K. Takayama, and T. Nagai. (1999). Thiomenthol derivatives as novel percutaneous absorption enhancers. Drug. Dev. Ind Pharm. 25:89–94.
- S. Tenjaria. (1999). Microemulsions: an overview and pharmaceutical applications. Crit. Rev. Ther. Drug Carrier Syst. 16:461–521.
- C. Valenta, and A. Kerner. (1996). Effect of various vehicles on ketoprofen permeation across artificial membranes and excised rat skin. Pharmazie 51:605–606.
- C. Valenta, M. Wanka, and J. Heidlas. (2000). Evaluation of novel soya-lecithin formulations for dermal use containing ketoprofen as a model drug. J. Control. Release 63:165–173.
- D. S. Yim, I. J. Jang, S. G. Shin, J. H. Yoo, and H. C. Eun. (1994). Pharmacokinetic and skin irritation of transdermal ketoprofen. Kor. J. Clin. Pharmacol. Ther. 2:21–27.
- Y. Yuan, S. M. Li, F. K. Mo, and D. F. Zhong. (2006). Investigation of microemulsion system for transdermal delivery of meloxicam. Int. J. Pharm. 321:117–123.
- X. Zhao, J. P. Liu, X. Zhang, and Y. Li. (2006). Enhancement of transdermal delivery of theophylline using microemulsion vehicle. Int. J. Pharm. 327:58–64.