Abstract
The main purpose of the present study was to investigate different cationic submicron emulsions as potential delivery for oral administration. Different submicron emulsion based formulations were prepared by standard procedures incorporating Chitosan, stearylamine, and protamine as charge inducer. Saquinavir (SQ) laden emulsions were characterized in terms of globule size, zeta potential, entrapment efficiency, release profile, cytotoxicity, LDH release, and stability studies. The prepared formulations were stable in terms of mean globule size, drug content, and tended to retain their cationic charge. Pay load efficiency was found to be pretty high (≈ 95–99%) in various formulations prepared. Sustained release phenomenon was more prominent in the case of chitosan emulsions (CE) followed by stearylamine emulsion (SE), Protamine emulsion (PE), and then plain emulsion (E) containing no charge inducer. The total amounts of drug released in 24 hr from CE, SE, PE, and E were 46%, 52%, 56%, and 62%, respectively. The induction of positive charge in emulsions resulted in enhanced absorption of drug through intestinal membrane. The apparent permeability coefficient through the intestinal sac was in the order of CE > SE > PE > E. The permeation flux of SQ through CE (1.0 μg/min) was more than twice compared to plain emulsion (0.46 μg/min) while it was almost three times (0.3 μg/min) compared to control. However, protamine based emulsion didn’t confer significant improvement in absorption when compared to plain emulsion formulation. By this study it can be concluded that induction of positive charge on submicron emulsions can be effective for improving oral absorption of drug safely, as it is evinced with low LDH release into the medium when intestinal tissue is treated with submicron emulsion.
Introduction
Submicron emulsion or submicron emulsion-based drug delivery has proven its potential in the field of drug delivery which can administered through different routes. Submicron emulsion bearing positive charge are nowadays a promising alternative formulation approach for delivery of drug by oral route; besides providing a controlled release system, it results in enhanced retention and permeability with improve bioavailability. Further, many authors have demonstrated the role of cationic emulsion in prolonging pharmacological effects of drugs by improving oral permeability, viz mainly by electrostatic and muco-adhesive approaches. Submicron emulsions are also used orally to increase bioavailability and prolong pharmacological effects of drugs with poor oral absorption or short biological half-lifes (Rubinstein et al. Citation1991; Myers and Stella Citation1992). It has been shown in numerous studies that the incorporation of a drug in o/w emulsion significantly increased the absorption compared with the equivalent aqueous solution of the drug when administered orally. An oleic acid o/w-type emulsion of a new tacrolimus formulation has shown an improvement in the oral absorption of the drug in rats (Uno et al. Citation1999). The enhanced interaction of oil droplets with the mucosal surface, resulting in increased adhesion of the positively charged droplets to rat intestinal mucosa, and thus increased mucosal uptake of the drug, may be explained in part by their electrostatic interaction with negatively charged sites existing in the epithelial surface (Gershanik, Benzeno, and Benita Citation1998). The cationic submicron emulsion bearing cationic charge has been reported in various literature preferable for ocular delivery (Yang and Benita Citation2000). However, the efficacy and safety of submicron emulsion using different cationic charge inducers like chitosan, protamine, and stearylamine for oral delivery has not been studied so far. Therefore, an attempt has been made to prepare a drug-loaded cationic submicron emulsion and evaluate its performance with respect to oral delivery. Chitosan and protamine are well known for their applications in biomedical research. To the best of the knowledge of the authors, for the first time chitosan- and protamine-based emulsions have been exploited as drug delivery vehicles.
SQ was taken as model drug as its oral bioavailability is limited both by poor solubility and permeability across intestinal membrane. SQ, a peptidomimetic analog, is a highly selective potent inhibitor of the HIV aspartyl protease. Saquinavir (SQ) bioavailability is variable in people, and, due to its inherent physicochemical properties, its bioavailability is restricted to limited value, which may be due to poor solubility, CYP3A4 metabolism inhibition, Pg-p mediated efflux transport (Kim et al. Citation1998). Scientists are consistently starving to develop an optimized delivery system which is compatible to cells and enhances absorption and bioavailability.
Materials and methods
Materials
Medium-chain triglyceride (MCT) was purchased from Sigma (MO). MCT consisted of not less than 95% the esterified fatty acids, comprising eight and 10 carbon atoms according to manufacturer specifications. Lecithin, Poloxamer 188 (Pluronic F68), protamine sulfate, and stearylamine (SA) were purchased from Sigma (MO) and was used as such without further purification. All other ingredients were pharmaceutical grade. Saquinavir was kindly provided by Cipla Pharma (Mumbai, India) as a gift sample.
Preparation of chitosan aqueous phase
The chitosan was dispersed in 2% solution of lactic acid. The resulting mixture was stirred vigorously without heating for 24 hr until complete solubility was reached. The pH of the resulting solution was adjusted at 5.5 with 0.1 N NaOH to avoid any flocculation of Chitosan. This solution was filtered (0.45 mm filter) to separate the non-soluble accompanying fibers. The pH of the final emulsions was measured directly in the emulsion using a pH meter (Thermoelectron Corporation, USA).
Emulsion preparation
Submicron emulsions were prepared by homogenization followed by ultrasonication, as described previously (Venkateswarlu and Manjunath Citation2004). Saquinavir (SQ), MCT oil, and phosphatidylcholine were dissolved in 10 ml of mixture of chloroform and methanol (1:1). Organic solvents were completely removed using a Buchi rotoevaporator (400 mbar, 60°C). Nitrogen was blown on to the lipid layer for 30 min to remove traces of vapors of organic solvents, if any. This oil phase (containing SQ, MCT oil, and phosphatidylcholine) was heated up to 70–80°C and maintained till the addition of aqueous phase. The aqueous phase was prepared by dissolving poloxamer 188 in triple distilled water and heated up to 70–80°C. Oil phase was slowly added to hot aqueous phase and Primary emulsion was prepared by homogenizing in mechanical an Ultra Turrax T-25 (IKA labortechnik, Germany) homogenizer at a speed of 11,000 rpm for 5 min. Coarse hot oil in water emulsion thus obtained was ultrasonicated (Sonics & Materials Inc, USA) for 15 min to get submicron emulsion (E). Stearylamine (a surface charge modifier) was incorporated in submicron emulsion (SE) by dissolving in oil phase, whereas chitosan (CE) and protamine sulfate (PE) charge modifiers were incorporated by dissolving in aqueous phase.
Physicochemical characterization
The emulsion zeta potential was measured with the Malvern Zetasizer (Malvern, UK) while its pH was recorded at given time intervals using a pH meter. The mean droplet size and particle size distribution of submicron emulsion were determined by a laser diffraction particle size analyzer (Malvern Mastersizer®, UK). Each emulsion sample was diluted in water to an appropriate concentration before measurement at room temperature.
Morphological examination
Morphological examination was carried out on a Nikon (Eclipse E200, Japan) microscope with ×1000 magnification; a few milliliters of sample were taken undiluted on glass slide, covered with cover slip. This precaution was taken to prevent the drying of emulsion sample during observation.
Drug entrapment
Entrapment efficiency was calculated by determining the amount of unentrapped drug (A2) after removal of unentrapped drug by dialysis over the total amount of drug (A1). Entrapment efficiency was calculated using the equation.
E.E (%) = (A2/A1) × 100
Drug analysis
SQ concentrations in the samples were measured by an HPLC method as previously described (Frappier et al. Citation1998) with a slight modification. The mobile phase consisted of a mixture of acetonitrile: 5 mM potassium phosphate dibasic buffer (55: 45, v/v) adjusted to pH 8 with phosphoric acid and the flow rate was 1 ml/min. The injection volume was 20 μl and the detection was made at 240 nm.
Stability assessment
The drug content, pH, and droplet size distribution were monitored over periods of time stored at 4°C and 37°C. The creaming and the phase separation were assessed visually at given time intervals. All other visible changes were recorded. To evaluate its mechanical and physical resistance, the emulsion was subjected to an accelerated mechanical stress and its globule size distribution was measured before and after shaking at 100 strokes per min over 48 hr at room temperature.
In-vitro drug release
The in vitro release was determined using dialysis tubing (Sigma, USA). Free drug was removed from the formulation by exhaustive dialysis for 24 hr against PBS buffer at 4°C. Then, 2 ml of dialysate were introduced into the prewashed dialysis tubing’s which were placed in a beaker containing 250 ml of PBS buffer (pH 7.4). Sink condition was maintained continuously and the medium was stirred with a magnetic stirrer. Sample aliquots were withdrawn periodically and analyzed by HPLC.
Cytotoxicity study
Cytotoxicity study was carried out using MTT assay on J 774 macrophage cell line. This is based on the fact that any living and early apoptotic cells with mitochondrial activity when incubated with MTT, formazan crystals are formed, while dead cells don’t produce formazan crystals. Briefly, 100 μl of J 774 macrophage cell suspension (usually 5 × 104/ml) was taken in 96-well tissue culture plate and incubated at 37°C in the presence of 5% CO2 with different formulation for a period of 12 hr. Thereafter, 10 μl of MTT solution (5 mg/ml) was added to each well. It was further incubated for 3–4 hr at 37°C in the presence of 5% CO2. The plate was placed on a shaker and agitated for 10–20 min, and inverted rapidly to remove medium. The formazan crystals formed were solubilized in 200 μl of organic solvent (DMSO: ethanol 1:1). Plates were read at 550–570 nm (L1) and 620–650 nm (L2) as reference on a scanning multiwell spectrophotometer. The formazan solution absorbs light at 550–570 nm and absorbance at 630–650 nm usually results from cell debris and well imperfections. Final optical density (OD) obtained from formazan formation can be calculated as OD = L1 − L2.
Percentage cell survival is expressed as: (Absorbance of treated wells/Absorbance of control wells) × 100%
In-vitro absorption study
This was carried out as described previously (Bouer et al. Citation1999). Briefly, adult male Sprague Drawley rats (250–300 g; 11–12 weeks) were starved for 24 hr, sacrificed by cervical dislocation, and the small intestine was removed and washed thoroughly with saline (0.9% NaCl solution) at room temperature. The intestine was immediately placed in oxygenated (O2/CO2, 95%:5%) medium TC 199 at 37°C. The intestine was everted using a glass rod (2.5 cm in diameter) by clamping at one end. Small sacs (2–2.5 cm in length) were made and then tied using silk braided sutures. Each sac was placed in a 50 ml Erlenmeyer flask containing 9.0 ml of oxygenated media at 37°C. One milliliter of medium containing SQ was then added having specified concentration. The flasks were stoppered with gas-tight silicon bungs. Finally, the resulting gut sacs were incubated at 37°C in an oscillating water bath (60 cycles/min). At the appropriate time points, sacs were removed, washed four times in saline (0.9% NaCl solution), and blotted dry. The sacs were cut open and the serosal fluid (sac contents) was drained into small tubes. Each sac was weighed before and after serosal fluid collection to calculate accurately the volume inside the sac and to correct the serosal fluid for the actual volume. The amount of drug permeated through each sac from each formulation (E, SE, CE, and PE) was determined through HPLC. Each experiment was carried out using the small intestine from one animal.
Biochemical evaluation of intestinal damage
The fresh ileal loop of small intestine of rat was treated with 20 ml of Krebs ringer oxygenated buffer (warmed to 37°C) and then flushed out with air. One milliliter of various formulations (submicron emulsions) in various segments starting from 1% triton x-100 as positive control and plain saline solution as negative control were used and compared with the formulations of cationic vesicles and formulation without stearylamine and was administered to the ileum and incubated in the segments for 2 hr. Then, the ileal loop was washed with 1.0 ml of PBS, and the intestinal fluid was collected. The concentration of lactate dehydrogenase (LDH) in the fluid was determined using a LDH-UV kit (Sigma).
Statistical analysis
Results obtained from these experiments were expressed as mean ± SEM and were compared with control. P-values were calculated with ANOVA followed by Dunnets test.
Results and discussion
HIV-1 protease inhibitors like SQ have made a tremendous impact as part of triple combination therapy for the treatment of HIV infection. The introduction of the soft gel formulation of SQ has improved the bioavailability of SQ (4% for hard gel; 12% for soft gel); however, there are still problems relating to its low oral bioavailability. This arises as a consequence of multiple factors including low aqueous solubility, extensive intestinal and hepatic first pass metabolism, and decreased absorption due to the presence of the active efflux pump P-glycoprotein (P-gp) in the intestinal epithelium (Venkateswarlu and Manjunath Citation2004).
An attempt has been made to prepare different cationic submicron emulsions bearing SQ and is evaluated for improvement in oral absorption if any. In order to prepare submicron emulsions, a well-established combined emulsification technique (Facklam et al. Citation2004) was employed which yielded a fine homogeneous emulsion. The incorporation of SQ in the emulsion did not affect the physicochemical properties of emulsion and remained practically unchanged. These formulations were found to be stable in terms of pH, droplet size, and size distribution, phase separation, creaming, and, therefore, used for further performance evaluation.
The optimized formulation contains lecithin and Pluronic as emulsifier and as co-emulsifiers, respectively. Phospholipids are usually the first candidates selected as emulsifiers because of their biocompatibility and application in commercial fat emulsions, in which vegetable oil is used as an oil phase. However, it was found that lecithin was not capable to emulsify SQ alone. This observation may be attributed to the fact that phospholipids being highly soluble in oil phase are not sufficiently available at the oil–water interface to impart stability. Interestingly, the stability was significantly improved on addition of hydrophilic co-emulsifier (Pluronic F-68) which could have rendered interfacial film stronger to prevent globule coalescence during physical and thermal process and due to strong steric repulsion (Jumaa and Muller Citation1998b). Moreover, Pluronic F-68 is known to prevent unfavorable interactions between free fatty acids present in oil phase and cationic agents.
The optimized composition of various formulations has been described in . Size distribution of different formulations was in submicron range and average globule size was found in the range of 200–400 nm (). The zeta potential of CE, SE, PE, and E formulation was found to be +15.83, +12.80, +12.84, and −11.0 mV respectively (). When stearylamine (0.5%) was incorporated in emulsion formulation, zeta potential values changes from negative (–11.0 mV) to positive (up to +12.8 mV). The addition of chitosan (0.5% w/w), stearylamine (0.5% w/w), and protamine (0.1% w/w) to lipid emulsions led to a change of surface charge of oil droplets from negative to positive values, i.e. from –11 to ≈ (+)16, ≈ (+)13 mV, and ≈ (+)13 mV for CE, SE, and PE, respectively. The zeta potential and mean droplet size did not differ markedly from batch to batch (data not shown), indicating that the experimental conditions were well controlled. The addition of chitosan and protamine beyond optimized concentration disrupted the stability of formulation due to formation of small particulate aggregates. This may be attributed to unfavorable interaction between positively charged component and the negatively-charged free fatty acids, contained in the MCT oil and the concentration of Pluronic employed may not be sufficient to prevent this phenomenon (Jumaa and Muller Citation1999). Moreover, it also resulted in an increase in viscosity of emulsion. It is well established that increasing the viscosity of emulsion phases led to increase in the globule size (Jumaa and Muller Citation1998a) and thus instability. However, in the case of the chitosan emulsion globule size of emulsion doesn’t increase with its viscosity. This behavior may be attributed to the interfacial tension properties of Chitosan (Jumaa and Muller Citation1998a). Positive zeta potential values are achieved in most of the emulsions prepared, this is helpful to develop a high-energy barrier which causes repulsion of adjacent droplets, resulting in the formation of stabilized emulsions.
Table 1. Compositions (%) (w/w) of different optimized submicron emulsion incorporating SQ.
Table 2. Zeta potentials; mean particle size and entrapment efficiency of submicron emulsion formulations.
For a submicron emulsion, which is identical in composition, an addition of cationic agent stearylamine, protamine, and chitosan did not result in any significant change in mean droplet size. However, a profound effect on the zeta potential of the emulsions was observed on incorporation of cationic inducer. However, substantial increase in positive zeta potential was observed beyond 0.5% w/w stearylamine, indicating the probable occurrence of a saturation coverage process of stearylamine at the o/w interface under the given experimental conditions. This observation is supported by other group of workers who have shown that there is a threshold for imparting a cationicity to the emulsion systems (Calvo, Vila-Jato, and Alonso Citation1997).
Stearylamine, a component of the formulation SE, is a cationic lipid and confers an overall positive charge to the formulation over a wide pH range. The electrical surface charge of droplets is produced by the ionization of the components forming the interfacial film. The positive surface potential value of the droplets of the SE emulsion depends mainly upon the extent of the ionization of stearylamine at the oil/water interface. Furthermore, the incorporation of SQ did not alter the properties of the cationic emulsions as other physical parameters (zeta potential and mean droplet size) were closed to control positively charged emulsion.
Encapsulation efficiency of submicron emulsion was more than 90% in all formulations, as shown in . Morphological examination of emulsion reveals that the emulsions formed were homogenous, globules were spherical to oval in shape, and size distribution was in submicron range, as shown in . There was no significant change in terms of globule size, zeta potential, and drug content on storage for 4 months, as shown in . When subjected to excessive shaking (100 strokes per min over 48 hr), no change in mean droplet size, phase separation, and/or creaming were observed on visual inspection. SQ release from different submicron emulsions prepared has been summarized in .
Figure 1. Normal phase contrast microscopy of various submicron emulsions (magnification ×100). E: Plain emulsion without and charge inducer. SE: Submicron emulsion containing stearylamine as charge inducer. PE: Submicron emulsion containing protamine as charge inducer. CE: Submiron emulsion containing chitosan as charge inducer.
Figure 2. Cumulative release profile of SQ from submicron emulsion in PBS, pH 7.4. The values represented are means of three individual experiments. The experiment was performed using dialysis tubing (with cut-off mol. wt. 12,000). CE: Submicron emulsion containing chitosan as charge inducer. PE: Submicron emulsion containing protamine as charge inducer. SE: Submicron emulsion containing stearylamine as charge inducer. E: Plain emulsion without any charge inducer.
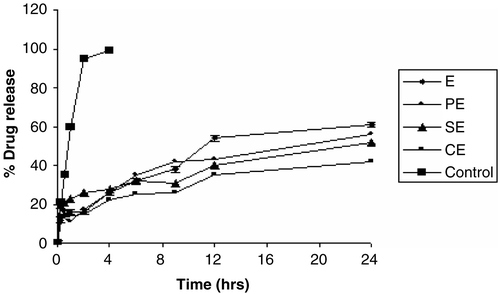
Table 3. Effect of storage time on stability of different submicron emulsion formulations at room temperature.
The release profile of SQ from different emulsion formulation was found to be sustained when studied for 24 hr. In contrast to this the control formulation (SQ dissolved in MCT oil) released almost 100% of the drug within 4 hr. Different formulations viz. CE, SE, PE, and E released 42%, 52%, 56%, and 62% of SQ, respectively, in 24 hr. This could be attributed to partitioning of the drug between the oily droplets and the external aqueous medium. SQ, being a highly lipophilic drug, diffuses out slowly from the oily phase to the external aqueous phase. The SQ release was lowest from CE formulation compared to SE, PE, and E. The presence of chitosan in formulation further sustains the release profile, as it is also known to acts as an emulsifier and present at the interface, forming the matrix around the oily core.
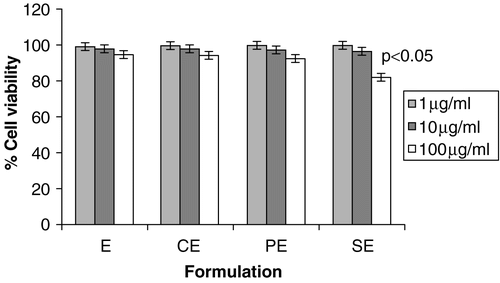
To assess any deleterious effect of submicron emulsion formulation on cells due to induction of cationic charge, a cell viability study was carried out on J-774 macrophage cell lines. As shown in the cell viability was more than 99% at lower concentrations (1 μg/ml, 10 μg/ml) of formulation, however at higher concentration (100 μg/ml) the viability was found to be in the order of E > CE > PE > SE. SE formulation showed more cytotoxicity compared to other cationic charge inducers, as stearylamine is known to possess high toxicity against cell system in-vitro. CE shows minimum cytotoxicity among cationic submicron emulsion, as chitosan is known to be a nontoxic cationic biopolymer, and their applications in different delivery systems are well documented (Kotze et al. Citation1998). However, it has been found that the concentration employed in proposed formulations did not have serious deleterious effects on cell viability. This study rules out the presumption that cationic polymers, by and large, induce the cytotoxic effects on cells. The polymers employed in the formulation for induction of cationicity are not toxic and are being used for various biomedical applications. Moreover, this study ascertains the applicability of these formulations when administered by an other than oral route which is under way in our laboratory.
Figure 3. Effect of different submicron emulsion on cell viability on J 774 MΦ at three different concentrations (A = 1 μg/ml; B = 10 μg/ml; C = 100 μg/ml) by MTT assay. The error bars indicates ± SD of three set of experiments (n = 3). CE: Submicron emulsion containing chitosan as charge inducer. PE: Submicron emulsion containing protamine as charge inducer. SE: Submicron emulsion containing stearylamine as charge inducer. E: Plain emulsion without any charge inducer.
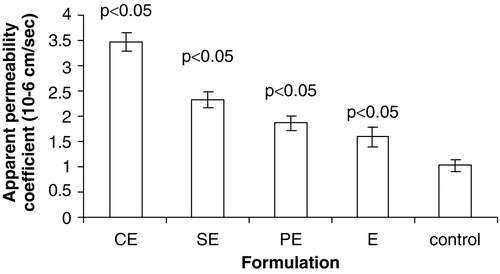
The absorption pattern of various formulations was found in the order of CE > SE > PE > E > Control, as shown in . The permeation flux of SQ from CE, SE, PE, E, and control were 1, 0.56, 0.54, 0.46, and 0.3 μg/min, respectively. The amount of drug permeated through a gut sac in the case of CE was almost four times that of control while it was around two times that of SE and PE after 120 min. The formulation CE showed maximum permeation as indicated by apparent permeability coefficient value of 3.49 × 10−6 cm/sec, which was significant when compared to others formulations. The apparent permeability coefficient values of SE, PE, and E were found to be 1.96 × 10−6 cm/sec, 1.89 × 10−6 cm/sec, 1.61 × 10−6 cm/sec, respectively, which were significant when compared to a control value of 1.05 × 10−6 cm/sec. The statistical analysis of data revealed that there were no significant differences (p < 0.05) in permeability coefficient of the SE, PE, and E formulations, while permeability coefficient and total flux of SQ were more with CE when compared with those of other formulation. The enhanced absorption behavior of all cationic emulsions is mainly due to interaction of oil droplets with the mucosal surface, resulting in increased adhesion of the positively charged droplets to rat intestinal mucosa and thus increased uptake of the drug. This may be explained in part by their electrostatic attraction to negatively charged sites existing in the epithelial surface (Gershanik, Benzeno, and Benita Citation1998). The intestinal membrane is negatively charged due to the presence of a variety of lipids and proteins. Thus, from these results, it was evident that, in spite of E having smallest globule size (251 nm) relative to that of CE (304 nm), shows lower permeability. This result shows that cationicity imparted to the formulations played a significant role even if they are in submicron size. The lower permeability through E could be attributed to hindrance in interaction of submicron particles with negatively charged surface of the intestinal mucosa as E contains phospholipids and free fatty acid present in MCT oil.
Figure 4. Apparent permeability coefficient (×10−6) of various submicron emulsions obtained using in-vitro everted intestinal sac method. The error bars indicates ± SD of three sets of experiments (n = 3). CE: Submicron emulsion containing chitosan as charge inducer. PE: Submicron emulsion containing protamine as charge inducer. SE: Submicron emulsion containing stearylamine as charge inducer. E: Plain emulsion without any charge inducer.
The permeability data revealed the maximum permeation has been shown by CE compared to other formulations, which indicates that the type of charge (+ve) and the presence of inherent permeation enhancer (chitosan) are the main factors responsible for improving the intestinal absorption of saquinavir. The CE displayed maximum zeta potential vis-à-vis other formulations which may have accounted for the higher permeability coefficient. Although there is not much difference in zeta potential values between SE and PE, the higher permeability coefficient of SE compared to PE implies that mere imparting surface positive charge may not be sufficient to improve the absorption, but some other factors still exist which may be accountable for such effects. Chitosan has long been recognized as a safe and effective absorption enhancing agent having mucoadhesion properties, which is known to open tight junctions between cells due to interaction of the positively charged amino groups present in chitosan with the negatively charged sialic groups of the membrane-bound glycol-proteins and thus facilitates paracellular absorption as well. Several studies have highlighted the potential application of chitosan as an absorption enhancing agents for the administration of hydrophilic drugs (Kotze et al. Citation1998). Nevertheless, for the interpretation of the favorable behavior of CE, it is important to take into account the previous studies that showed increase in permeability through nasal (Illum, Farraj, and Davis Citation1994), ocular (Calvo, Vila-Jato, and Alonso Citation1997), and intestinal (Kotze et al. Citation1999) epithelia by chitosan. The authors suggested a combined mechanism of mucoadhesion and ability to open the tight junctions of epithelial cells to allow for a paracellular transport pathway. In addition, chitosan induces a redistribution of cytoskeletal F-actin and the tight junction protein ZO-1 (Schipper et al. Citation1997), thereby altering the integrity of the tight junctions which is associated directly or indirectly with the F-actin filaments, to allow an increase of the paracellular permeability (Thongborisute et al. Citation2006). Furthermore, chitosan salts caused an immediate and pronounced lowering in the transepithelial electric resistance of the intestinal cell monolayers in a reversible way to allow for paracellular transport (Kotze et al. Citation1998). Moreover; its mucoadhesive properties might have improved its residence time at absorption site and resulted in enhanced penetration through intestine. The higher permeation flux of SE compared to PE, despite being identical surface may be attributed to interaction of cationic lipid stearylamine with negative surface groups available in GI tract without hindering active transport process. In addition, the stearylamine being more cytotoxic compared to protamine (as shown in cell viability study) could have disrupted the cell membrane and resulted in improved absorption. However, there was no significant difference in apparent permeability coefficient of protamine based emulsion and a formulation devoid of positive charge inducers, these may be due to its inhibitory action on active transport processes in rat small intestine in vitro, but it tends to increase passive diffusion processes in the rat. It is interesting to note that emulsion based formulation enhances the intestinal permeability on imparting positive charge, but it doesn’t affect intestinal membrane damage and confer any cellular toxicity, which implies safety of these formulations.
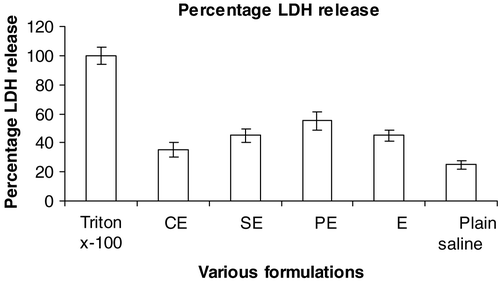
It was anticipated that improvement in permeability could be attributed to disruption of intestinal membrane. But interestingly it has been observed that the LDH release (an indicator of membrane damage) was not significant in any of the cationic submicron emulsions. The order of LDH release was found in the order of PE > SE > E > CE, as demonstrated in , indicating that CE emulsion is safer for administration of SQ for improved absorption, since it does not damage intestinal membrane integrity. This data is also in agreement with our cytotoxic data which showed insignificant toxicity compared to other cationic charge inducer.
Figure 5. Effect of different formulations on lactate dehydrogenase (LDH) in ileal loop of rat small intestine. The error bars indicates ± SD of three set of experiments (n = 3). CE: Submicron emulsion containing chitosan as charge inducer. PE: Submicron emulsion containing protamine as charge inducer. SE: Submicron emulsion containing stearylamine as charge inducer. E: Plain emulsion without any charge inducer. Triton x-100 and plain saline was taken as positive and negative control, respectively.
Conclusion
Submicron emulsion based drug delivery has proven its potential in the field of drug delivery which can be administered through different routes. The cationic submicron emulsion bearing cationic charge has been reported in various literatures preferable for ocular delivery. However, the efficacy and safety of submicron emulsion using different cationic charge inducers like chitosan, protamine, and stearylamine for oral delivery has not been studied so far. Therefore, an attempt has been made to prepare drug loaded cationic submicron emulsion and evaluate its performance with respect to oral delivery. In this study, SQ laden cationic submicron emulsions were successfully prepared by ultrasonication method. The prepared optimized formulations were stable with desired physiochemical profile. It has been shown that the incorporation of cationic charge inducers were crucial for the improved permeability through intestine. The enhanced permeability of SQ is probably due to interaction of the positively charged droplets with negatively charged sites existing in the epithelial surface in GI tract, which resulted in increased mucosal uptake of the SQ. However, chitosan based submicron emulsion showed maximum permeability followed by stearylamine, protamine, and negatively charged submicron emulsion. Therefore, the fraction of molecules which may penetrate through the intestine depends not only on the physicochemical properties of the droplets (positive or negative charge) but also on nature and type of cationic charge inducer. These results clearly indicate that cationic submicron emulsions are promising carrier systems for the oral delivery of saquinavir.
Acknowledgment
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bouer A, Werther K, Catao-Dias JL, Nunes AL 1999. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: in vitro studies using the rat everted intestinal sac. Fundam. Clin. Pharmacol. 13, 494–500.
- Calvo P, Vila-Jato JL, Alonso MJ 1997. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int. J. Pharm. 153, 41–50.
- Facklam MM, Burhenne J, Ding R, Fricker R, Mikus G, Zaman N T, Tam YK, Tawfik S, Wiltshire H 2004. Factors responsible for the variability of Saquinavir absorption: studies using an instrumented dog model. Pharm. Res. 21, 436–442.
- Frappier S, Breilh D, Diarte E, Ba B, Ducint, D, Pellegrin JL, Saux MC 1998. Simultaneous determination of ritonavir and saquinavir, two human immunodeficiency virus protease inhibitors, in human serum by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 714, 384–389.
- Gershanik T, Benzeno S, Benita S 1998. Interaction of a self emulsifying lipid drug delivery system with the everted rat intestinal mucosa as a function of droplet size and surface charge. Pharm. Res. 15, 863–869.
- Illum L, Farraj NF, Davis SS 1994. Chitosan as a novel nasal delivery system for peptide drugs. Pharm. Res. 11, 1186–1189.
- Jumaa M Muller BW, 1998a. The effect of oil components and homogenization conditions on the physicochemical properties and stability of parenteral fat emulsions. Int. J. Pharm. 163, 81–89.
- Jumaa M, Muller BW 1998b. The stabilization of parenteral fat emulsion using non-ionic ABA copolymer surfactant. Int. J. Pharm. 174, 29–37.
- Jumaa M, Muller BW, 1999. Physicochemical properties of chitosan-lipid emulsions and their stability during the autoclaving process. Int. J. Pharm. 183, 175–184.
- Kim RB, Fromm, MF, Wandel, C, Leake B, Wood, AJJ, Roden DM, Wilkinson GR 1998. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Invest. 101, 289–294.
- Kotze AF, Lueβen HL, De BJ, Boer AG, Verhoef JC, Junginger, H 1998. Comparison of the effect of different chitosan salts and N-trimethylchitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J. Contr. Rel. 51:35–46.
- Kozte AF, Thanou MM, Lueßen HL, Boer AG, Verhoef JC, Junginger HE 1999. Effect of degree of quaternization of N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). Eur. J. Pharm. Biopharm. 47, 269–274.
- Myers RA, Stella VJ 1992. Systemic bioavailability of penclomedine (NSC-338720) from oil-in-water emulsions administered intraduodenally to rats. Int. J. Pharm. 78, 217–220.
- Rubinstein A, Pathak YV, Kleinstein J, Reches A, Benita S 1991. In vitro release and intestinal absorption of physostigmine salicylate from submicron emulsion. J. Pharm. Sci. 80, 643–647.
- Schipper NGM, Olsson S, Hoogstraate JA, Boer AG, Varum KM, Artursson P 1997. Chitosan as absorption enhancers for poorly absorbable drugs: mechanism of absorption enhancement. Pharm. Res. 14, 923–929.
- Thongborisute J, Takeuchi H, Yamamoto H,Kawashima Y 2006. Visualization of the penetrative and mucoadhesive properties of chitosan and chitosan-coated liposomes through the rat intestine. J. Liposome Res. 16, 127–141.
- Uno T, Kazui T, Suzuki Y, Hashimoto H, Suzuki K, Muhammad, BA, 1999. Pharmacokinetic advantages of a newly developed tacrolimus oil-in-water-type emulsion via the enteral route. Lipids 34, 249–254.
- Venkateswarlu V, Manjunath M, 2004. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Contr. Rel. 95, 627–638.
- Yang SC, Benita S, 2000. Enhanced absorption and drug targeting by positively charged submicron emulsions. Drug Dev. Res. 50, 476–486.