Abstract
Non-invasive transdermal delivery using microneedle arrays was recently introduced to deliver a variety of large and hydrophilic compounds into the skin, including proteins and DNA. In this study, a microneedle array was applied to the delivery of a hydrophobic drug, ketoprofen, to determine if transdermal delivery in rats can be improved without the need for permeation enhancers. The ability of a microneedle to increase the skin permeability of ketoprofen was tested using the following procedure. A microneedle array was inserted into the lower back skin of a rat using a clip for 10 min. Subsequently, 24 mg/kg of a ketoprofen gel was loaded on the same site where the microneedle had been applied. Simultaneously, the microneedle was coated with 24 mg/kg of a ketoprofen gel, and inserted into the skin using a clip for 10 min. As a negative control experiment, only 24 mg/kg of the ketoprofen gel was applied to the shaved lower back of a rat. Blood samples were taken at the indicated times. The plasma concentration (Cp) was obtained as a function of time (t), and the pharmacokinetic parameters were calculated using the BE program. The group loaded with the microneedle coated with ketoprofen gel showed a 1.86-fold and 2.86-fold increase in the AUC and Cmax compared with the ketoprofen gel alone group. These results suggest that a microneedle can be an ideal tool for transdermal delivery products.
Keywords::
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly administereded drugs worldwide, and are used primarily for the symptoms associated with osteoarthritis and other chronic musculoskeletal conditions (Marie Citation1998; Timothy Citation2002). Ketoprofen, 2-(3-benzoylphenyl)-propionic acid, is a hydrophobic NSAID that is widely used to treat rheumatoid arthritis and related diseases (Yalcin, Gulgun, and Umit Citation1999; Kim and Choi Citation2002; Charles, Simon, and John Citation2003). However, it is associated with a variety of adverse side effects when administered orally, such as gastrointestinal irritation (CitationFries et al. 1993). Since ketoprofen is usually administered to patients over an extended period, several attempts have been made to reduce its adverse side effects. One promising method is to administer the drug through the skin, and various transdermal dosage forms of ketoprofen including patches (Mazieres Citation2005), gels (Gallagher and Heard Citation2005; Bowen and Heard Citation2006), creams (Li et al. Citation1996), and ointments (CitationJaeckle, Schaefer, and Loth 2003) have been reported. A variety of physicochemical methods, such as the development of appropriate vehicles (Garcia et al. Citation2006), iontophoresis (Tashiro et al. Citation2000), and the use of permeation enhancers (Thomas and Heard Citation2005) have been developed in an attempt to improve the skin permeation of ketoprofen.
Recently, a new concept, non-invasive transdermal delivery, was introduced as a sequel to developments in the microelectronics industry (Henry et al. Citation1998; Prausnitz, Citation2004; Reed and Lye Citation2004; Prausnitz, Mikszta, and Raeder-Devens Citation2006). The skin barrier can be overcome through the formation of mechanically produced conduits through the stratum corneum using an array of small needles, i.e. microneedle arrays using biocompatible polycarbonate (Han et al. Citation2007) to deliver a variety of large and hydrophilic compounds into the skin, including proteins and DNA (Lin et al. Citation2001; Chabri et al. Citation2004).
Ketoprofen is an already commercialized transdermal product. However, most ketoprofen products in use employ permeation enhancers, which might induce skin irritation. Therefore, a microneedle array was applied to ketoprofen to determine if it can improve transdermal delivery in rats without the need for permeation enhancers. This is the first report showing the effect of a microneedle array on the transdermal pharmacokinetics of ketoprofen.
Materials and methods
Materials
Ketoprofen, phenoprofen, and carbomer 940 were obtained from Hwail Pharm. Co. (Hwasung, Korea), and used as received without further purification. Poloxamer 188 (Lutrol® F68) was purchased from BASF (France).
The acetonitrile used in this study was of HPLC grade and supplied by J. T. Baker Inc. (USA). All other chemicals were of reagent grade and used without further purification. The microneedle was supplied by KAIST (Daejeon, Korea).
Preparation of ketoprofen gel
The ketoprofen gel was prepared with carbomer 940 (2%), poloxamer 188 (0.5%), tween 80 (5%), ketoprofen (3%), triethanolamine (0.1%) using two mixtures of an ethanol and distilled water solution. After complete hydration of carbomer 940, a 40% ethanol solution of ketoprofen containing tween 80 was added to the carbomer 940 solutions. The mixtures were stirred with a magnetic stirrer, and the ketoprofen gel was formed with triethanolamine.
HPLC analysis of ketoprofen
An Agilent 1100 liquid chromatography system, an autosampler, and UV detector were used for HPLC analysis. The column used was a C18 column (3.9 × 150 mm, 4 μm particle size, Nova-pak®, Waters, USA). The flow rate of the mobile phase was 1 ml/min and the detection wavelength was set to 256 nm. The mobile phase was a mixture of acetonitrile and a pH 3.0 phosphate buffer (78:22 V/V). All procedures were carried out at ambient temperature.
Animal experiments and drug administration
All animal studies were conducted in accordance with the ‘Guiding Principles in the Use of Animals in Toxicology’ adopted by the Society of Toxicology (USA) and the experimental protocols were approved by the Animal Care Committee of Chungnam National University. Male Sprague-Dawley rats weighing 240–260 g were purchased from the Dae Han Laboratory Animal Research Co. (Chungbuk, Korea). The rats were given commercial rat chow diet (No. 322-7-1) purchased from the Superfeed Co. (Gangwon, Korea) and tap water ad libitum. The animals were housed three per cage in laminar flow cages that were maintained at 22 ± 2°C, and 50–60% relative humidity. The animals were kept in these facilities for at least 1 week prior to the experiment and were fasted for at least 24 hr before commencing the experiments. The ability of the microneedle to increase the skin permeability to ketoprofen was tested using the following procedure. A microneedle array was inserted into the lower back skin of a rat using a clip for 10 min. Subsequently, 24 mg/kg of the ketoprofen gel was loaded to the same site where the microneedle had been applied. Simultaneously, 24 mg/kg of the ketoprofen gel was coated on the microneedle and inserted into the skin of the lower back using a clip for 10 min. As a negative control, only 24 mg/kg of the ketoprofen gel was loaded onto the shaved lower back of the rat. Blood samples (0.6 ml) were collected at 0, 1, 2, 3, 4, 5, 6, 8, 10, 12, 22, and 24 hr after administration. The blood samples were centrifuged at 3000 rpm for 10 min, and the plasma samples were stored at −70°C until analyzed by HPLC. The ketoprofen was extracted using a modification of the procedure reported by Onishi, Takahash, and Machida (2006). One hundred μl of the plasma sample was placed into a 15 ml centrifuge tube, and phenoprofen (62.5 μg/ml, internal standard) and 100 μl of 1 N HCl were added. They were then extracted with 5 ml of ether. After vortexing for 10 min, the mixture was centrifuged at 4000 rpm for 15 min. The organic phase was transferred to the other tubes and evaporated to dryness using a vacuum system. The residues were dissolved in 400 μl of acetonitrile and 20 μl aliquots were injected into the HPLC system for analysis. The linear relationship between the area ratio (ketoprofen/IS) vs the concentration was calculated. In addition, the amount of ketoprofen remaining was measured by recollecting the ketoprofen gel onto the rat skin and microneedle after drug administration during 10 min. The recollected ketoprofen gels were then resuspended in the methanol solution and sonicated for 10 min. Subsequently, the resuspended ketoprofen was centrifuged at 3000 rpm for 10 min, and the supernatants were filtered and injected into the HPLC system.
Pharmacokinetic analysis
Noncompartmental pharmacokinetic analysis was carried out using the BE computer program which calculates the AUC (Area under the curve) of the plasma concentration (Cp) as a function of time (t). The maximum plasma concentration (Cmax) and the time to reach the maximum plasma concentration (Tmax) were obtained from the experimental data. The area under the plasma concentration curve as a function of time (AUC0–t) from time zero to the time of the last measured concentration (Clast) was calculated using the linear trapezoidal rule. The AUC zero to infinite (AUC0–∞) was obtained by adding the AUC0–t, and the extrapolated area was determined by Clast/Kel. The total plasma clearance (CL/F) was calculated by dividing the dose by the AUC.
Statistical analysis
All the mean values are presented with their standard deviation (Mean ± SD). The pharmacokinetic parameters were compared using a one-way ANOVA, followed by a posteriori testing using a Dunnett correction. A p-value < 0.05 was considered significant.
Results and discussion
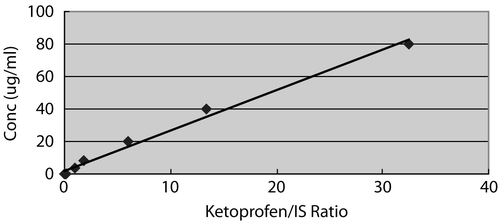
The amount of ketoprofen in the plasma was measured by reverse phase HPLC, as mentioned in the materials and methods section. Based on the chromatogram, the retention time of ketoprofen and the internal standard were 3.4 min and 5.7 min, respectively. The calibration curve was linear over the concentration range, 0.02~40 μg/ml, and the regression curve was y = 2.9943x + 0.7074 (R2 = 0.9976) ().
The biocompatible microneedle was fabricated by Han et al. (Citation2007). Optical microscopy was used to identify the piercing effect of an array of 500 μm-depth-microneedles with a density of 154/cm2 by piercing the rat skin with the microneedle for 10 min and staining the holes made by the microneedle array (data not shown). In addition, a skin transport study of the ketoprofen gel was carried out to determine the effect of the modes for applying the microneedle on the transdermal delivery of ketoprofen. This experiment was carried out to determine if synchronized or separate systems between the microneedle array and ketoprofen gel can be optimizing for the development of a transdermal drug delivery system. The application modes are as follows. The ketoprofen gel alone was applied to the rat skin as a control. The ketoprofen gel was loaded onto the rat skin after applying the microneedle. The ketoprofen gel was then applied directly with a microneedle onto the rat skin. shows the mean plasma concentration-time profiles of ketoprofen according to the methods used to apply the microneedle in the rats. summarizes the mean pharmacokinetic parameters. After the s.c. administration of the ketoprofen gel (24 mg/kg of ketoprofen), the peak plasma concentration of ketoprofen was 2.87 μg/ml at 5.0 hr, which decreased gradually to 2.04 μg/ml at 24 hr. When the microneedle and ketoprofen gel was coupled, the AUC and Cmax of ketoprofen were increased by 186% and 286%, respectively, and the total plasma clearance (CL/F) of ketoprofen was decreased by 92% compared with the control (p < 0.05). When the microneedle array was applied for 10 min, followed by administration of the ketoprofen gel, the AUC and Cmax of ketoprofen were increased slightly by 109% and 129%, respectively, and the total plasma clearance (CL/F) of ketoprofen was decreased slightly by 94.9% compared with the control. Consequently, the relative bioavailability of ketoprofen in the rats treated with the ketoprofen gel coupled directly with the microneedle array was higher than those from the control group (the ketoprofen gel alone group) (p < 0.05). There were no significant changes in the Kel and terminal plasma half-life (T1/2) of ketoprofen, irrespective of the application of the microneedle.
Figure 2. Mean plasma concentration-time profiles of ketoprofen according to the method for applying the microneedle and ketoprofen gel. The microneedle was pierced into the skin before loading the ketoprofen gel onto the rat skin (•), ketoprofen gel coupled with the microneedle was applied to the rat skin (o), simple ketoprofen gel was loaded on the rat skin (▾).
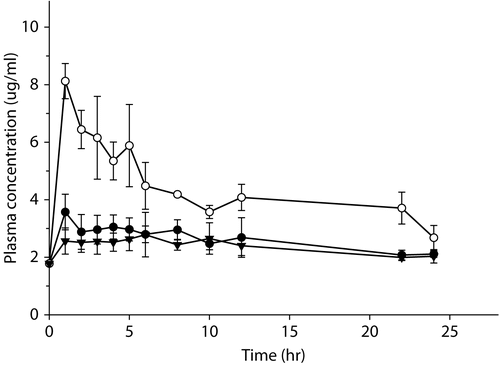
Table 1. Mean pharmacokinetic parameters of ketoprofen after transdermal delivery.
In addition, the ketoprofen remaining on the rat skin and microneedle was measured by collecting the ketoprofen gel remaining after administering the drug for 10 min. The correlation between the amount of ketoprofen absorbed into the rat and the amount of ketoprofen remaining on the rat skin and microneedle after drug administration for 10 min was determine using a recovery experiment. shows that the amount of ketoprofen was lowest when the microneedle and ketoprofen gel were coupled. The amount of ketoprofen remaining was reduced by 53.2% compared with the control. In addition, when the microneedle array was applied for 10 min, followed by administration of the ketoprofen gel, the amount of ketoprofen remaining was reduced by 88.9% compared with the control. Overall, these results suggest that the amount of ketoprofen remaining after drug administration decreased with increasing amount of ketoprofen absorbed with a corresponding higher AUC being observed. Although the therapeutically useful rates of ketoprofen can be obtained easily from conventional formulations (gel, cream, etc), these results suggest that the transdermal delivery of ketoprofen was clearly improved using the microneedle, which indicates that less ketoprofen is required for formulations applied using this method. This study is the first to report the effect of the application of microneedles on the pharmacokinetics of ketoprofen in rats.
Table 2. Amount of ketoprofen remaining in the microneedle and on the rat skin after drug administration.
Conclusion
The means for overcoming the skin barrier reported in this study is based on the formation of mechanically produced conduits through the stratum corneum using an array of small biocompatible polycarbonate needles. The optimal application modes between the microneedle and ketoprofen were examined in an attempt to achieve improved transdermal delivery of ketoprofen. When the microneedle and ketoprofen gel were coupled, the AUC and Cmax of ketoprofen were increased by 186% and 286%, respectively, and the total plasma clearance (CL/F) of ketoprofen was decreased by 92% compared with the control (p < 0.05). The amount of ketoprofen remaining was 53.2% lower than the control. These results indicate that the amount of ketoprofen remaining after drug administration decreased because more ketoprofen was absorbed and there was a higher AUC. This study suggests that a microneedle array may be a supplement for transdermal drug delivery systems.
Acknowledgment
This research has been supported by the Intelligent Microsystem Center (IMC; http://www.microsystem. re.kr), which carries out one of the 21st century’s Frontier R&D Projects sponsored by the Korea Ministry of Knowledge Economy and by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (NO. R01-2008-000-20071-0).
References
- Bowen JL, Heard CM 2006. Film drying and complexation effects in the simultaneous skin permeation of ketoprofen and propylene glycol from simple gel formulations. Int. J. Pharm. 307, 251–257.
- Chabri F, Bouris K, Jones T, Barrow D, Hann A, Allender C, Brain K, Birchall J 2004. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br. J. Dermatol. 150, 869–877.
- Charles MH, Simon JG, John H 2003. The in vitro delivery of NSAIDs across skin was in proportion to the delivery of essential fatty acids in the vehicle. Evidence that solutions permeate skin associated with their salvation cages. Int. J. Pharm. 261, 165–169.
- Denis MM 1999. Comparative toxicity of nonsteroidal anti- inflammatory drugs. Am. J. Med. 107, 37S.
- Gabriel SE, Jaakkimainnen RL, Bombarier C 1991. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Ann. Intern. Med. 115, 787.
- Fries JF, Williams CA, Ramey D, Blach DA 1993. The relative toxicity of disease-modifying antirheumatic drugs. Arthritis Rheum. 36, 297–306.
- Gallagher SJ, Heard CM 2005. Solvent content and macroviscosity effects on the in vitro transcutaneous delivery and skin distribution of ketoprofen from simple gel formulations. Skin Pharmacol. Physiol. 18, 186–194.
- Garcia MT, da Silvia CH, de Oliveira DC, Braga EC, Thomazini JA, Bentley MV 2006. Transdermal delivery of ketoprofen: the influence of drug-dioleylphosphatidylcholine interactions, Pharm. Res. 23, 1776–1785.
- Han MH, Hyun DH, Park HH, Lee SS, Kim CH, Kim CG 2007. Anoble fabrication process for out-of-plane microneedle sheets of biocompatible polymer. J. Micromech. Microeng. 17, 1184–1191
- Henry S, McAllister DV, Allen MG, Prausnitz MR 1998. Fabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 87, 922–925.
- Jaeckle E, Schaefer UF, Loth H 2003. Comparison of effects of different ointment bases on the penetration of ketoprofen through heat-separated human epidermis and artificial lipid barriers. J. Pharm. Sci. 92, 1396–1406.
- Kim JH, Choi HK 2002. Effect of additives on the crystallization and the permeation of ketoprofen from adhesive matrix. Int. J. Pharm. 236, 81–85.
- Li KL, Vogel R, Jeffcoat MK, Alfano MC, Smith MA, Collins JG, and Offenbacher S 1996. The effect of ketoprofen creams on periodontal disease in rhesus monkeys. J. Periodontal Res., 31, 525–532.
- Lin W, Cormier M, Samiee A, Griffin A, Johnson B, Teng CL, Hardee GE, Daddona PE 2001. Transdermal delivery of antisense oligonucleotides with microprojection patch (macroflux) technology. Pharm. Res. 18, 1789–1793.
- Marie RG 1998. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. Am. J. Med. 104, 23S.
- Mazieres B 2005. Topical ketoprofen patch. Drug R.D. 6, 337–344.
- Onishi H, Takahashi M, Machida M 2005. PLGA implant tablet of ketoprofen: comparison of in vitro and in vivo releases. Biol. Pharm. Bull. 28, 2011–2015.
- Prausnitz MR 2004. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56, 581–587.
- Prausnitz MR, Mikszta JA, Raeder-Devens J 2006. Microneedles. In, Percutaneous Penetration Enhancers, eds. Smith EW, Maibach HI , 239–256. Boca Raton, FL; CRC Press.
- Reed ML, Lye WK 2004. Microsystems for drug and gene delivery. Proc. IEEE. 92, 56–75.
- Tashiro Y, Kato Y, Hayakawa E, Ito K 2000. Iontophoretic transdermal delivery of ketoprofen: effect of iontophoresis on drug transfer from skin to cutaneous blood. Biol. Pharm. Bull. 23, 1486–1490.
- Thomas CP, Heard CM 2005. In vitro transcutaneous delivery of ketoprofen and essential polyunsaturated fatty acids from a fish oil vehicle incorporation 1,8-cineole. Drug Deliv. 12, 7–14.
- Timothy AC 2002. Nonsteroidal anti-inflammatory drugs, apoptosis, colon-cancer chemoprevention. Lancet Oncol. 3, 166.
- Yalcin T, Gulgun Y, Umit G 1999. Inclusion of ketoprofen with skimmed milk by freeze-drying. Farmaco 54, 648–652.