Abstract
The aim of this investigation was to prepare and characterize microemulsions/mucoadhesive microemulsions of Diazepam (D), Lorazepam (L) and Alprazolam (A), evaluate their pharmacodynamic performances by performing comparative sleep induction studies in male albino rats to assess their role in effective management of insomnia patients. Microemulsions of Diazepam (DME), Lorazepam (LME) and Alprazolam (AME) were prepared by titration method and characterized for drug content, globule size distribution and zeta potential, nasal toxicity and sleep induction. DME, LME and AME were transparent and stable with mean globule size and zeta potential in the range of 95.6 nm to 141.7 nm and -2.205 to -0.111 mV respectively. The prepared microemulsions exhibited reversible nasal toxicity. Onset of sleep and duration of sleep were observed in the following order: Lorazepam > Alprazolam>Diazepam. Faster onset of sleep following intranasal administration of microemulsions (<20 min) compared to oral administration (29-33 min) and control group (>45 min) for all three drugs suggested selective nose-to-brain transport of drug(s). Intranasal administration of microemulsion based formulations resulted in even faster onset of sleep (<12 min) with intranasal mucoadhesive microemulsion(s) resulting in fastest onset of sleep (<9 min). Duration of sleep was longest with the intranasal mucoadhesive microemulsions. These results are suggestive of larger extent of distribution of drug(s) to brain after intranasal administration of mucoadhesive microemulsion(s). These results are further corroborated with by loss or rightening reflex and startle reflex at earlier time points (within 10 min and 15 min respectively) with mucoadhesive microemulsions. Thus, the results of this investigation indicated rapid and larger extent of drug transport to the rat brain resulting in rapid induction of sleep followed by prolonged duration of sleep in rats following intranasal administration of mucoadhesive microemulsion(s). However, the role of microemulsion based formulations developed in this investigation in clinical practice can only be established after animal studies in two different animal models followed by extensive clinical trials.
Introduction
Insomnia is a troublesome physiological condition associated with inadequate sleep, difficulty in falling asleep, frequent episodes of sleeplessness, trouble in resuming the sleep, sleep apnoea, narcolepsy, restless leg syndrome etc. Population-based studies indicate that approximately 30% of the general population has complaints of sleep disruption, whereas approximately 10% has associated symptoms of daytime functional impairment consistent with the diagnosis of insomnia. The National Sleep Foundation’s (2005) Sleep in America Poll indicates that 54% of those surveyed reported that they experienced at least one symptom of insomnia one or more nights a week, with 33% reporting at least one symptom every night or almost every night (CitationNewer options in the management of insomnia). People of both sexes and all age groups are affected by insomnia, even though it is most common in women and senior citizens. Insomnia affects about one-third of senior adults and up to two-thirds of individuals over the age of fifty have one type of sleep problem or another according to the International Longevity Centre (CitationNewer options in the management of insomnia). About 60 million Americans each year suffer from insomnia leading to serious sleep deficits. It tends to increase with age and affects about 40% of females and 30% males (CitationNewer options in the management of insomnia).
Pathophysiological mechanisms of the various types of insomnia are not fully understood, but by and large, endogenous neurotransmitters and neurohormones contribute to modulation of the sleep-wake cycle. Wakefulness and sleep are antagonistic states competing for control of brain activity. Wakefulness-promoting neurotransmitters include norepinephrine (NE), dopamine (DA), histamine, glutamate, and acetylcholine. Sleep-promoting neurotransmitters include serotonin, gamma-amino butyric acid and naturally occurring opiate peptides, such as enkephalins and endorphins. The supra-chiasmic nucleus (SCN) of the hypothalamus regulates the sleep-wake cycle by receiving input from the retina (light/dark), the prefrontal cortex and cortex NE, DA, dorsal raphe nucleus (5-Hydroxytryptamine), and pineal gland (melatonin), among other brain regions. It is hypothesized that neurochemical imbalance in these regions of the brain results in disturbing the homeostasis of the brain, leading to insomnia. Enhanced levels of wakefulness promoting neurotransmitters or/and deficits of circulating inhibitory neurotransmitters are mainly held responsible for genesis of insomnia. The pathophysiology of insomnia is characterized by hyperarousal affecting both REM (Rapid Eyeball Movement) and NREM (Non Rapid Eyeball Movement) sleep. It is suggested that the progressive hyper polarization of the thalamo-cortical neurons as sleep deepens is slower in the insomnia patient population.
The most commonly reported symptoms of insomnia, often experienced at least a few nights a week in the past years, included waking up feeling unrefreshed (38%) and waking up frequently during the night (32%). Less commonly reported symptoms included difficulty falling asleep (21%) and waking up too early and not being able to fall back asleep (21%)1
Benzodiazepines have been widely and popularly prescribed for the treatment of insomnia. They reduce the proportion of REM sleep by acting very selectively on GABA-A receptors, which mediate the fast inhibitory synaptic response produced by activity in GABAnergic neurons. Benzodiazepines enhance the response to GABA, by facilitating the opening of GABA-activated chloride channels, leading to hyper polarization of neuronal membrane, thus depressing the entire Central Nervous System (CNS) and producing a state of deep sleep.
Benzodiazepine derivatives are GABA agonists available in the market in oral tablets and intravenous injections for the treatment of insomnia. Although intravenous administration helps in achieving rapid hypnosis, an alternative route of drug delivery is needed since oral and intravenous routes for delivering drugs are sometimes impractical and/or inconvenient (CitationRey et al., 1999), for instance, because of a delay in hospitalization of the patient, lack of an available hospital facility or a patient condition incompatible with oral ingestion of a tablet dosage form.
Predominant limitations associated with oral dosage forms of benzodiazepines are mainly the delayed onset of action leading to higher latent period and shorter duration of sleep, non-specific drug distribution leading to various side effects along with poor uptake of anxiolytic drugs in the brain leading to poor therapeutic benefit to the patient. Hence, an alternative drug delivery system that surpasses all the drawbacks associated with the conventional dosage forms needs to be explored and established for the management of insomnia. Previous studies have demonstrated that intranasal administration offers a practical, non invasive, alternative route of administration for drug delivery to the brain (CitationBigal et al., 2003; CitationWermling et al., 2001; CitationDorman et al., 2002; CitationDragphia et al., 1995). Intranasal drug delivery also offers the advantages that drugs can be administered simply, cost effectively, and conveniently (CitationLiu et al., 2001). Direct transport of drugs to the brain circumventing the brain-barriers following intranasal administration provides a unique feature and better option to target drugs to brain (Illum Lisbeth, Citation2000, Citation2002; CitationVyas et al., 2005). However, to enhance effectiveness of the drug, a few issues should be carefully considered by the formulator when designing intranasal drug delivery (CitationVyas et al., 2005; CitationDhanda et al., 2005). The formulation should be designed so as to provide rapid transport of drug across nasal mucosa and longer residence time in nasal cavity (CitationBehl et al., 1998). Microemulsions, by virtue of their lipophilic nature and having low globule size, are widely explored as a delivery system to enhance uptake across mucosa (CitationHu et al., 2001). Addition of a mucoadhesive agent such as a polyelectrolyte polymer helps in retention of the formulation on the nasal mucosa (CitationUgwoke et al., 2001). Evidences of intranasal drug delivery systems formulated using mucoadhesive agent and its benefits in enhancing nose-to-brain drug transport have been reported by various scientists in the literature (CitationAlpar et al., 2005; CitationGavini et al., 2005). Intranasal administration allows transport of drugs to the brain circumventing BBB, thus providing a unique feature and better alternative to target the drugs to the brain. Systemic drug delivery through nasal route has been demonstrated as a potentially promising alternative to IV injection for rapid onset delivery of CNS medications owing to extensive microcirculation underneath nasal mucosa and preferential delivery to brain (CitationSakane et al., 1999). However, reports in the literature reveal erratic and highly variable bioavailability in case of intranasal administrations of various drugs used in the treatment of CNS disorders. Therefore, there is a need to design a delivery system that can provide rapid transport of drugs across nasal mucosa and longer residence time in the nasal cavity (CitationBehl et al., 1998). Microemulsions have been explored widely as a delivery system by virtue of having considerable potential to enhance transport of a wide range of drug molecules (CitationLawrence and Rees, 2000). The addition of a mucoadhesive agent such as polyelectrolyte polymer helps in retention of the formulation in the nasal cavity (CitationUgwoke et al., 2001; CitationAlpar et al., 2005; CitationGavini et al., 2005; CitationSakane et al., 1999; CitationLawrence and Rees, 2000; CitationSinswat and Tengamnuay, 2003).
The objective of this investigation was to prepare and characterize microemulsion/mucoadhesive microemulsions of benzodiazepines, by assessing their efficacy in nose-to-brain delivery and comparing their consequent effect on sleep induction pattern with that produced by drug solutions and marketed formulations in rats. It was hypothesized that the microemulsions-/mucoadhesive microemulsion-based alternative drug delivery systems would result in rapid nose-to-brain transport of benzodiazepines and therefore greater drug transport and distribution into and within the brain which in turn would help to maximize the therapeutic benefit of the drug, reduce side effects, decrease the dose and frequency of dosing, and perhaps, even the cost of the therapy.
Materials and methods
Materials
Diazepam (Molecular weight- 284.75 g/mole (CitationKlaus Florey, 1997), solubility-50mg/L) was gifted by Bharat Parenterals Ltd., Vadodara, India. Lorazepam (Molecular weight-321.20 (CitationKlaus Florey, 1997a) g/mole, solubility-80 mg/L) and Alprazolam (Molecular weight-308.76 g/mole, solubility-40mg/L at pH=7 and 12 mg/L at pH=2) were kindly gifted by Gujarat Laboratories, Ahmedabad,India. Capmul MCM and Captex 200P were provided as gift sample by Abitec Corporation Limited, Columbus, Ohio. Other Chemicals were of analytical grade and were used as received. Tablets of Lorazepam, Alprazolam and Diazepam were purchased from the local market.
Methods
Preparation and characterization of microemulsions
Diazepam and Lorazepam solutions were prepared by dissolving diazepam (15 mg/mL) and lorazepam (6.2 mg/mL) respectively in a mixture of ethanol: propylene glycol: distilled water (1:4.5:4.5) with continuous stirring. Alprazolam solution was prepared by dissolving alprazolam (1.6 mg/mL) in mixture of ethanol: propylene glycol: distilled water (1:6:3) with continuous stirring.
Diazepam microemulsion (DME, 15 mg/mL), lorazepam microemulsion (LME, 6.2 mg/mL) and alprazolam microemulsion (AME, 1.6 mg/mL) were prepared by water titration method using Capmul MCM as an oil phase (20% w/w), Tween 80 as surfactant ( S-26.3% w/w), Captex 200P as a co-surfactant (CoS-13.7 % w/w), ethanol (10 % w/v) as a co-solvent and distilled water (40 % w/w) as continuous aqueous phase. Formulations were prepared by dissolving the above mentioned drugs in oil phase containing S, CoS and co-solvent with continuous stirring and titrating slowly with aqueous phase at ambient temperature until a clear, homogeneous transparent microemulsion (% T > 99%) was obtained. Compositions of resultant microemulsions are shown in . Mucoadhesive microemulsions (DMME, LMME and AMME) were prepared by addition of polycarbophil (0.5 % w/w) to DME, LME and AME respectively with continuous stirring for 30 min at ambient temperature.
Table 1. Composition and Characterization of Diazepam, Lorazepam and Alprazolam Microemulsions*.
Drug content in the prepared microemulsion formulations was determined using UV-Visible spectrophotometer (UV, 1601, 220X Shimadzu, Japan) at λmax 228.0 nm, 285.0 nm and 220.0 nm for Lorazepam, Diazepam and Alprazolam respectively using methanol as blank (CitationKlaus Florey, 1997). Briefly, the prepared microemulsion(s) of the drug(s) in question was diluted suitably with methanol and estimated spectrophotometrically at their respective wavelength(s). The globule size determination (CitationRoland et al., 2003) was performed using photon correlation spectroscopy with in-built Zetasizer (Model: Nano ZS, Malvern Instruments, Worcestershire, UK WR141XZ) at 633 nm, Helium-neon gas laser having intensity of 4 mW was the light source. The equipment was programmed to provide 18 mm laser width. Measured electrophoretic mobility (mm/s) using small volume disposable zeta cell was converted to zeta potential (CitationRoland et al., 2003) by in-built software based on Helmholtz- Smoluchowski equation. % Transmittance at 630 nm was checked against distilled water using UV visible spectrophotometer (UV, 1601, 220X Shimadzu, Japan). The results are recorded in .
Table 2. Sleep pattern of Diazepam formulations (Misra et al).
Nasal toxicity studies
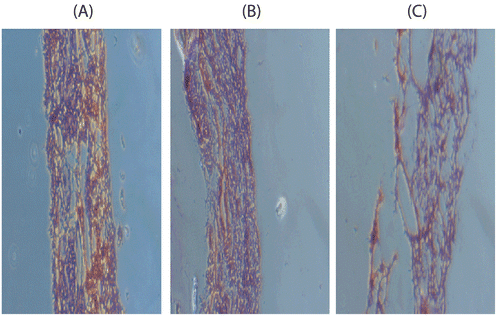
Nasal ciliotoxicity of excipients was assessed by exposing the sheep nasal mucous membrane to the prepared microemulsions. In brief, freshly excised sheep nasal mucosal pieces of uniform thickness of 0.2 mm were mounted on Franz diffusion cell, treated with 0.5 ml blank microemulsions and then rinsed with Phosphate buffer saline-PBS (pH 6.4) after 1 hr. The sheep nasal mucosae were examined with an optical microscope. PBS (pH 6.4) and isopropyl alcohol (a serious nasal mucociliary toxic agent) were used as a negative and positive control respectively followed by washing with PBS (pH 6.4) after 1 hr and photomicrographs of the prepared slides were taken (Olympus, Model BX10, Japan) and shown in .
Sleep induction test
To evaluate the influence of the developed formulations on sedation, sleep induction test in rats was performed by evaluating onset of sleep, duration of sleep, loss of Rightening Reflex (RR) and Startle Reflex (SR). An RR was defined as spontaneous return of the rat on its four paws within 15 seconds when placed on its back and an SR was defined as any movement of the body upon the sudden noise (CitationBol et al., 2000). A hand clap, out of sight of the rat, was used to assess the SR. RR was measured at the regular interval of 10 min and SR was measured at the interval of 15 min. All experiments conducted on animals were approved by the Committee for the purpose of control and supervision of experiments on animals, Ministry of Social Justice and Empowerment, Government of India, New Delhi, India. The rats were deprived of food 12 hr before the commencement of the experiments and water ad libitum was provided. All experiments started between 10 and 11 A.M (CitationBol et al., 2000).
Selected animals were divided into five different groups for all three drug candidates. Group I (Control-Untreated), Group II- treated intranasally with developed microemulsion (ME) of drugs, Group III- treated intranasally with mucoadhesive microemulsions (MME) of the drugs, Group IV – treated intranasally with drug solution (DS) and Group V - treated orally with tablets (marketed formulations) of the respective drug candidates.
Intranasal administration of the prepared formulations was carried out using microlitre syringe (1 to 20 μl) fitted with 4” blunt needle. On top of the 4” syringe 0.10 mm polypropylene tube about 0.10” in length was fitted (CitationVyas et al., 2005). 25 μl dose was administered in divided fashion. Initially 5 μl of each of the test formulations was administered and then the syringe was perfused using 2 μl PBS and this cycle was repeated for 5 times at an interval of 5 seconds and switched over from one nostril to other one in-between. Once the dosing was completed, 10 μl of PBS was administered into the rat nostrils for uniform distribution of the administered formulations. Animals were observed for onset of sleep, duration of sleep, and loss of RR and SR and results were recorded in , and .
Table 3. Sleep pattern with various Lorazepam formulations.
Table 4. Sleep induction pattern with various Alprazolam formulations.
Results and discussion
The drug contents were found to be 97.77%, 98.31 % and 98.44% for DME, LME and AME respectively. The mean globule size and zeta potential for DME, LME and AME were found to be 95.2 nm, 141.7 nm and 106.7 nm and -0.209,-2.205 and -0.111 respectively (). Nasal ciliotoxicity studies () revealed that nasal mucosa treated with PBS pH 6.4 (negative control) showed intact epithelium layer without any damage while nasal mucosa treated with Isopropyl alcohol (positive control - mucociliary toxic agent) showed complete destruction of epithelium layer and even the dipper tissue part were also destroyed. The nasal mucosa treated with blank microemulsion and rinsed with PBS pH 6.4 after 1 hr showed reversible contraction of epithelial layer and no damage to the other parts of mucosa suggesting that the effects of excipients of microemulsion formulation on the sheep nasal mucosa are reversible.
The sleep inducing effects of developed formulations were evaluated by measuring the onset of sleep, duration of sleep, loss of RR and SR in rats. In case of Lorazepam, Group II to V (drug treated group) resulted in faster onset of sleep (6-30 min) and longer duration of sleep (>5.5 hr) as compared to control group (Group I) (>45 min and <1.0 hr respectively). Amongst Group II to V, intranasal administration resulted in faster onset of sleep (<15 min) compared to oral tablet (29.33 ± 2.08 min). These results are suggestive of rapid and direct nose-to-brain transport of lorazepam following intranasal administration. Intranasal administration of microemulsion based formulations of lorazepam resulted in even faster onset of sleep (<10 min) with LMMEi.n. resulting in fastest onset of sleep (6.65 ± 0.73 min). This may be attributed to the fact that microemulsion enhances transport of drug across nasal mucosa. These findings are in congruence with the observations reported by CitationQizhi et al (2004) and CitationLianli et al (2002) that microemulsion enhances transport of drug across nasal mucosa resulting in direct nose-to-brain transport of the drugs. Duration of sleep was found to be longer in case of LToral (6.6 ± 0.36 hr) compared to LMEi.n. (6.5 ± 0.5 hr) and LSi.n. (5.76 ± 0.61 hr). Faster onset of sleep (10-15 min) accompanied with relatively shorter duration of sleep (<6.5 hr) was observed in case of LMEi.n. and LSi.n. as compared to slower onset of sleep (29.33 ± 2.08 min) followed by longer duration of sleep (6.6 ± 0.36 hr) in case of LToral. This may be attributed to rapid absorption of drug from microemulsion/solution followed by fast wash out from the nasal mucosa due to rapid mucociliary clearance which results in faster absorption rate but lesser extent of absorption. These findings also suggest more steady absorption of drug in case of LToral due to relatively slow wash out of drug in solid dosage forms like tablets from the absorption site and ultimately leading to more reliable and enhanced extent of drug absorption resulting in longer duration of sleep. Duration of sleep was found to be longest (7.13 ± 0.16 hr) with LMMEi.n. This may be attributed to the increased residence time of formulation in the nasal cavity due to addition of mucoadhesive agent. Above findings suggest greater extent of selective transport of lorazepam to the brain in case of mucoadhesive microemulsion based systems by intranasal route. Similar observations were made by Citationvyas et al (2006a, Citationb) and CitationJogani et al (2007). These results are further corroborated by loss of RR and SR at earlier time points (within 10 min and 15 min respectively) in case of LMMEi.n. Similar results were observed in case of diazepam and alprazolam formulations with DMMEi.n. and AMMEi.n. resulting in rapid and greater extent of direct nose-to-brain transport of respective drug(s) ( and respectively) and further corroborating the fact that intranasal administration of mucoadhesive microemulsions of drug results in improved rate and extent of drug absorption to the brain.
Apart from role played by intranasal route in improving the rate and extent of drug absorption in the brain, lipophilicity of drug candidate(s) was also found to be a major contributor in enhancing the rate and extent of drug uptake in the brain following intranasal administration of various microemulsion based systems. Onset of sleep (<7.4 min) and duration of sleep (>7 hr) in case of LMME, onset of sleep (<8 min) and duration of sleep (>6 hr) with AMME and onset of sleep (>9 min) and duration of sleep (<7 hr) were seen with DMME. These findings are suggestive of increased rate and extent of drug uptake in the brain following intranasal administration of drug candidates in order of drug lipophilicity. These findings also stress upon the importance of incorporation of mucoadhesive agent (polycarbophil) in increasing the nose-to-brain drug transport by virtue of increased mucoadhesion which in turn, leads to increased residence time of drugs in nasal cavity facilitating efficacious brain targeting as corroborated by faster onset of sleep followed by prolonged duration of sleep in case of MMEs as compared to microemulsions not incorporated with mucoadhesive agents.
Higher lipophilicity of Lorazepam, as compared to other two drug candidates, may have favoured the faster and enhanced uptake of the drug in the brain, facilitating rapid onset and prolonged duration of sleep. Extended duration of sleep compared with control group and other groups treated with solution and tablet dosage forms suggest role of microemulsion in delaying the mucociliary clearance of a lipophilic molecule and lesser extent to hydrophilic molecule. (tablets and solutions). Better brain targeting efficiency may be attributed to substantial direct nose-to-brain transport.
Conclusion
The studies demonstrated rapid and larger extent of selective nose-to-brain transport compared with solution and conventional dosage forms (tablets) of benzodiazepines in rats. Enhanced rate and extent of transport of Diazepam, Lorazepam and Alprazolam following intranasal administration of ME and MME may help in decreasing the dose and frequency of dosing and possibly maximize the therapeutic benefit, thereby providing safe, patient friendly, efficacious and economic drug delivery. However, thorough animal studies using different species followed by extensive clinical trials and toxicological evaluation need to be conducted to establish the appropriateness of these formulations in clinical practice.
Acknowledgments
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Newer options in the management of insomnia, Dopheide JA, and Kushida AC, http://www.uspharmacist.com.
- Rey E, Treuler JM, and Pons G, “Pharmacokinetic optimization of benzodiazepine therapy for acute seizures: Focus on delivery routes, Clin Pharmacokinet 36(6) (1999), 409–424.
- Bigal MEBordini CA, Antoniazzi AL, and Speciali JG, “The triptan formulations: a critical evaluation”, Arq Neuropsiqiatr 61(2A) (2003), 313–320.
- Wermling DP, Miller JP, Archer SM, Manaligod JM, and Rudy AC, “Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous and intramuscular administration”, J ClinPharmacology 41(2001), 1225–1231.
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, and Wong BA“Olfactory transport: A direct route of delivery of inhaled manganese Phosphate to the rat brain”, J Toxicol Environ Health 65(20) (2002), 1493–1511.
- Dragphia R, Caillaud C, Manicom R, Pavirani A, Kahn A, and Poenaru L, “Gene delivery into the central nervous system by nasal instillation in rats”, Gene Ther. 2 (6) (1995), 418–423.
- Liu XF, Fawcett JR, Thorne RG, and Frey WH. “2nd intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage”, J Neurol Sci. 187(1) (2001), 91–97.
- Lisbeth Illum “Transport of drugs from the nasal cavity to the central nervous system”, Eur J Pharm Sci. 11(1) (2000), 1–189
- Lisbeth Illum, “Nasal Drug Delivery: new developments and strategies”, Drug Discovery Today. 7(23) (2002), 1184–1189.
- Vyas TK, Shahiwala A, Marathe S, and Misra A, “Intranasal drug delivery for brain targeting”, Curr Drug Deliv. 2(2) (2005), 165–175.
- Dhanda DS, Frey WH, Leopold Donald, and Kompella UB, “Approaches for drug deposition in the human olfactory epithelium”, Drug Del Technol. 5(4) (2005).
- Behl CR, Pimplaskar HK, Sileno AP, Meireles JDe, and VDRomeo, “Effects of physicochemical properties and other factors on systemic nasal delivery”, Adv Drug Del Rev. 29(1-2) (1998), 89–116.
- Hu Z, Tawa R, Konishi T, Shibata N, and Takada K, “A novel emulsifier, labrasol, enhances gastrointestinal absorption of gentamicin”, Life Sci. 69(24) (2001), 2899–2910.
- Ugwoke MI, Verbeke N, and Kinget R, “The biopharmaceutical aspects of nasal mucoadhesive drug delivery”, J. Pharm Pharmacol. 53(2001), 3–22(20).
- Alpar HO, Somavarapu S, Atuah KN, and Bramwell VW, “Bio- degradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery”, Adv Drug Del Rev. 57(3) (2005), 411–430.
- Gavini E, Rassu G, Sanna V, Cossu M, and Giunchedi P, “Mucoadhesive microspheres for nasal administration of an antiemetic drug, metoclopramide: In-vitro/ex-vivo studies”, J Pharm Pharmacol. 57(2005), 287–294.
- Sakane T, Yamashita S, Yata N, and Sezaki H “Transnasal delivery of 5-fluorouracil to the brain in the rat”, J Drug target. 7(3) (1999), 233–240.
- Lawrence MJ, and Rees GD, “Microemulsion based media as novel drug delivery systems”, Adv. Drug Deliv. Rev. 45(1)(2000), 89–121.
- Sinswat P, and Tengamnuay P, “Enhancing effect of chitosan on nasal absorption of salmon calcitonin in rats; comparison with hydroxypropyl and dimethyl-beta-cyclodextrins”, Int. J. Pharm. 257 (2003), 15–22.
- Florey Klaus, Analytical Profiles of Drug substances, Academic press, New York, Harcourt Brace Jonovich Publishers, 1(81) (1997).
- Florey Klaus, Analytical Profiles of Drug substances, Academic press, New York, Harcourt Brace Jonovich Publishers, 4(398) (1997a).
- Roland I, Piel G, Delattre L, and Evrard B. “Systemic characterization of oil-in-water emulsions for formulation design”, Int J Pharm. 263(1) (2003), 85–94.
- Bol CJ, Vogelaar JG, W JP, Tang JP, and Mandema JW, “Quantification of pharmacodynamic interactions between dexmedetomidine and midazolam in the rat”, J. Pharmacol. Exp. Ther. 294(1) (2000), 347–355.
- Zhang Qizhi, Jiang Xinguo, Xiang Wenming, Lu Wei, Su Lina, and Shi Zhenqui, “Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation of the targeting efficiency to brain”, Int J Pharm 275(1,2) (2004), 85–96.
- Lianli Li, Nandi I, and Kim KH, “Development of an ethyl laurate-based microemulsion for rapid-onset intranasal administration of diazepam”, Int J Pharm. 237(1, 2) (2002), 77–85.
- Vyas TK, Sharma RK, and Misra A, “Preliminary brain targeting studies of intranasal mucoadhesive microemulsions of sumatriptan”, AAPS Pharm Sci Tech. 7(1) (2006), Article 8.
- Vyas TK, Sharma RK, and Misra A, “Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting”, J Pharm Sci. 95(3) (2006), 570–580.
- Jogani ViralV, Shah PranavJ, Mishra Pushpa, Mishra AnilKumar, and Misra Ambikanandan, “Intranasal Mucoadhesive Microemulsion of Tacrine to Improve Brain Targeting”, Alzheimer Dis Assoc Disord, (2007).