Abstract
The present study was to investigate the potentials of DNA/chitosan nanocomplexes as a carrier for small drug delivery. Two highly water-soluble chitosans (87 kDa and 18 kDa) were prepared and labeled with fluorescein isothiocyanate (FITC). DNA/chitosan nanocomplexes were prepared by mixing salmon testes DNA and the FITC labeled chitosan (FITC-chitosan) and their biophysical properties and biodistribution in vivo were then investigated. The molecular weight of chitosan and the ratio of the positive amino group of chitosan to the negative phosphate group of DNA (N/P ratio) influenced the physical properties of the nanocomplexes. The fluorescence intensity of both types of the free FITC-chitosan decreased rapidly within 4 hr post-injection. In contrast, the DNA/chitosan nanocomplexes were accumulated in the liver and kidneys and remained at a relatively high stable level in these tissues and in blood up to 24 hr post-injection. This study also assessed the stability of the anti-cancer drug doxorubicin (DOX) when it was conjugated to chitosan to form a chitosan-doxorubicin conjugate (chi-DOX), which was then mixed with DNA to form a DNA/chitosan-doxorubicin nanocomplex (DNA/chi-DOX). Both the chi-DOX and DNA/chi-DOX complexes exerted cytotoxic effects on HeLa, HepG2, QGY-7703, and L02 cells, while the non-malignant L02 cells were less sensitive to the DNA-containing complex than to the chi-DOX complex, suggesting possible selectivity. Studies in tumor-bearing animals demonstrated that DNA/chi-DOX could efficiently deliver doxorubicin to the tumor and liver, implying that the DNA/chitosan nanocomplex may represent a novel drug carrier.
Introduction
Among many factors affecting therapeutic effectiveness and safety of therapeutic agents are the ultimate fate and the clearance of the administered drugs (CitationMoghimi et al., 2001). Perhaps, the most feasible approach to overcome unfavorable biodistribution or clearance is to develop a drug delivery system that can protect the drug from degradation and deliver it to inaccessible target cells in a controlled manner. Numerous biomaterials have been explored as drug carriers, including liposomes, nanoparticles, and micelles (CitationMaitani et al., 2008; CitationLiu et al., 2008; CitationLacoeuille et al., 2007). Liposomes have been widely researched for their biocompatible and biodegradable properties. However, rapid clearance from the blood, an unsuitable surface structure, and osmotic fragility have limited their use in vivo (CitationLi and Huang, 1997; CitationOkuda et al., 2006). Nanoparticles, a different encapsulation system, have been introduced in order to improve the control of drug delivery. Many researchers have used anionic or neutral polymers as nano-particulate carriers for proteins, peptides, gene constructs, and drugs. These polymers include poly (D, L-lactic-co-glycolic acid) (PLGA), poloxamer, dextran, and merhylmethacrylate copolymer (CitationYadav et al., 2007; CitationNimesh et al., 2006).
As a drug delivery system, however, drugs within polyelectrolyte nanoparticles are difficult to exploit in vivo because the complex is quickly phagocytosed by cells of the reticuloendothelial system (RES) due to their large size and surface hydrophobicity (CitationGref et al., 1994; CitationSantander-Ortega et al., 2007). As a result, they have a relatively short retention in the blood. Recently, cationic polymers have been synthesized and used to deliver DNA without producing hydrophobic collapse, and have been able to evade the RES to achieve improved in vivo retention (CitationHoward et al., 2000). However, despite their improved stability, the techniques used to generate these nanoparticle-encapsulated drugs and to accomplish the surface modifications are complex and impractical for large-scale applications.
Chitosan is a deacetylated derivative of a natural cationic polysaccharide consisting of repeated glucosamine units. This biopolymer has favorable biocompatibility characteristics (CitationHirano et al., 1989), low cytotoxicity (CitationLee et al., 1998), and the ability to increase membrane permeability (CitationArtursson et al., 1994). Chitosan has been investigated extensively as an in vitro and in vivo gene delivery system, since it can interact with DNA via a high positive charge density in the polysaccharide backbone (CitationSato et al., 2001; CitationCorsi et al., 2003; CitationSatoh et al., 2007). Moreover, water-soluble chitosan and its derivatives (such as N-trimethylated chitosan chloride) have been used to deliver protein or peptide drugs (CitationThanou et al., 2000). The potential of microspheres or nanospheres synthesized with chitosan for entrapping and delivering drugs has also been evaluated (CitationJanes et al., 2001; CitationBanerjee et al., 2002). For example, glutaraldehyde-crosslinked chitosan microspheres and chitosan nanoparticles made by ionotropic gelation with sodium tripolyphosphate (TPP) were used to load horseradish peroxidase (HRP) and doxorubicin, respectively, and showed effective controlled release of their drugs without any decrease in their activity. However, these synthetic micro- or nano-spheres were usually cytotoxic at most concentrations used, and sometimes were not so effective in disease models (CitationJanes et al., 2001). DNA/chitosan nanocomplexes, which can be prepared readily via a self-assembled process, have been demonstrated to have lower cytotoxicity, representing a novel (and potentially more effective) means to improve drug delivery.
In the present study, we investigated the use of DNA/chitosan nanocomplexes for delivering small drugs. For this purpose, FITC, used as a model drug, was conjugated to chitosan and DNA/chitosan nanocomplexes obtained by a coacervation process. The biophysical properties and the biodistribution of the DNA/chitosan nanocomplexes were studied in vitro and in vivo. The in vivo results showed that the DNA/chitosan nanocomplexes had a longer retention time in blood, and higher uptake rate by the liver than FITC-chitosan. In addition, to further evaluate the efficiency of DNA/chitosan nanocomplexes as a drug carrier, the anti-cancer drug doxorubicin (DOX) was conjugated to chitosan (in place of FITC) to form chitosan-doxorubicin (chi-DOX) and DNA/chitosan-doxorubicin nanocomplexes (DNA/chi-DOX). The in vitro results indicated that both chi-DOX and DNA/chi-DOX had cytotoxicity to HeLa, HepG2, QGY-7703 and L02 cells compared with DOX. The in vivo study in tumor-bearing animals demonstrated that DNA/chi-DOX not only extended the half-life of DOX in the body, but also efficiently delivered doxorubicin to the tumor and liver, suggesting that DNA/chitosan nanocomplexes can potentially be used as novel drug carriers that can specifically target tumor tissues or the liver.
Materials and methods
Materials and cell lines
High molecular weight chitosan, FITC, DNA (Sodium salt, from salmon testes), and 1-Ethyl-3- (3-dimethylaminopropyl)-carbodiimide (EDC) were purchased from Sigma Chemical Co. (St. Louis, MO). Doxorubicin hydrochloride (purity > 98%) was from Standard Pharma. Ltd. (China). Tissue freezing medium was obtained from Leica Instruments (Germany). Heparin for blood sample collection was from Gibco. B.R.L. (Grand Island, NY). All other reagents were of analytical grade.
Human cancer cell lines, HeLa, and HepG2, were obtained from the American Type Culture Collection (ATCC). Human non-malignant hepatocellular L02 and human hepatocellular carcinoma QGY-7703 cells were obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Science (CBTCCCAS).
Animals and tumor models
ICR mice (18–22 g) were obtained from the Experiment Animal Center of Nanjing Medical University (Nanjing, China), and maintained in plastic cages at 21 ± 2°C with free access to food and water. The mice were kept on a 12 hr light/dark cycle. This study complied with the current ethical regulations on animal research of Nanjing University, and all mice used in the experiment received humane care.
Sarcoma-180 (S180) tumor cells were maintained in peritoneal cavities of ICR mice obtained from Medical College of Nanjing University (Nanjing, China). Mice were anesthetized with anhydrous ether and subcutaneously inoculated with S180 cells (2.4 × 106 cells/mouse) at the axillary region to generate sarcoma-bearing mice.
NaNO2 degradation of chitosan and preparation of FITC-labeled chitosan
High molecular weight chitosan (2 g) was dissolved in 0.1 N acetic-acid. NaNO2 (60 mg) was added, and the mixtures were stirred at room temperature for an appropriate time of 1.5 hr or 10 hr. The solution was then adjusted to above pH 7.0 with 1 N NaOH, and the depolymerized chitosan was precipitated using a double volume of ethanol. Precipitated materials were centrifuged at 10,000 rpm for 15 min, then the supernatant was discarded. The pellets were washed with Milli Q water three times, and were re-suspended in 50 ml of Milli Q water, frozen, and lyophilized.
The preparation of FITC-labeled chitosan was described previously (CitationQaqish and Amiji, 1999). Briefly, the depolymerized chitosan (150 mg) was dissolved in 15 ml of 0.1 N acetic-acid and 15 ml of dehydrated methanol was added to the solution with continuous stirring. FITC (8 mg), dissolved in 8 ml of methanol, was slowly dropped into the chitosan solution. The reaction proceeded overnight in the dark at room temperature. FITC-chitosan was precipitated in 0.1 N NaOH. The precipitate was washed extensively with Milli Q water until the free FITC fluorescence signal in the washing medium was not detected. The polymer was then frozen and lyophilized.
The chitosan and FITC-chitosan used in this study were re-dissolved using 0.1 N acetic- acid and adjusted to pH 6.0 with 0.1 N NaOH, and the 1 mg/ml solutions were stored in the dark at 4°C.
Measurement of molecular weight and amino group content of chitosan
The molecular weight (MW) of chitosan was determined as reported previously (CitationGao et al., 2003). The amino group content of each sample was determined by metachromatic titration using Xylidine Tonceau 2R (C.I. Acid Red 26) according to the modified method used by Gummow and Roberts (CitationGummow and Roberts, 1985).
Preparation and characterization of DNA/chitosan nanocomplexes
The typical preparation of DNA/chitosan nanocomplex was as follows: the solutions of DNA and chitosan were diluted with PBS to 0.8 mg/ml. After 15 min, different volumes of chitosan solution were added to the DNA solution, and the mixed solutions (pH 6.5) were left for 30 min at room temperature. The ratio of the positive amino group of chitosan to the negative phosphate group of DNA (N/P ratio) was calculated from the weight ratio of DNA and chitosan and the -NH2 group content of chitosan.
The particle size and surface zeta potential of the DNA/chitosan nanocomplexes were analyzed by light scattering using a 90 Plus Particle Sizer (Brookhaven Instruments, Holtsville, NY). The physical stability of the complexes at various N/P ratios was studied by 1% agarose gel electrophoresis with 2 μg of salmon testes DNA loaded. The morphology of the DNA/chitosan nanocomplex at N/P = 4 was observed using a JEM-200 CX (KV200) transmission electron microscope (TEM) (Japan). One drop of DNA/chitosan nanocomplex was placed on a copper grid and negatively stained with 1% phosphotungstic acid solution for 30 s. The grid was allowed to dry further for 10 min and then examined with TEM.
Biodistribution of FITC-chitosan and DNA/chitosan nanocomplexes
The solutions of DNA and FITC-chitosan were both diluted with PBS to a concentration of 0.8 mg/ml. Normal mice received 128 μg FITC-chitosan (160 μl FITC-chitosan solution + 40 μl PBS) or DNA/chitosan nanocomplex, which consisted of 128 μg FITC-chitosan and 32 μg DNA with an N/P ratio of 4 (160 μl FITC-chitosan solution + 40 μl DNA solution), via the lateral tail vein without anesthetization, and 200 μl PBS was administrated to the control mouse. At 5 min, 2, 4, 18, 24, and 50 hr after injection, blood samples were drawn into cuvettes through retroorbital bleed, and several tissues, including heart, liver, spleen, lung, and kidney, were excised following sacrifice. Each organ was washed with PBS, gently blotted using filter paper, and weighed. Then the appropriate volume of PBS was added, and the mixture was homogenized. The supernatant was obtained by centrifugation twice at 12,000 rpm for 10 min, and the fluorescence intensity of each sample was measured (Ex = 489.7 nm, Em = 520 nm) using a Hitachi M850 fluorescence spectrophotometer (Japan). Blood samples were centrifuged similarly to tissues. The concentration of FITC-chitosan in the samples was determined from the standard calibration curve, and was corrected by the according recovery ratio of each tissue or blood in vitro. The amount of drug present in the blood was calculated based on an estimation of the blood volume of mice as 2.18 ml/25 g (CitationKataoka et al., 2000).
Preparation and characterization of the chi-DOX conjugate and the DNA/Chi-DOX nanocomplex
Doxorubicin was conjugated to chitosan through a succinic spacer. Succinylated chitosan was prepared as reported (CitationYamaguchi et al., 1981; CitationSong et al., 1996). Briefly, chitosan (18 kDa, 0.5 g) was dissolved in 0.1 N acetic acid (10 ml), and diluted with methanol (40 ml). Succinic anhydride (60 mg) dissolved in acetone (10 ml) was added, and the mixture was stirred overnight. The solution was dialyzed against distilled water and lyophilized to obtain N-succinylated chitosan. Then N-succinylated chitosan (80 mg) was dissolved in 15 ml distilled water with 60 mg EDC. Doxorubicin hydrochloride (16 mg) dissolved in minimal water was added. The solution was stirred in the dark for 24 hr to obtain the chi-DOX conjugate. The degree of succinylation was calculated by elemental analysis. Doxorubicin content was determined by UV absorbance at 480 nm.
The DNA/chi-DOX nanocomplex was made using different volume ratios of DNA to chi-DOX (0.1 mg/ml). The particle size and stability of the nanocomplex were studied as described for DNA/chitosan.
In vitro cytotoxicity of chi-DOX conjugate and DNA/chi-DOX nanocomplex
The cytotoxicity of the chitosan-DOX conjugate and DNA/chi-DOX nanocomplex was measured using the MTT dye reduction assay. HeLa, HepG2, QGY-7703, and L02 cells were seeded at a density of 1.0 × 104 cells/well in 96-well plates and incubated for 24 hr. The cells were incubated in 100 μl serum-free medium containing 100 U/ml of penicillin and 100 μg/ml of streptomycin and treated with 2 μg (DOX eq.)/ml of chitosan-DOX conjugate, DNA/chi-DOX nanocomplex, or free DOX for 72 hr at 37°C. Then the cells were washed, and cell viabilities were measured by the MTT assay. The percentage of cell viability was determined using untreated cells as the control.
Tissue distribution study of DNA/chi-DOX nanocomplex
Tumor-bearing mice were used to study the distribution of drugs 6 days after inoculation of S180 cells. Doxorubicin hydrochloride, chi-DOX, and DNA/chi-DOX complexes were dissolved in PBS and administered intravenously to S180-bearing mice at a dose of 5 mg (DOX eq.)/kg. Four hours after injection, blood and various tissues, including the liver, lung, heart, spleen, kidneys, and tumor, were removed to determine doxorubicin content based on fluorometric assays (CitationSchwartz, 1973).
Results and discussion
Depolymerization of chitosan
High molecular weight chitosan cannot be used under physiological conditions because of its low solubility and high viscosity. Therefore, water-soluble and low molecular weight chitosan were prepared to obtain optimal delivery in vivo.
summarizes the molecular weight and the -NH2 group content of the chitosan used in the study. The molecular weight of chitosan decreases with the duration of NaNO2 depolymerization. The average molecular weights of depolymerized chitosan are 87 kDa (chi-87K) and 18 kDa (chi-18K), respectively, after 1.5 or 10 hr NaNO2 treatment. With depolymerization, the -NH2 content of chitosan also decreased, from 71% to 51% over the 10 hr of treatment. This may have been due to the oxidation of -NH2 groups during the depolymerization process.
Table 1. Properties of chitosan used in the study.
Biophysical properties of DNA/chitosan nanocomplexes
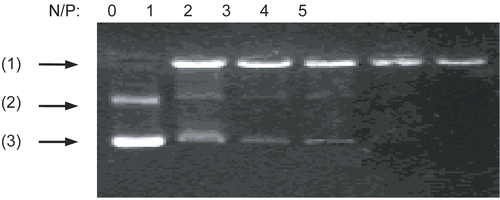
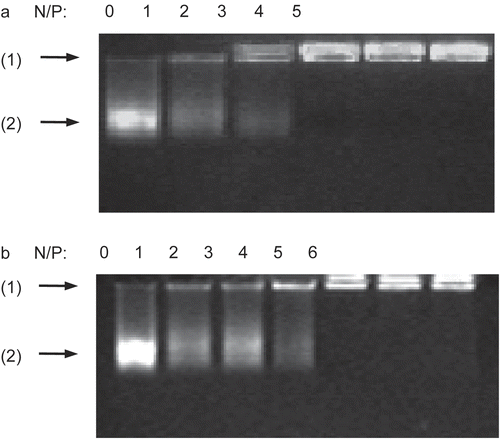
DNA (2 μg of salmon testes DNA) was blended with chi-87K or chi-18K to form nanocomplexes at various N/P ratios, then the complexes were analyzed by 1% agarose gel electrophoresis. As shown in , there was a decrease in the staining intensity of DNA in the gel as the amount of chitosan increased. It should be noted that there are many continuous and sparse bands of stained DNA in the gel due to the wide molecular weight range of the salmon testes DNA. The movement of the DNA was completely retarded by chi-87K at an N/P ratio of 3:1, as shown in , while the N/P ratio for chi-18K to achieve the same retardation effect was 4:1 (). This indicates that chi-87K and chi-18K are both able to condense DNA to form a stable electrostatic complex. Moreover, chi-87K, with its higher molecular density of positive charges in the backbone, can interact with and thus condense DNA more efficiently than chi-18K.
Figure 1. Gel retardation assay of the DNA/chitosan complexes. Complexes containing 2 μg of salmon testes DNA were analyzed by 1% agarose gel electrophoresis at various N/P ratios: (A) DNA/chi-87K complexes, (B) DNA/chi-18K complexes. Arrows indicate: (1) loading position, (2) unretarded DNA.
We also examined the particle size and zeta potential for the DNA/chitosan nanocomplexes (). These experiments demonstrate the influence of the charge ratio on the particle size and zeta potential of the complex. The particles have a negative surface charge when the complexes with chi-87K and chi-18K are made at N/P ratios below 1. At N/P = 1, the particles formed from the two kinds of chitosan are nearly neutral. However, the surface zeta potential becomes positively charged when the N/P ratio is above 1, up to approximately +30 mV at N/P = 6. This indicates that the molecular weight of chitosan does not have a major impact on the zeta potential of the complex.
Figure 2. Zeta potential and particle size as a function of the N/P ratio of DNA/chitosan complexes prepared at the concentration of 0.2 mg/ml of DNA and chitosan. ▪ DNA/chi-87K complexes, o DNA/chi-18K complexes. The results are expressed as the mean ± SE (n = 5).
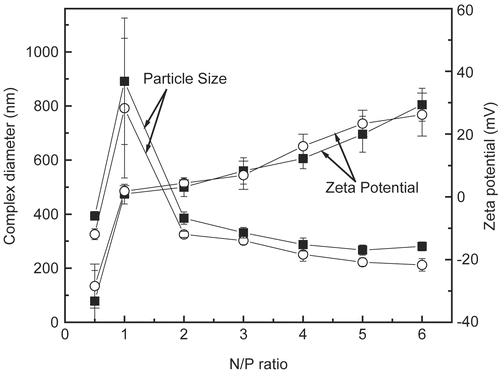
Evaluation of particle sizes showed a trend toward a slight decrease in the size of the complex as the N/P ratio increased, with the exception of when N/P = 1 for both kinds of chitosan. DNA/chitosan nanocomplexes with various N/P ratios are all within the size range from 200 nm to 400 nm, and are homogenous with low standard errors. As expected, there was also a slight increase in particle size as the molecular weight of chitosan increased. For example, the nanocomplex formed with chi-87K at each N/P ratio was nearly 50 nm greater than that of chi-18K. When the complexes formed from both kinds of chitosan had a 1:1 N/P ratio, the complex was considerably larger than at other ratios because the complex forms aggregates in solution. This result is in agreement with the zeta potential measurements. Zeta potential measurements clearly showed that the complex is nearly neutral at N/P = 1, and thus has a tendency to aggregate.
The stability, size, and charge characteristics of DNA/chitosan nanocomplexes are important factors that influence their delivery efficiency. In the past, the low stability, large particle diameter, and unsuitable surface charge properties of the complexes have hindered their use for drug delivery in biological milieu (CitationCorsi et al., 2003). However, the present results suggest that the complex at N/P = 4 has a better ability to condense DNA than complexes with other N/P ratios, indicating that it might be suitable for in vivo use. Thus, the complexes formed at N/P = 4 were used as the test delivery system in the subsequent in vivo experiments.
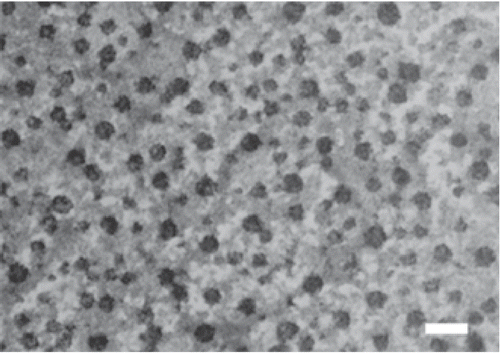
In order to further assess the morphology and size of the DNA/chitosan nanocomplexes, TEM (transmission electron microscopy) was employed. shows the image of the particles formed by DNA and chi-87K in the complex at N/P = 4. The particles appear nearly spherical, with a consistent diameter range of 20–50 nm, and the particles were dispersed from each other. It should be noted that the particle size recorded using TEM was much smaller than that from laser light scattering. This is likely due to the fact that the measurement of light scattering is accomplished in aqueous solution, where the particles are hydrated, thus increasing their the average size compared to the size of the dehydrated particles used for TEM measurement.
In vivo study of FITC-chitosan and DNA/chitosan nanocomplexes
Chitosan and its derivatives labeled with FITC have previously been administrated intraperitoneally by other researchers (CitationOnishi et al., 1999), and the FITC-polymer linkage is considered to be stable in the body. In the present study, the behavior of DNA condensed FITC-chitosan at N/P = 4, as well as free FITC-chitosan, was evaluated in mice following tail vein injection. shows the tissue distribution of the free FITC-chitosan and its DNA complex 4 hr after injection. As shown in , FITC-chi-87K and FITC-chi-18K both distributed mainly to the liver and kidneys. In the case of the DNA/chitosan nanocomplexes, the distribution to the kidneys and liver increased significantly compared with that of the free FITC-chitosan (). DNA/chitosan nanocomplexes also accumulated more than free FITC-chitosan in other tissues, likely because the DNA/chitosan nanocomplexes were retained in systemic circulation for a longer period of time ().
Figure 4. Tissue distribution of FITC-chitosan (A) and DNA/chitosan nanocomplexes (B) 4 hr after tail vain injection. ▪ FITC-chi-18K, □ FITC-chi-87K. Results are expressed as the mean ± SE (n = 5).
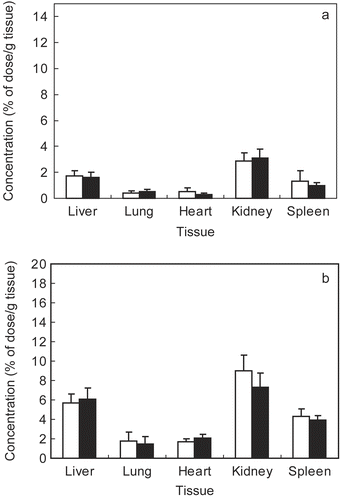
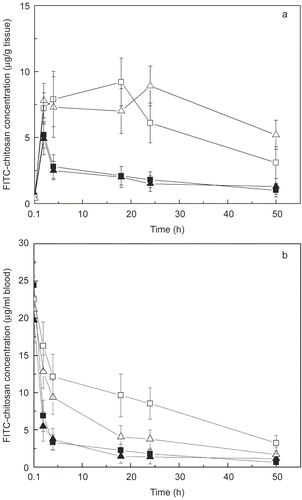
Figure 5. Time-course of FITC-chitosan concentrations in mouse liver (A) and blood (B) after tail vain injection. ▪ FITC-chi-87K, ▴ FITC-chi-18K, □ DNA/chi-87K nanocomplexes, △ DNA/chi-18K nanocomplexes. Results are expressed as the mean ± SE (n = 5).
Because it is positively charged, chitosan can interact with cells, including red blood cells. This suggests that the FITC-chitosan complexes may interact with various cells in the blood, and thus will be quickly transferred to kidneys and excreted (CitationOnishi and Machida, 1999). In agreement with this, the FITC-chitosan complexes were largely distributed to the kidneys by 4 hr after administration ().
In contrast to FITC-chitosan complexes, DNA/chitosan nanocomplexes are distributed more to the liver than kidneys. shows the time course of accumulation of DNA/chitosan nanocomplexes and FITC-chitosan in the liver after injection. The DNA/chitosan nanocomplexes have an increased accumulation in liver compared to the FITC-chitosan. This may result from the fact that the complexes are taken up to a significant extent by hepatocytes or Kupffer cells in the liver in vivo because hepatocytes possess the asialoglycoprotein receptor (ASGR), which binds and internalizes galactose terminal asialoglycoprotein (CitationWall et al., 1980), or it may specifically recognize and interact with the glucose moiety on mammalian liver (CitationNishikawa et al., 1992; CitationKaneo et al., 2001).
shows the time course of DNA/chitosan nanocomplexes and the FITC-chitosan complexes in blood after injection. During the first 24 hr post-injection, both the concentration and retention of the DNA/chi-87K and DNA/chi-18K complexes were significantly higher in the blood compared with the FITC-chitosan complexes. For the FITC-chitosan complexes, the fluorescence intensity sharply decreased in the 4 hr after injection, and the complexes were almost completely cleared from the blood by 18 hr post-injection. These results demonstrate that both DNA/chi-18K and DNA/chi-87K complexes have a prolonged residence time in blood compared to unconjugated complexes, although the DNA/chi-18K complex showed a more rapid clearance from the circulation than the DNA/chi-87K complex.
Nanoparticles have a special role in drug delivery that is largely attributed to their particle size and surface features. The major obstacle to active targeting of the nanoparticles in vivo is their rapid removal by the reticoendothelial system (RES) following intravenous administration (CitationMoghimi et al., 2001). Poor surface solubility of the nanoparticles, together with non-specific charge-mediated interactions with proteins and the components of the RES, are additional factors that contribute to their rapid clearance from the bloodstream. Many researchers have demonstrated that nanoparticles modified with hydrophilic polymers, such as PEG, PEO, or poloxamers, have a prolonged circulating time in the systemic circulation and reduced uptake by the RES because of the spatial barrier effect (CitationGref et al., 1994; CitationSantander-Ortega et al., 2007; CitationStolnik et al., 2001; CitationAvgoustakis et al., 2003). It has also been demonstrated that the smaller the liposome, the larger the contribution of hepatocytes in total hepatic uptake (CitationRomero et al., 1999), suggesting that smaller nanoparticles can evade clearance by the endothelial system by being targeted to the liver, thus exhibiting a longer retention in systemic circulation.
Since both chi-18K and chi-87K are low molecular weight chitosan molecules that are highly water-soluble, the DNA/chitosan nanocomplexes have adequate hydrophilic surfaces for in vivo administration. In addition, the DNA/chitosan nanocomplexes show relatively low surface charge densities after interacting with DNA. Based on these properties, the DNA/chitosan nanoparticles can avoid the tendency to aggregate. On the other hand, these properties help decrease the interaction with proteins and cells in the bloodstream, thus increasing their retention in systemic circulation.
The stability of nanoparticles in vivo is also an important factor that influences their clearance. Although the gel retardation assay shows that DNA can be completely condensed by both two kinds of chitosan at N/P = 4, the DNA/chitosan nanocomplexes gradually disassemble in blood, and are then excreted via the kidney, like the FITC-chitosan complexes. The stability of the DNA/chi-87K nanocomplex might be higher than the DNA/chi-18K nanocomplex in vivo due to its stronger electrostatic interaction with DNA. It is reasonable to infer that the DNA/chi-18K nanocomplex disassembles more rapidly in the blood than DNA/chi-87K nanocomplex, and as a result, is cleared more rapidly ().
Characterization of chi-DOX conjugate and DNA/ chi-DOX nanocomplexes
In order to evaluate the DNA/chitosan nanocomplexes as potential carriers for drug delivery, doxorubicin, an important anti-tumor drug with an amino group of sugar residue was chosen for this study. Succinic anhydride was used to link the amino groups of doxorubicin to chi-18K. As calculated from the C/N ratio in elemental analysis, the degree of N-succinylation of chitosan is 59% against the amino group. The UV spectrum for the chitosan-doxorubicin conjugate showed an absorption at 480 nm, which is the same as doxorubicin. A calibration curve was made using solutions with various concentrations of doxorubicin. As determined using the calibration curve, the doxorubicin content of the chi-DOX conjugate was approximately 27 wt%.
The DNA/chi-DOX nanocomplex was formed not only by electrostatic attraction between chitosan and DNA, but also by doxorubicin intercalating into the GC base pairs of DNA (CitationAndre et al., 1972). shows the stability of the DNA/chi-DOX nanocomplexes as determined using 1% agarose gel electrophoresis. When the N/P ratio of the chitosan-doxorubicin conjugate to DNA reaches 4, the movement of the complex is completely blocked. This occurs with an average particle size of 285 nm and a zeta potential of 2.6 mV.
Cytotoxic effects of the chi-DOX conjugate and DNA/chi-DOX nanocomplex
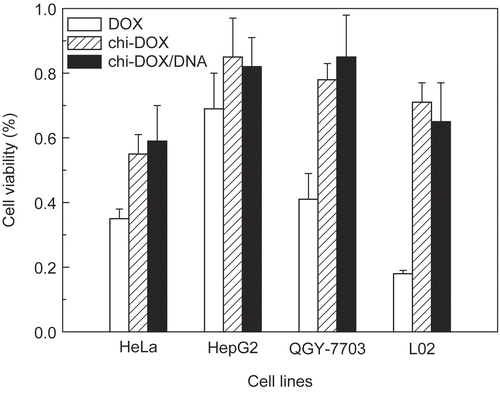
The cytotoxic effects of the chi-DOX conjugate and DNA/chi-DOX nanocomplex on HeLa, HepG2, QGY-7703, and L02 cells were examined by MTT assay (). The chi-DOX conjugate was cytotoxic to all of the tested cell lines, but was less cytotoxic than unconjugated doxorubicin at a 2 μg/ml doxorubicin equivalent concentration. This profile is consistent with many other polymer drugs (CitationChytil et al., 2006; CitationRodrigues et al., 1999), since the release of the free drugs from polymer drugs is a controlled process and occurs over a longer time course. The cytostatic activity of both doxorubicin and the chi-DOX conjugate depends on cell type (). For example, the human non-malignant L02 hepatocytes exhibited a greater difference in sensitivity to the chi-DOX conjugate versus doxorubicin than the other cell lines. This suggests that the chi-DOX conjugate may be less toxic to normal tissues than doxorubicin. The Chi-DOX/DNA nanocomplex shows similar cytotoxic effects as the chi-DOX conjugate on these cell lines, but was less cytotoxic than free doxorubicin.
Distribution of the DNA/chi-DOX nanocomplex
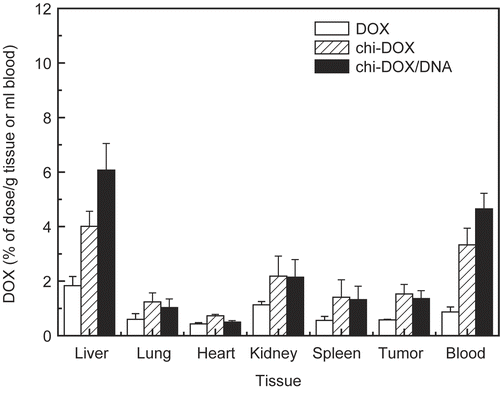
shows the tissue distribution of free doxorubicin, the chi-DOX conjugate, and the DNA/chi-DOX nanocomplex 4 hr after injection in S180-bearing mice. Both the chi-DOX conjugate and the DNA/chi-DOX nanocomplex were able to increase the doxorubicin content in the tested tissues, with increases in their distribution to the liver and in the blood compared with free DOX. The DNA/chi-DOX nanocomplex also exhibited a higher liver uptake and blood retention than the chi-DOX conjugate, possibly due to the decreased surface charge and the size of the particles. In addition, the chi-DOX conjugate and the DNA/chi-DOX nanocomplex both accumulated to a greater extent in the tumors because of their enhanced membrane permeability and increased retention. The relatively high blood retention and liver accumulation of the chi-DOX conjugate () may be a result of the free succinyl residue in the chi-DOX conjugate. This result is consistent with a previous report, in which chitosan had a longer duration in systemic circulation when a succinyl group was introduced (CitationKato et al., 2000). Compared to the chi-DOX conjugate, the DNA/chi-DOX nanocomplex shows even better delivery of doxorubicin to the liver, blood, and the tumor.
Conclusion
In the present study, we prepared DNA/chitosan nanoparticles by mixing highly water-soluble chitosan and salmon testes DNA and evaluate their potential as a drug carrier. The physical properties of the DNA/chitosan nanoparticles were affected by the molecular weight of chitosan and the N/P ratio. The DNA/chitosan nanocomplexes generated using 18kDa- and 87kDa-chitosans were highly stable, and had size and charge characteristics at N/P = 4 that made them well-suited for in vivo applications. After intravenous administration, the complexes accumulated in liver, blood, and kidneys 4 hr post-injection, and had a much longer retention in these tissues 24 hr post-injection compared with the FITC-chitosan. These findings suggest that the complexes may be suitable for drug delivery. In addition, the anti-tumor drug doxorubicin was conjugated to chitosan to form chi-DOX conjugates, then mixed with DNA to generate DNA/chi-DOX nanocomplexes. Both of the agents effectively inhibited the growth of HeLa, HepG2, QGY-7703, and L02 cells. Although extensive studies are needed to determine their full potential, the easily-made DNA/chi-DOX nanocomplexes can efficiently deliver doxorubicin to tumor and liver tissues, indicating that the DNA/chitosan nanocomplexes may serve as promising carriers for delivering small drugs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 50673041 and 30771036), the National Basic Research Foundation of China (973 Programs 2006CB503908 and 2004CB518603), a Young Investigator Grant (NCET-04-0466), the Chinese National Programs for High Technology Research and Development (863 Program 2006AA02Z177), and the 111 project from the Chinese Ministry of Education.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Moghimi SM, Hunter AC, Murray JC. (2001). Long-circulating and target-specific nanoparticles: therory to practice. Pharmacol Rev, 53, 283–318.
- Maitani Y, Aso Y, Yamada A, Yoshioka S. (2008). Effect of sugars on storage stability of lyophilized liposome/DNA complexes with high transfection efficiency. Int J Pharm, 356, 69–75.
- Liu BR, Yang M, Li RT, Ding YT, Qian XP, Yu LX, Jiang XQ. (2008). The antitumor effect of novel docetaxel-loaded thermosensitive micelles. Eur J Pharm Biopharm, 69, 527–34.
- Lacoeuille F, Hindre F, Moa F, Roux J, Passirani C, Couturier O, Cales P, Le Jeune JJ, Lamprecht A, Benoit JP. (2007). In vivo evaluation of lipid nanocapsules as a promising colloidal carrier for paclitaxel. Int J Pharm, 344, 143–9.
- Li S, Huang L. (1997). In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther, 4, 891–900.
- Okuda T, Kawakami S, Akimoto N, Niidome T, Yamashita F, Hashida M. (2006). PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J Contr Rel, 116, 330–6.
- Yadav AK, Mishra P, Mishra AK, Mishra P, Jain S, Agrawal GP. (2007). Development and characterization of hyaluronic acid–anchored PLGA nanoparticulate carriers of doxorubicin. Nanomed Nanotech Biol Med, 3, 246–57.
- Nimesh S, Kumar R, Chandra R. (2006). Novel polyallylamine– dextran sulfate–DNA nanoplexes: highly efficient non-viral vector for gene delivery. Int J Pharm, 320, 143–9.
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. (1994). Biodegradable long-circulating polymeric nanospheres. Science, 263, 1600–3.
- Santander-Ortega MJ, Csaba N, Alonso MJ, Ortega-Vinuesa JL, Bastos-González D. (2007). Stability and physicochemical characteristics of PLGA, PLGA:poloxamer and PLGA:poloxamine blend nanoparticles: a comparative study. Colloid Surf A Physicochem Eng Asp, 296, 132–40.
- Howard KA, Dash PR, Read ML, Ward K, Tomkins LM, Nazarova O, Ulbrich K, Seymour LW. (2000). Influence of hydrophilicity of cationic polymers on the biophysical properties of polyelectrolyte complexes formed by self-assembly with DNA. Biochim Biophys Acta, 1475, 245–55.
- Hirano S, Seino H, Akiyama Y, Nonaka I. (1989). Biocompatibility of chitosan by oral and intravenous administration. Polym Eng Sci, 59, 897–901.
- Lee KY, Kwon IC, Kim YH, Jo WH, Jeong SY. (1998). Preparation of chitosan self-aggregates as a gene delivery system. J Contr Rel, 51, 213–20.
- Artursson P., Lindmark T., Davis S.S., Illum L. (1994). Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res, 11:1358–61.
- Sato T, Ishii T, Okahata Y. (2001). In vitro gene delivery mediated by chitosan. Effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials, 22, 2075–80.
- Corsi K, Chellat F, Yahia LH, Fernandes JC. (2003). Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials, 24, 1255–64.
- Satoh T, Kakimoto S, Kano H, Nakatani M, Shinkai S, Nagasaki T. (2007). In vitro gene delivery to HepG2 cells using galactosylated 6-amino-6-deoxychitosan as a DNA carrier. Carbohyd Res, 342, 1427–33.
- Thanou M, Florea BI, Langemeÿer MWE, Verhoef JC, Junginger HE. (2000). N-Trimethylated chitosan chloride (TMC) improves the intestinal permeation of the peptide drug buserelin in vitro (Caco-s cells) and in vivo (Rats). Pharm Res, 17, 27–31.
- Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ. (2001). Chitosan nanoparticles as delivery systems for doxorubicin. J Contr Rel, 73, 255–67.
- Banerjee T, Mitra S, Singh AK, Sharma RK, Maitra A. (2002). Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int J Pharm, 243, 93–105.
- Qaqish RB, Amiji MM. (1999). Synthesis of a fluorescent chitosan derivative and its application for the study of chitosan-mucin interactions. Carbohyd Polym, 38, 99–107.
- Gao SY, Chen JN, Xu XR, Ding Z, Yang YH, Hua ZC, Zhang JF. (2003). Galactosylated low molecular weight chitosan as DNA carrier for hepatocyte-targeting. Int J Pharm, 255, 57–68.
- Gummow BD, Roberts GAF. (1985). Studies on chitosan-induced metachromasy, 1. Metachromatic behavior of sodium 2’-hydroxy-1, 1’-azonaphthalene-4-sulfonate in the presence of chitosan. Makromol Chem, 186, 1239–44.
- Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y. (2000). Doxorubicin-loaded poly(ethylene glycol)-poly(β- benzyl-L-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. J Contr Rel, 64, 143–53.
- Yamaguchi R, Arai Y, Itoh T. (1981). Preparation of partially N-succinylated chitosans and their cross-linked gels. Carbohydr Res, 88, 172–5.
- Song Y, Onishi H, Machida Y, Nagai T. (1996). Drug release and antitumor characteristics of N-succinyl-chitosan-mitomycin C as an implant. J Contr Rel, 42, 93–100.
- Schwartz HS. (1973). A fluorometric assay for daunomycin and adriamycin in animal tissues. Biochem Med, 7, 396–404.
- Onishi H, Machida Y. (1999). Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials, 20, 175–82.
- Wall DA, Wilson G, Hubbard AL. (1980). The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell, 21, 79–93.
- Nishikawa M, Ohtsubo Y, Ohno J, Fujita T, Koyama Y, Yamashita F, Hashida M, Sezaki H. (1992). Pharmacokinetics of receptor-mediated hepatic uptake of glycosylated albumin in mice. Int J Pharm, 85, 75–85.
- Kaneo Y, Tanaka T, Nakano T, Yamaguchi Y. (2001). Evidence for receptor-mediated hepatic uptake of pullulan in rats. J Contr Rel, 70, 365–73.
- Stolnik S, Daudali B, Arien A, Whetstone J, Heald CR, Garnett MC, Davis SS, Illum L. (2001). The effect of surface coverage and conformation of poly(ethylene oxide) (PEO) chains of poloxamer 407 on the biological fate of model colloidal drug carriers. Biochim Biophys Acta, 1514, 261–79.
- Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Livaniou E, Evangelatos G, Ithakissios DS. (2003). Effect of copolymer composition on the physicochemical characteristics, in vitro stability, and biodistribution of PLGA-mPEG nanoparticles. Int J Pharm, 259, 115–27.
- Romero EL, Morilla MJ, Regts J, Koning GA, Scherphof GL. (1999). On the mechanism of hepatic transendothelial passage of larger liposomes. FEBS Lett, 448, 193–6.
- Andre T, Daniele DC, Christian DD. (1972). Chemotherapy through lysosomes with a DNA-daunorubicin complex. Nature New Biol, 239, 110–2.
- Chytil P, Etrych T, Koňák č, šírová M, Mrkvan T, říhová B, Ulbrich K. (2006). Properties of HPMA copolymer–doxorubicin conjugates with pH-controlled activation: Effect of polymer chain modification. J Contr Rel, 115, 26–36.
- Rodrigues PCA, Beyer U, Schumacher P, Roth T, Fiebig HH, Unger C, Messori L, Orioli P, Paper DH, Mülhaupt R, Kratz F. (1999). Acid-sensitive polyethylene glycol conjugates of doxorubicin: preparation, in vitro efficacy and intracellular distribution. Bioorgan Med Chem, 7, 2517–24.
- Kato Y, Onishi H, Machida Y. (2000). Evaluation of N-succinyl-chitosan as a systemic long-circulating polymer. Biomaterials, 21, 1579–85.