Abstract
The objective of the present study was to design controlled release ophthalmic delivery systems for ciprofloxacin based on polymeric carriers that undergo sol-to-gel transition upon change in pH or in the presence of cations in an attempt to prolong the effect of ciprofloxacin and improve its ocular bioavailability. Carbopol and alginates polymers were used to confer gelation properties to the formulations. Hydroxypropyl methylcellulose and methylcellulose were combined with carbopol to increase the viscosity of the gels and to reduce the concentration of the incorporated carbopol. The release exponents (n) for the designed systems were close to 1, indicating that the drug release occurred by zero-order kinetics. Controlled release in situ gels consisting of carbopol and cellulose derivatives showed an increase in viscosity, gelling capacity, and adhesiveness as the concentration of each polymeric component was increased. On the other hand, these parameters possessed lowest values when alginate was used as an in situ gelling agent. The antimicrobial efficiency of the selected formulation against gram-positive and gram-negative organisms including Echerichia coli, staphylococcus strains and Pseudomonas aeruginosa confirmed that the designed formulation has prolonged the antimicrobial effect of ciprofloxacin and retained its properties against bacteria.
Introduction
Development of controlled release delivery systems for ocular therapy is a challenging task. Conventional eye drops results in poor bioavailability and therapeutic response owing to drainage by gravity-induced lacrimal and normal tear turnover (Schoenwald, Citation1990). Frequent dosing therefore is necessary to compensate for the decreased pre- corneal drug administration, however it’s usually associated with non-patient compliance. Inclusion of excess drug in the formulation in an attempt to overcome the bioavailability problem is potentially dangerous if the drug solution drained from the eye is systemically absorbed from the nasolacrimal duct (Middleton et al., Citation1990). It has been suggested that incorporation of polymers into aqueous vehicle can increase its viscosity and slow down the drug elimination from the conjuctival sac; however, limited improvement in bioavailability has been obtained (Patton and Robinson, Citation1975; Zaki et al., Citation1986). Mucoadhesive property of the vehicle is also a critical factor that affects the corneal contact for drugs (Greaves and Wilson, Citation1993).
Ophthalmic delivery systems based on in situ gel have gained increasing interest. These systems consist of polymers that undergo sol-to-gel phase transition upon change in the physical conditions (pH, temperature). The gelation can be triggered by a shift in temperature as poloxamer (Miller and Donovan, Citation1982) and ethyl (hydroxy ethyl) cellulose (Lindell and Engstrom, Citation1993), a shift in pH as for cellulose acetate phthalate (Gurny, Citation1981) and carbopol (Srividya et al., Citation2001), or by presence of cations as for deacetylated gellan gum (Carfors et al., Citation1998) and alginate (Cohen et al., Citation1997; Liu et al., Citation2006). In situ gel system is formulated as liquid preparation suitable to be instilled into eyes which upon exposure to the physiologic environment changes to gel, thus increasing the precorneal residence time of the delivery system, and enhances the ocular bioavailability of the drug.
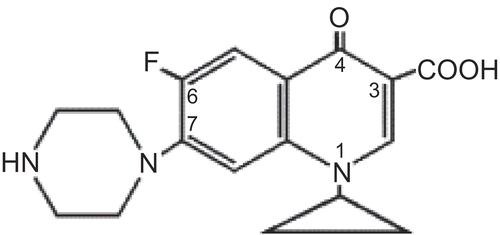
Ciprofloxacin (CPFX) (Scheme 1) is a synthetic fluoroquinolone antibiotic with a broad spectrum antimicrobial activity (Goodman and Gilman, Citation1996). It is effective against external infection of the eye, such as conjunctivitis and kerato conjunctivitis (Appelbaum and Hunter, Citation2000). The solubility of ciprofloxacin is pH-dependant and is ranging from 6.19 mg/ml at pH 5 to 0.15 mg/ml at pH 7 and 37°C. The low aqueous solubility of this compound at pH close to 7 is due to that in aqueous solution, ciprofloxacin exists in its zwitterionic form due to the acid/base interaction between the basic nitrogen of the piperazine and the carboxylic acid groups (Vilches et al., Citation2002). This interaction is the main reason that prevents the design of liquid dosage forms such as ophthalmic and parenteral solution, because the aqueous compatibility of these drugs occurs at rather basic or acidic pH (Allemandi et al., Citation1999). The efficacy of market fluoroquinolone products, mostly aqueous solubility, is limited by poor bioavailability (Lin et al., Citation1996). When ciprofloxacin 0.3% solution was used to treat bacterial corneal ulcers, crystalline precipitates appeared in the superficial portion of the corneal defect (Thomson Micromedex, Citation2003).
Therefore, and based on what has been mentioned above, the main objective of the present investigation was to control the rate of dissolution of ciprofloxacin in an attempt to control its rate of absorption from the cornea, improve its ocular bioavailability, and reduce its side-effects by incorporating it into a controlled release in situ gelling system based on polymeric carriers. Carbopol and alginate were investigated as vehicles for formulation of ciprofloxacin eye drops which undergo gelation when instilled into the cul de sac. Carbopol (CP) is widely used in in-situ gel forming systems, owing to the property of its aqueous solution to transform into gel when the pH is raised. However, the concentration of CP to form stiff gel results in highly acidic solutions which are not easily neutralized by the buffer action of the tear fluid. Therefore, viscosity-enhancing agents such as cellulose derivatives were used in order to reduce CP concentration without compromising the gelling capacity and the rheological properties of the prepared systems. In the present study hydroxypropylmethylcellulose (HPMC) and methyl cellulose have been used as viscosity-enhancing agents to carbopol, and their effects on the mechanical properties and the release characteristics of ciprofloxacin from the gel formulations were studied. A further objective of the study was to evaluate alginate as an ocular delivery system of ciprofloxacin which has the ability to gel in the presence of cations.
Materials and methods
Materials
Ciprofloxacin was obtained from Sigma Chemical Co (USA). Carbopol 934 and Carbopol 971 were purchased from BF Goodrich (USA). Sodium alginate was obtained from Hopkins and Williams Ltd. (UK). Hyroxy propyl methyl cellulose, Methyl cellulose, and Tween 80 were obtained from Sigma Chemical Co. (USA). All other chemicals were commercially available products of analytical grade.
Preparation of in-situ ciprofloxacin formulations
shows the composition of controlled released in-situ ciprofloxacin gels. Ciprofloxacin gels based on HPMC and Cabopol (CP) were prepared as follows. Initially, HPMC was dispersed in pH 6 phosphate buffer containing 1% (w/w) Tween 80 using a mechanical stirrer. Following complete hydration, CP was sprinkled over the solution and allowed to hydrate over night. Ciprofloxacin was then dissolved in 0.5 N NaOH solution and incorporated manually into the HPMC/CP mixture until homogeneity was attained. Alginate gels were prepared by dispersing the required amount of alginate polymer in pH 6 phosphate buffer containing 1% w/v Tween 80. Ciprofloxacin was then added to the mixture after dissolving it in 0.1 N NaOH. The samples were then transferred into amber ointment jars after adjusting the pH to 6 and stored at 4°C prior to further analysis.
Table 1. Composition of prolonged-action in situ ciprofloxacin eye gels.
Uniformity of drug content and pH measurements
Drug content of ciprofloxacin formulations was determined by dissolving an accurately weighed quantity of formulation (1 g) in 50 ml of pH 6 phosphate buffer. The solutions were then filtered through 0.45 m membrane filter and analyzed for ciprofloxacin content by UV spectrophotometer at 270 nm.
The pH was measured for each formulation using a pH meter (Sartorius, USA) which was calibrated before use with buffered solution at pH 4, 7, and 10.
Gelling capacity
The gelling capacity of ciprofloxacin formulations was determined by placing a drop of the prepared systems in a vial containing 2 ml of pH 7.4 artificial tear fluid freshly prepared and equilibrated at 37°C. The gelling capacity was determined visually by assessing the gel formation and noting the time taken for the gel formed to remain intact in tear fluid before complete dissolution took place. The composition of artificial tear fluid was 0.670 g Sodium Chloride, 0.2 g Sodium bicarbonate, 0.008 g Calcium Cloride.2H2O, deionized water q.s. 100 g (Vo’oleghem, Citation1993).
Viscosity measurement
A Viscosimeter (Brookfield R.V II, UK) was used to measure the viscosities of the developed CPs/Cellulose derivatives—ciprofloxacin formulations (pH 6). The spindle (number 21) was rotated at 10 rpm. The viscosity was also measured for ciprofloxacin formulations after raising their pHs to 7.4 using 0.5 N NaOH solution. The change in viscosities of the formulation based on alginate was evaluated after mixing it with artificial tear fluid.
Adhesion study
The adhesion study of controlled release ciprofloxacin formulations, after gelling, was measured using the apparatus described by Agrwal and Mishra (Citation1990). The apparatus consisted of two circular aluminium discs each of 3 cm diameter. One disc was allowed to hang on an iron stand with the help of an aluminium wire fastened with a hook provided on the back side of the disc. The other disc was connected to a pre-weighed lightweight plastic bag using a hook attached on its back. The studied gel was placed between the two discs, which were then kept under constant pressure for 5 min (preload time) to initiate adhesion bond. After that, water was added to the plastic bag through an intravenous infusion set at a rate of 1 drop/min until the two adhered discs became detached from each other. The water collected in the bag was weighed. The weights of collected water were converted into force required for detachment.
In vitro diffusion studies of ciprofloxacin through a membrane
The in vitro diffusion of the drug through a membrane was carried out in a system composed of a glass tube in which a cellophane membrane (soaked over night in artificial tear fluid, pH 7.4) was stretched and securely fastened with a rubber band; 1 g of the 0.3% w/w formulation was placed in the tube (phase I or the donor phase). This was hung vertically in a beaker containing 50 ml artificial tear solution, pH 7.4 (phase II or the acceptor phase). The diffusion system was placed in a thermostatically shaking water bath at 37°C ± 1. At predetermined time intervals, 1 ml of the solutions were removed from the acceptor phase and analyzed for ciprofloxacin using a UV spectrophotometer at 270 nm and an equal volume of fresh, pre-warmed artificial tear was replaced into the dissolution vessels. The presence of formulation excipients did not interfere with the analysis. The amount of ciprofloxacin released at each time was calculated from a calibration curve (2–10 μg/ml, r > 0.99 with zero intercept).
Antimicrobial efficiency of controlled release ciprofloxacin gel
The antimicrobial efficiency and prolonged effect of selected controlled release ciprofloxacin gel were determined on Echerichia coli, staphylococcus strains, and Pseudomonas aeruginosa as a function of time. The inhibitory effect of ciprofloxacin formulation on the studied microorganisms was evaluated using agar diffusion test. Wells were punched into the agar (Mueller Hinton agar) previously seeded with test organisms (105 cfu/ml by Macferland method) and wells were filled with 100 μl of the samples which were collected from the in vitro diffusion test of ciprofloxacin gel formulation. After incubation for 24 h at 37°C the diameters of inhibition zones were measured. The results were compared with control (artificial tear fluid).
Results and discussion
Formulation of the gels
The pH of the buffer has a major contribution in formulation of a stable and therapeutically effective ophthalmic delivery system. It has been reported that ocular penetration of levofloxacin, which is a derivative of ciprofloxacin, is at a maximum at pH 6.5 (Kawazu et al., Citation1996). In addition, and as mentioned earlier in the introduction, the aqueous solubility of ciprofloxacin occurs at acidic or basic pHs. Therefore, the pHs of ciprofloxacin formulations prepared in this study were adjusted to 6.
Physicochemical properties of controlled release ciprofloxacin ophthalmic in-situ gels
Drug content and pH
All prepared ciprofloxacin formulations possessed satisfactory pHs, ranging from 6–6.2. Drug content values ranged from 98–100%.
Mechanical properties of ciprofloxacin ophthalmic gels
The desired outcome of a prolonged action gel must be to preserve it’s integrity without erosion in the tear fluid for a long period of time. However, it has been reported that a prolonged precorneal residence of formulations containing the in situ gelling polymer, alginic acid, was looked for, not only based on its ability to gel in the eye but also because of its mucoadhesive properties (Smart et al., Citation1984). For these reasons, mechanical properties of ciprofloxacin ophthalmic formulations, including the gelling capacity, viscosity at pH 7.4, and the mucoadhesive properties, were evaluated and the results are presented in . The gelling capacity was expressed as the time it takes the gel to remain intact in tear fluid before erosion takes place. It should be noted that all formulations underwent immediate transition into gel phase in contact with the tear fluid (pH 7.4). The type of in situ gelling polymer, its concentration, and the type of cellulose derivative had a significant effect on the gelling capacity of ciprofloxacin formulations. Minimum gelling capacity was exhibited by formulations containing alginate. On the other hand, formulations based on Carbopol and methylcellulose exhibited the maximum gelling capacity.
Table 2. Physical properties of prolonged action in in situ ciprofloxacin eye gels.
shows the viscosity of ciprofloxacin formulations consisting of CPs/cellulose derivatives when their pHs were raised to 7.4 and alginate formulation after it was mixed with artificial tear fluid. In formulations based on HPMC and CP, increasing the concentration of each polymeric component significantly increased the formulation viscosity. Carbopol and HPMC are water-swellable polymers. The extent of swelling of these polymers was dependent on the amount of free water in the formulations. Hence, in formulations containing high concentrations of HPMC or CP, their extent of swelling was decreased. Furthermore, in formulations containing high concentrations of HPMC or formulations containing high concentrations of CP, a greater proportion of theses polymers was present as unswollen suspended solid compared to formulations containing lower concentrations of these polymers. It is proposed that the physical states of these polymers, i.e. swollen or unswollen, were directly responsible for the mechanical properties, including the viscosity of the prescribed formulations. Ferrari et al. (Citation1994) reported that gel strength of HPMC gels increased as the polymer concentration was increased. Jones et al. (Citation2000) revealed that increasing the concentration of polymer components of the gels resulted in an increase in the hardness, adhesiveness, compressibility, and syringeability of the formulations. The effect of HPMC and CP on the viscosity of in situ prolonged action gels under examination may also be explained by their effect on the product viscoelasticity following dispersion of swollen or unswollen solids. Increasing the product viscoelasticity, particularly elasticity, produced an increased resistance to product deformation during viscosity measurements. The lowest viscosity value was observed from formulation 4, containing alginate, indicating complete swelling of the polymer in the buffer system. On the other hand, the formulation consisting of MC and CP (F3) showed lower viscosity data than formulations containing HPMC and CP, which may be attributed to the improved swelling property of methylcellulose in the vehicles used for preparation of ciprofloxacin formulations.
One important characteristic of the prolonged action eye gel is its ability to exhibit retention in the eye. The polymers used in this study have been described as mucoadhesives. Therefore, they would be expected to show retention through formation of adhesive bonds with ocular tissues.
The adhesiveness of ciprofloxacin formulations, after they were gelled, are presented in . In the method used to evaluate the mucoadhesive properties of the ciprofloxacin gels, the work required to overcome the attractive force developed between the surface of the sample and the surfaces of the discs was expressed as adhesiveness. The bioadhesive force is known to be dependent on the nature and the concentration of the bioadhesive polymer. In formulations consisting of HPMC and CP, the adhesiveness of the gel increases as the concentration of each polymer increases. This may be attributed to the increased ability of these polymers to interact with the surface of the discs, but may also be a function of the increased tack of each formulation. Furthermore, the physical state of the polymeric component was observed to significantly affect the adhesiveness of the formulations. Hence, in formulations where CP or HPMC existed as suspended unswollen solids, the adhesiveness was greater than in formulations in which these polymers exhibited a greater degree of swelling. The increased mass of unswollen particles in formulations containing CP 0.5%(w/w) compared to those containing CP 0.4% (w/w) or those containing HPMC 1.5% (w/w) compared to those containing HPMC 1% (w/w) has a direct effect on the adhesive strength. These results are in agreement with Choi et al. (Citation1998), who reported an increase of bioadhesive forces of the gels as the carbopol concentration was increased. also reveals that the impact of HPMC on the bioadhesive force of the gels was lessened by the presence of CP971. Keeping the concentration of HPMC constant, the formulation containing 0.5% (w/w) of CP 971 possessed lower adhesive force than formulation containing 0.5% (w/w) of CP 943. On the other hand, methylcellulose has enhanced the gel adhesiveness more efficiently than the other systems examined. This could be due to the strong adhesive nature of methylcellulose when it is combined with CP in a formulation. Of all the systems prepared, the formulation containing 2% of alginate possessed the poorest adhesive properties, and would therefore be susceptible to easy removal from the cornea after administration. Thus, these results suggest that addition of viscosity-enhancing agent to alginate gel may improve its adhesiveness and other mechanical properties.
In vitro diffusion study
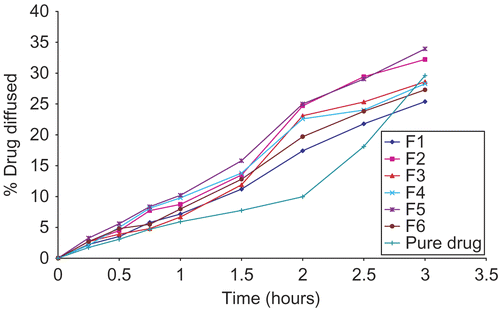
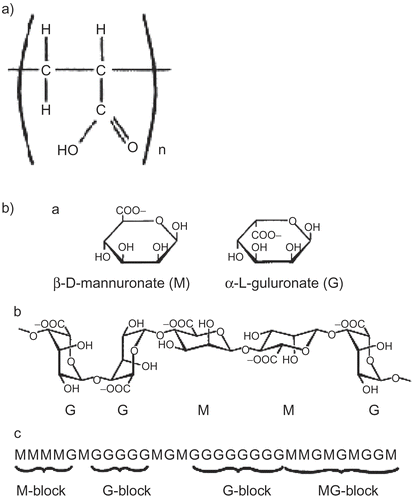
The in vitro diffusion results of the drug release obtained with the ciprofloxacin formulations in comparison with the ciprofloxacin solution are shown in . A slow diffusion rate was observed from ciprofloxacin solution which could be attributed to the low aqueous solubility of ciprofloxacin at pH close to 7. Surprisingly, incorporation of ciprofloxacin into gels caused an increase in its release and permeation rates. In an attempt to explain the reasons for these findings, the chemical structures of alginates and carbopol, the polymers used in gels formulations, were analyzed ((a) and (b)). The chemical structures suggest formation of ionic pairs between the zwitterionic species of ciprofloxacin and the carboxylic groups of carbopol and alginates. During the diffusion studies, Na+, K+, HCO3−, and Cl− ions present in the acceptor phase (artificial tear fluid) diffused into the gel and Na+ and K+ promoted the exchange of the cationic and zwitterionic species attached to carbopol and alginate polymers. The dissociation of ionic pairs has contributed in facilitating the drug release from gel matrices. Vilches et al. (Citation2002) reported that ionic pairing between fluoroquinolones and carbopol is the main interaction that determines the enhanced aqueous compatibility and releasing properties of the drug. Moreover, loading carbopol with fluoroquinolone together with an appropriate amount Na+ yields physically stable dispersion that behaves as a molecular matrix of fluoroquinolones, able to deliver the drug at a constant rate (zero order) in contact with a biological fluid-like solution.
Figure 2. (a) General structure of Carbopol polymers. (b) schematic diagram of the structure of alginate showing (a) the constituent sugars, (b) their relevant linkages, and (c) possible intramolecular patterns of the different sugars.
shows the time required for 20% of the ciprofloxacin to be released (T20%) from the various gel formulations. Increasing the concentration of HPMC from 1 to 1.5% and the CP from 0.4 to 0.5% has markedly increased T20%. The maximum T20% value was 135 min, and was associated with F1 (formulation containing highest concentration of HPMC and CP). On the other hand, the minimum T20% value was associated with the formulation containing lowest CP and HPMC concentrations (F5), and was 105 min. F3 (formulation containing methylcellulose) and F4 (formulation containing alginate) exhibited T20% values between the two. It is thought that these effects are the function of interaction of two parameters: the polymer type and its concentration. As the polymer concentration increases, the diffusion of ciprofloxacin through the formulation reduces due to the more entangled nature of the polymeric network. In addition, the ingress of water into the formulation containing high concentration of polymer was reduced, thus lowering the rates of both dissolution/erosion. Also, the density of the chain structure of the gels increases at higher polymeric concentrations, and this limits the active substance movement area (Wang et al., Citation2001). Finally, the degree of swelling increases as the concentration of the suspended solids increases, thus decreasing the diffusion of ciprofloxacin from the swollen matrix. The swelling phenomenon may be directly responsible for the effects of the type of polymer on the drug release from the formulation.
Table 3. The time required for the release of 20% of the original mass of ciprofloxacin from gel formulation.
The data generated from these release studies were fitted to the general release equation (CitationKorsmeyer et al.,) using the logarithmic transformation and least-square regression analysis, as described below, in an attempt to investigate the mechanism of ciprofloxacin release.
log Mt/M∞ = log k + n log t
where Mt is the amount of drug released at time t; M∞ is the total drug content; k is a constant incorporating structural and geometrical characteristics of the device; and n is the release exponent which may indicate the mechanism of drug release.
The kinetic parameters n and k were calculated and tabulated (). For the formulations under investigation, the release exponents (n) were close to 1, suggesting that ciprofloxacin was delivered from gels by zero order kinetics. Similarly, Vilches et al. (Citation2002) reported zero-order kinetics for the release of ciprofloxacin from carbomer hydrogels. The slight deviation of the n-value that was observed in some cases could be attributed to the swelling properties of these formulations following the ingress of water.
Table 4. Kinetic parameters obtained from the release equation.
It has been reported that as the k-value becomes higher, the release rate becomes faster (Choi et al., Citation1998). Analysis of k-value of the various formulations revealed that the release rate of ciprofloxacin was decreased as the concentration of each polymeric component was increased. These results confirm our earlier finding based on T20% data. Moreover, replacing HPMC with MC has slightly accelerated drug release rate, as indicated by k-values, 0.1215 and 0.1246 for formulations containing HPMC and MC, respectively. Based on the mechanical properties and release characteristics of the investigated formulations, formulation F3 containing Carbopol 934 and methyl cellulose in addition to ciprofloxacin was selected for the microbiological studies.
Antimicrobial efficiency studies
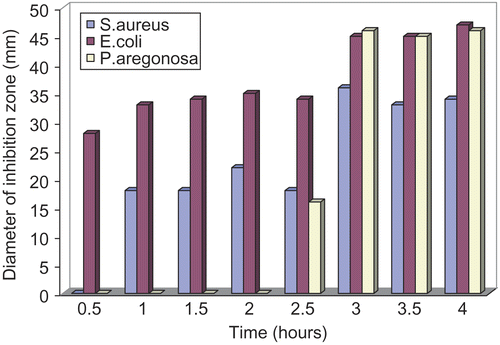
The antimicrobial efficiency of the selected controlled release ciprofloxacin formulation was evaluated against gram-positive and gram-negative organisms including Echerichia coli, staphylococcus strains, and Pseudomonas aeruginosa. The inhibition zones produced by ciprofloxacin gel was measured in bacteriological medium and the results are presented in . The inhibition zones were evaluated every 30 min and reduction in the growth of microorganisms was clearly observed. Comparison of the diameters of the zone of growth inhibition () reveals that controlled release ciprofloxacin formulation was most effective against E. coli. The growth inhibition was observed in the first 30 min of the experiment and increased as the amount of ciprofloxacin diffused from gel was increased. Ciprofloxacin gel has also shown activity against staphylococcus stains but to a lesser extent than E. coli; however its antimicrobial activity was sustained for 4 h. On the other hand, the result of ciprofloxacin formulation against P. aeruginosa showed that the microorganism has resisted ciprofloxacin in the first 2 h, but rapid increase in the rate of growth inhibition was observed afterwards.
Figure 3. Evaluation of the antimicrobial efficiency of ciprofloxacin gel on (a) E. coli, (b) S. aureus, and (c) P. aeruginosa as a function of time.

Figure 4. Comparison of the diameters of the inhibition zones of E. coli, S. aureus, and P. aeruginosa.
In conclusion, various controlled release in situ gelling systems for ciprofloxacin were designed and characterized in terms of ciprofloxacin release, mechanical, and mucoadhesive properties. Based on the results obtained from the in vitro characterization techniques, a candidate formulation containing carbopol, methyl cellulose in addition to ciprofloxacin was selected for microbiological evaluation. The selected formulation offered compromise between optimal ciprofloxacin release and adhesiveness and rheological properties, and showed a prolonged antimicrobial effect against gram-positive and gram-negative organisms. The designed controlled release in situ gelling system is a viable alternative to conventional eye drops as a result of its ability to enhance bioavailability through its prolonged drug release.
Acknowledgement
The authors would like to thank Miss Fadheela Al-Anzy for her valuable help in the microbiological studies.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Schoenwald R. Ocular drug delivery: pharmacokinetics considerations. Clin Pharmacokinet 1990;184:255–269.
- Middleton D, Leung S, Robinson J. In: Lenaerts V, Gurny R, editors. Bioadhesive drug delivery systems. Boca Raton, FL: CRC Press;1990. p 179–202.
- Patton T, Robinson J.Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J Pharm Sci 1975;64:1312–1316.
- Zaki I, Fitzgerald P, Hardy J, Wolson C.A comparison of the effect of viscosity on the precorneal residence of solutions in rabbit and man. J Pharm Pharmacol 1986;3:463–466.
- Greaves J, Wilson C.Treatment of diseases of the eyes with mucoadhesive delivery systems. Adv Drug Deliv Rev 1993;11: 349–383.
- Miller S, Donovan M.Effect of polaxomer 407 gel in the miotic activity of polocarpine nitrate in rabbits. Int J Pharm 1982;12:147–152.
- Lindell K, Engstrom S.In vitro release of timolol maleate from an in situ gelling polymer system. Int J Pharm 1993;95:219–228.
- Gurny R.Preliminary study of prolonged acting drug delivery system for the treatment of glaucoma. Pharm Acta Helv 1981;56:130–132.
- Srividya B, Cardoza R, Amin P.Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Contr Rel 2001;73:205–211.
- Carfors J, Edsman K, Petersson R, Jornving K.Rheological evaluation of gelrite in situ gels for ophthalmic use. Eur J Pharm Sci 1998;6:113–119.
- Cohen S, Lobel E, Trevgoda A, Peled Y.A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Contr Rel 1997;44:201–208.
- Liu Z, Li J, Nie S, Liu H, Ding P, Pan W.Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm 2006;315:12–17.
- Goodman L, Gilman A. The pharmacological basis of therapeutics, 9th ed. New York: McGraw-Hill;1996. p 1065.
- Appelbaum P, Hunter P.The fluoroquinolone antibacterials: past, present, and future perspectives. Int J Antimicrob Agents 2000;16:5–15.
- Vilches A, Jilmenez-Kairuz A, Alovero F, Olivera M, Allemandi D, Manzo R.Release kinetics and uptake studies model fluoroquinolones from carbomer hydrogels. Int J Pharm 2002;246:17–24.
- Allemandi D, Alovero F, Manzo R.Formulation of a neutral solution of ciprofloxacin upon complexation with aluminium. II Farmaco 1999;54:758–760.
- Lin H, Ko S, Hsu L, Tsai Y.The preparation of norfloxacin-loaded liposomes and their in-vitro evaluation in pig’s eye. J Pharm Pharmacol 1996;48:801–805.
- Thomson Micromedex. MICROMEDEX (R), Healthcare series, 116.: Thomson Micromedex, Washington, D. C., USA;2003.
- Vo’oleghem M. In: Edman P, editor. Biopharmaceutics of ocular drug delivery. Boca Raton, FL: CRC Press;1993. p 27–41.
- Agrwal V, Mishra B.Design, development and biopharmaceutical properties of buccoadhesive compacts of pentazocine. Drug Dev Ind Pharm 1990;25:701–709.
- Kawazu K, Takashina H, Kawashima Y, Usui M, Mitsui Y.Effect of pH on the ocular penetration of levofloxacin. Atarashil Ganka 1996;13:259–262.
- Smart J, Kellaway I, Worthington H.An in vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J Pharm Pharmacol 1984;36:259–299.
- Ferrari F, Bertoni M, Caramella C, Lamanna A.Description and validation of an apparatus for gel strength measurements. Int J Pharm 1994;109:115–124.
- Jones D, Woolfson A, Brown A, Coulter W, McClelland C, Irwin C.Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal diseases. J Contr Rel 2000;67:357–368.
- Choi H, Hung J, Ryu J, Yoon S, Oh Y, Kim C.Development of insitu gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm 1998;165:33–44.
- Wang Y, Hong C, Chiu W, Fan J.In vitro and in vivo evaluations of topically applied capsaicin and nonivamide from hydrogels. Int J Pharm 2001;224:89–104.
- Korsmeyer R, Gurny R, Doelker E, Buri P, Peppas N.Mechanism of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25–35.
- Choi H, Oh Y, Kim C.In situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int J Pharm 1998;165:23–32.