Abstract
The aim of this study was to explore the use of Pharmasolve® as a new kind of permeability enhancer in ophthalmic drug delivery systems. The ocular irritation of different concentrations of Pharmasolve® on rabbit eyes was evaluated in detail. Four drugs ranging from hydrophilic to lipophilic, namely ribavirin, puerarin, enoxacin, and ibuprofen, were used as model compounds to investigate the effects of different concentrations of Pharmasolve® on the corneal permeability. The mechanism of ocular permeation enhancement of drugs by Pharmasolve® was also discussed. The results showed that Pharmasolve® presented no irritation when the concentration used was lower than 10%. Pharmasolve® could enhance the ocular permeability of the four test drugs; the maximum enhancement in Papp was 4.04, 2.76, and 2.67-fold for ribavirin, enoxacin, and puerarin, respectively; 2.5% (v/v) Pharmasovle® increased the Papp by about 1.47-fold for ibuprofen; which suggested that it would have a great potential to be used as a safe and effective penetration enhancer in ocular drug delivery systems in the future.
Introduction
Due to the presence of the blood-aqueous barrier and the blood-retinal barrier, which prevents drugs from entering the aqueous humor and the extravascular retinal space and the vitreous body, respectively, systemic drug administration for their ocular action was hard to carry out. So, topical administration was still an important route of ocular disease therapeutics (Citation1). A successful topical delivery of drugs to the eye is extremely difficult because the eye is protected by a series of complex defense mechanisms which make it difficult to achieve an effective concentration of drug within the target area of the eye. The cornea, the most important one of the defense mechanisms, has both hydrophilic and lipophilic structures, and presents an effective barrier to the absorption of both hydrophilic and lipophilic compounds.
Different methods have been used to overcome the barrier property of the cornea; one effective approach is to increase the transcorneal passage of drugs by incorporating penetration enhancers into the formulations. Ideally, an enhancer should be chemically and pharmacologically inert, non-toxic, and non-irritant, have a rapid and reversible onset of action, be potent at low concentrations, and compatible with the formulation ingredients.
There were many studies on the selecting of penetration enhancers. For example: the effects of hexamethylene louramide and Azone on the corneal permeability of eight drugs in vitro were studied (Citation2); however, the ocular irritation produced by them was not investigated. Besides, the effects of benzalkonium chloride, EDTA, non-ionic surfactants, surface-active heteroglycoside, and bile salts on the corneal permeability of four drugs were evaluated; the results showed that some of these enhancers had a significant irritation at low concentration (Citation3). Hence, it is very important to carry out ocular irritation studies when employing new penetration enhancers, and to evaluate their suitability for use in ocular pharmaceutical preparations. As is reported by Liu et al. (Citation4), the previous work accomplished in our laboratory has systematically investigated the enhancement effect and ocular irritation of a new kind of permeability enhancer, an interesting result about Transcutol P had been shown for a good, safe, and effective permeability enhancer in ocular drug delivery system. For further searching for other novelty ocular permeability enhancers, more investigation was carried on.
Pharmasolve® (N-methyl-2-pyrrolidone, NMP), a short chain amphiphile, has been suggested for use in human pharmaceutical formulations as solvents and solubilizer in oral and topical applications. It appears to be a type of effective and non-irritant penetration enhancer when applied to skin and mucous membranes (Citation5–7); people added NMP in drug transdermal delivery systems and got a satisfactory enhancement of drug skin permeation (Citation8–10).
To our knowledge, there was no report on use of Pharmasolve® as a penetration enhancer for an ocular drug delivery system. In the present investigation, we have tried to explore the use of Pharmasolve® in ophthalmic delivery system for both hydrophilic and lipophilic drugs, and, meanwhile, to discuss the mechanism of ocular permeation enhancement of drugs by Pharmasolve®. In addition, the ocular irritation of different concentrations of Pharmasolve® was evaluated.
Materials and methods
Materials
Pharmasolve® was kindly gifted by International Specialty Products (ISP); Ibuprofen was kindly sponsored by DongYu Pharmaceutical Manufacture (Shenyang, China); Enoxacin was purchased from Wuhan Pharmaceutical Manufacture (Wuhan, China); Ribavirin was bought from HuBei Qiangjiang Pharmaceutical Manufacture (Qiangjiang, China) and puerarin was obtained from HuBei Hengding Pharmaceutical Manufacture (Wuhan, China). All the other chemicals were acquired from Yuwang chemical company (Tianjin, China).
Animals
New Zealand White rabbits weighing 2.5–3.0 kg were used. They were provided by the Animal Experimental Center of Shenyang Pharmaceutical University. The animals were housed in standard cages in a light-controlled room at 19 ± 1°C and 50 ± 5% relative humidity separately, and given a standard pellet diet and water. During the experiments the rabbits were placed in restraining boxes, where they could move their heads and eyes freely. All studies were conducted in accordance with the Principles of Laboratory Animal Care (NIH publication no. 92–93, revised in 1985), and were approved by the Department of Laboratory Animal Research at Shenyang Pharmaceutical University. The procedures with animals were reviewed and approved by the Animal Ethical Committee at Shenyang Pharmaceutical University.
Measurement of solubility and partition coefficients
The saturated solubility of the four tested drugs in phosphate buffer solutions at pH 7.4 with different concentrations of Pharmasolve® was measured. Excess amount of drugs was added to phosphate buffer at pH 7.4 with 0%, 2.5%, 5%, and 10% (v/v) Pharmasolve®, respectively. These solutions were shaken in a water bath at 34°C for 72 h. Samples were centrifuged for 15 min at 3000 rpm and then the concentrations of the four tested drugs in the saturated solutions were measured.
Partition coefficients between n-octanol and buffer were determined by the modified shake-flask method. Briefly, a certain amount of compounds were dissolved in phosphate buffer at pH 7.4 (saturated with n-octanol) and agitated with n-octanol (saturated with phosphate buffer at pH 7.4) for 30 min. The two phases were allowed to equilibrate for at least 1 h. The partition coefficients were the ratio of the drug concentrations in the two phases.
Ocular irritation test
Ocular irritation studies were performed according to the Draize et al. (Citation11) technique. This technique has been used by the FDA to evaluate the safety of several substances (Citation11,Citation12).
All rabbits were divided into six groups of six, based on the concentrations of Pharmasolve®. The test solutions, consisting of 0%, 2.5%, 5%, 10%, 15%, 20% (v/v) Pharmasolve® in phosphate buffer at pH 7.4, were instilled into the left eyes, 100 μL every time, five times a day, for a period of 7 days. The condition of the ocular tissue was observed at 1, 12, 24, 48, and 72 h after the last instillation. The corneal opacity and iris hyperemia were graded on a scale from 0–4, and conjunctival congestion, swelling, and discharge were graded from 0–3, 0–4, and 0–3, respectively. The mean values of six tested eyes were calculated for each test solution. The evaluation criteria used in accordance with the technique mentioned above were non-irritant from 0–3.9, slightly irritant from 4–8.9, moderately irritant from 9–12.9, and seriously irritant from 13–16.
Corneal permeation experiment
After the animals were sacrificed by injecting intravenous air through a marginal ear vein, freshly excised rabbit corneas were weighted and immediately mounted in Franz-type diffusion cells which were maintained at 34°C under magnetic stirring at 600 rpm (79HW-1, Zhejiang, China). The area available for diffusion was 0.70 cm2. Preheated (34°C) test solution, drugs in phosphate buffer (PBS) at pH 7.4, with Pharmasolve® at various concentrations, 1.0 mL was added to the epithelial (donor cell) and glutathione bicarbonate Ringer (GBR) buffer 7.8 mL to the endothelial (receptor cell) compartment. Samples from the endothelial side were withdrawn every 60 min and replaced with fresh GBR of equal quantity and temperature. Each experiment was continued for 6 h and repeated four times. The concentration of the tested drugs was determined by RP-HPLC method.
The apparent corneal permeability coefficients (Papp) were calculated as followed (Citation13,Citation14):
where ΔQ/Δt is the steady-state slope of the linear portion of the plot of the amount of drug in the receiving chamber (Q) vs time (t), A the exposed corneal surface area (0.7 cm2), C0 the initial concentration of drug in the donor cell, and 60 the conversion of minutes to seconds.
The amount of each drug permeating through the cornea during a sampling interval was calculated based on the measured receptor-phase concentration and volume. The cumulative amount of drug permeating per unit area vs time was plotted. All data were calculated and presented as mean ± SE. The lag-time was determined by extrapolation of the linear portion of the cumulative amount of drug permeated vs time plot to the abscissa.
Determination of corneal hydration levels (HL)
Each test cornea was carefully removed from the scleral ring and weighed. The wet corneal weight was recorded as Wa, it was then desiccated at 70°C for 6 h to determine the corresponding dry corneal weight Wb. The corneal hydration level (HL%), defined as [1 – (Wb/Wa)]·100, was determined for both untreated corneas (removed no later than 30 min after the death of the animal) and corneas recovered from the tests.
Analytical methods
Ribavirin, puerarin, enoxacin, and ibuprofen were assayed by RP-HPLC (Shimadzu LC-10ATvp, Japan). The chromatographic conditions were given in previous studies (Citation15–18). The peak area correlating linearly with the concentrations was in the range of 5.000~150.0 μg/mL (r2 = 1), 0.200~15.00 μg/mL (r2 = 1), 0.212~31.80 μg/mL (r2 = 0.9997), and 5.000~200.0 μg/mL (r2 = 0.9999), respectively.
Results and discussion
Results of solubility and partition coefficients measurement
The solubility of the four test drugs in phosphate buffer solutions at pH 7.4 with different concentrations of Pharmasolve® was exhibited in , and the partition coefficients of ribavirin, puerarin, enoxacin, and ibuprofen were −2.6, −1.28, −0.65, and 1.27, respectively.
Table 1. Solubility of the four test drugs in PBS at pH 7.4 with different concentrations of Pharmasolve®.
As is reported, NMP is a good solvent and the solubility parameter of NMP (23.0 MPa1/2) is similar to those of ethanol (26.7 MPa1/2) and dimethyl sulfoxide (DMSO) (26.5 MPa1/2). In view of partial solubility parameters, NMP is similar to DMSO, with δD; δP, and δH of NMP at 18.0, 12.3, and 7.2 MPa1/2, respectively, and those of DMSO at 18.4, 16.4, and 10.2 MPa1/2, respectively (Citation19). In this study, the result showed that the solubility of the four drugs significantly increased after adding Pharmasolve®. For example, the solubility of puerarin and ribavirin in 10% Pharmasolve® was 4.78-fold and 3.64-fold of them in phosphate buffer solution at pH 7.4, respectively. The solubility of ibuprofen and enoxacin was enhanced by 2.47-fold and 1.98-fold in 10% Pharmasolve®. From the results we could see that Pharmasolve® was an effective solvent for both hydrophilic and hydrophobic drugs.
Ocular irritation of Pharmasolve® on rabbit eyes
According to the evaluation criterion in the Draize technique, that is cornea, disperse opacity; iris, hyperaemia; conjunctiva, redness of palpebral conjunctivae, swelling, and discharge with moistening of the lids, Pharmasolve® was non-irritant at concentrations of 0–10% (v/v), slightly irritant at 15% (v/v), and moderately irritant at 20% (v/v). The average scope of ocular irritation test was 0, 0, 0, 0, 4 ± 0.5, and 9 ± 3 from 0–20% (v/v) Pharmasovle®, respectively. This was possibly because of the fact that the pH values for 15% and 20% v/v Pharmasovle® aqueous solution were about 9–10, which exceeded the endurable extent of eyes.
There were no visible ocular damage involving the cornea or irises at all concentrations, but the conjunctiva were slightly damaged, so that congestion and swelling could be observed when the concentration of Pharmasolve® was at 15% (v/v) and 20% (v/v). Based on these results, the concentrations of Pharmasolve® selected for the corneal permeation test were 0%, 2.5%, 5%, and 10% (v/v).
The effect of Pharmasolve® on corneal permeation of test drugs
and displayed the effect of Pharmasolve® on the Papp values of four test compounds. showed the lag-time of drugs in different concentrations of Pharmasolve®. The permeability of ribavirin, puerarin, and enoxacin had little change when the concentration of Pharmasolve® was lower than 2.5% (v/v), and significantly increased when the concentration of Pharmasovle® increased from 2.5% up to 10%. In the case of ibuprofen, a distinguished change on Papp was observed when 2.5% Pharmasovle® was added, but a further increase in Pharmasovle® concentration was not able to change the permeability markedly. The maximum enhancement in Papp was 4.04-, 5.09-, and 2.67-fold for ribavirin, enoxacin, and puerarin, respectively. 2.5% (v/v) Pharmasovle® increased the Papp by about 1.47-fold for ibuprofen. The result indicated that Pharmasolve® could enhance the Papp of hydrophilic drugs to a great extent.
Figure 1. Corneal permeability coefficients change of ribavirin via the concentration of Pharmasolve®.
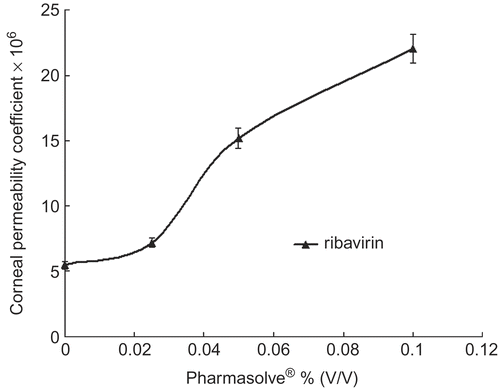
Figure 2. Corneal permeability coefficients change of enoxacin via the concentration of Pharmasolve®.
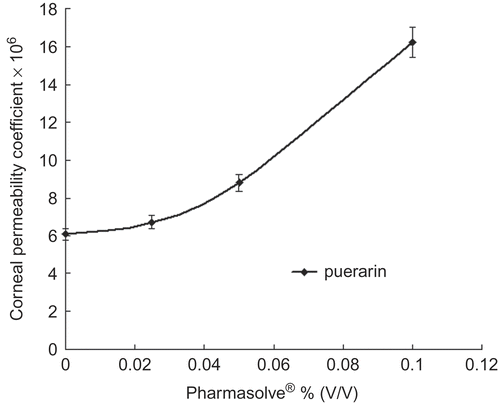
Figure 3. Corneal permeability coefficients change of ibuprofen via the concentration of Pharmasolve®.
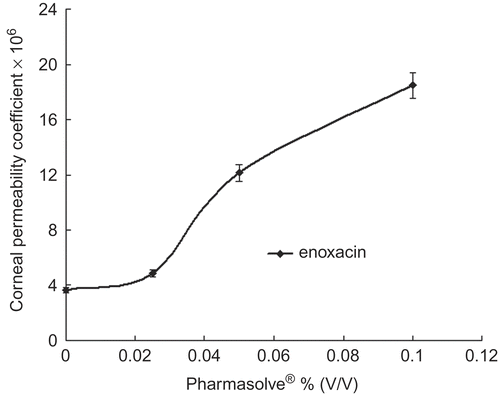
Figure 4. Corneal permeability coefficients change of puerarin via the concentration of Pharmasolve®.
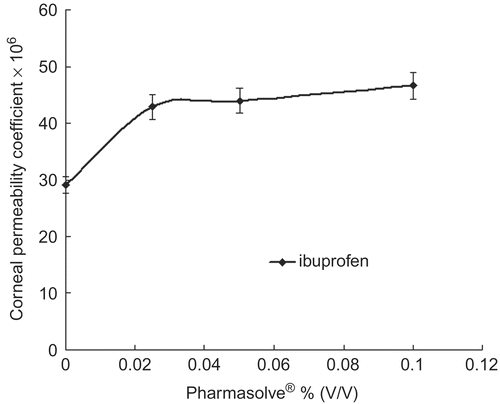
Table 2. Effect of Pharmasolve® on the corneal permeability of test drugs (n = 4).
Table 3. Lag-time of drugs in different concentrations of Pharmasolve® (n = 4).
There have been some reports on the possible mechanism of the enhancement effect of N-methyl-2-pyrrolidone (NMP) on skin permeation: (Citation1) solubilization of drug in vehicle and skin; (2) reducing the diffusional resistance, increasing thermodynamic activity in vehicle; Akhter and Barry (Citation20) reported NMP enhanced the permeation of flurbiprofen and ibuprofen applied as solid drug film because the dissolution step was removed, and thermodynamic activity of the drug remaining in the solution increased after rapid permeation of NMP and NMP changed the diffusional resistance; (3) fluidization of lipid in stratum corneum; Yoneto et al. (Citation21,Citation22) reported that N-alkyl-pyrrolidone derivatives increased drug solubility in skin and also fluidized lipids in stratum corneum; and (4) acting as a carrier for some drugs and co-permeation of NMP and drug (Citation23).
The cornea, which has similar structure and construction to the skin, accounting for its poor permeability characteristics, can be considered to be comprised of three layers: (1) the outer epithelium, which is lipophilic in nature; (2) the stroma, which constitutes approximately 90% of the thickness of cornea and is hydrophilic; and (3) the inner endothelium consisting of a single layer of flattened epithelium-like cells. Since the cornea has both hydrophilic and lipophilic structures, it presents an effective barrier to the absorption of both hydrophilic and lipophilic compounds.
According to the fact described above and the results of this study, the mechanism of Pharmasolve® increasing the corneal penetration of drugs can be explain as following: (1) As a surfactant, Pharmasolve® can increase the solubility of the test drugs in phosphate buffer at pH 7.4, which makes it possible that the water-insoluble drugs form a solution instead of suspension; (2) Pharmasolve® can incorporate into the lipid bilayer in the outer cell membrane of corneal epithelial cells, forming polar defects which change the physical properties of the cell membrane, accordingly changing the structure of the epithelium and leading to membrane solubilization and cornea solubilization; (3) The micelles formed by Pharmasolve® resulted in the removal of phospholipids from the epithelial cell membranes, forming a passage for the transcorneal passing of drugs, thereby leading to an increase in drug corneal permeability; and (4) Pharmasolve® may loosen the epithelium cell junctions and facilitate the influx of hydrophilic compounds by forming a hydration carrier (2); it can also explain why the corneal permeability enhancement of water-soluble drug is more significant.
Corneal hydration levels
The percentage corneal hydration is a parameter frequently used to evaluate damage to this tissue. Normal cornea has a hydration level of 76–80% (Citation24). As is indicated (Citation13), an 83–92% hydration level denotes damage to the epithelium and/or endothelium. As is shown in , all of the corneal hydration of drugs after the test was lower than 83%. This indicated that the Pharmasolve® did not cause any damage to the cornea at test concentration, which accords with the result of the ocular irritation study.
Table 4. The corneal hydration of drugs in different concentrations of Pharmasolve® (n = 4).
Conclusion
Pharmasolve® is non-irritant at concentrations of 0–10% (v/v); it is an effective solvent for both hydrophilic and lipophilic drugs and can enhance the Papp of the test drugs to different extents. It has the potential to be used as a safe permeability enhancer in ocular drug delivery systems in the future.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 30500638), and the N-methyl-2-pyrrolidone sample (Pharmasolve®) was a kind gift of ISP.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stjernschantz J, Astin M. (1993). In: Edman P, ed. Biopharmaceutics of Ocular Drug Delivery. Boca Raton, FL: CRC Press, 1.
- Diane D-S, Tang L, Joseph BR, Robert JW, Harun T. (1994). Effects of four penetration enhancers on corneal permeability of drugs in vitro. J Pharm Sci, 83, 85–90.
- Fabrizio SM Patrizia C, Riccardo C, Gabriela M, Laura B. (1996). Evaluation of ocular permeation enhancers: in vitro effects on corneal transport of four β-blockers, and in vitro/in vivo toxic activity. Int J Pharm, 142, 103–13.
- Liu ZD, Li JW, Nie SF, Guo H, Pan WS. (2006). Effects of Transcutol P on the corneal permeability of drugs and evaluation of its ocular irritation of rabbit eyes. J Pharm Pharmacol, 58, 45–50.
- Sasaki H, Kojima M, Mori Y, Nakamura J, Shibasaki J. (1988). Enhancing effect of pyrrolidone derivatives on transdermal drug delivery I. Int J Pharm, 44, 15–24.
- Sasaki H, Kojima M, Nakamura J, Shibasaki J. (1990). Acute toxicity and skin irritation of pyrrolidone derivatives as transdermal penetration enhancer. Chem Pharm Bull, 38, 2308–10.
- Yoneto K, Ghanem AH, Higuchi WI, Peck KD, Li SK. (1995). Mechanistic studies of the 1-alkyl-2-pyrrolidones as skin permeation enhancers. J Pharm Sci, 84, 312–7.
- Koizumi A, Fujii M, Kondoh M, Watanabe Y. (2004). Effect of N-methyl-2-pyrrolidone on skin permeation of estradiol. Eur J Pharm Biol, 57, 473–8.
- Ren C, Fang L, Li T, Wang M, Zhao L, He Z. 2006. Effect of permeation enhancers and organic acids on the skin permeation of indapamide. Int J Pharm, 142, 103–13.
- Lee PJ, Ahmad N, Langer R, Mitragotri S, Shastri VP. (2006). Evaluation of chemical enhancers in the transdermal delivery of lidocaine. Int J Pharm, 308, 33–9.
- Draize JH, Woodard G, Calvery HO. (1944). Methods for the study of irritation and toxicity of substances. J Pharmacol Exp Ther, 82, 377–90.
- Fitzhugh OG, Woodard G, Braun HA. (1946). The toxicities of compounds related to 2,3-dimercaptopropanol(BAL) with a note on their relative therapeutic efficiency. J Pharm Exp Ther, 87 (suppl), 23–7.
- Schoenwald RD, Huang HS. (1983). Corneal penetration behavior of β-blocking agents I: Physico chemical factors. J Pharm Sci, 72, 1266–72.
- Camber O. (1985). An in vitro model for determination of drug permeability through the cornea. Acta Pharm Suec, 78, 815–9.
- Zhang L, Qi G, Zhu H. (2000). Study on pharmacokinetics of transdermal ionphoretic ibuprofen in rabbits. Chin Pharm J, 35, 189–91.
- Liu F. (2001). Determination of ribavirin in ribavirin eye drops. Chin Pharm, 12, 368–9.
- Liu ZD, Pan WS, Nie SF, Li JW, Liu HF. (2004). Investigation on the corneal permeability of enoxacin in vitro. Chin J New Drugs, 13, 1000–2.
- Song C, Yang X, Liu Z, Zhang Z, Pan W. (2007). Study on release profile of puerarin ophthalmic gel in vitro. Chin J New Drugs, 16, 2045–56.
- Hansen CM, Just L. 2001. Prediction of environmental stress cracking in plastics with Hansen solubility parameters. Ind Eng Chem Res, 40, 21–5.
- Akhter S, Barry BW. (1985). Absorption through human skin of ibuprofen and flurbiprofen; effect of dose variation, deposited drug film, occlusion and the penetration enhancer N-methyl-2-pyrrolidone. J Pharm Pharmacol, 37, 27–37.
- Yoneto K, Li SK, Ghanem AH, Crommelin DJ, Higuchi WI. (1995). A mechanistic study of the effects of the 1-alkyl-2-pyrrolidones on bilayer permeability of stratum corneum lipid liposomes: a comparison with hairless mouse skin studies. J Pharm Sci, 84, 853–60.
- Yoneto K, Li SK, Higuchi WI, Shimabayashi S. (1997). Influence of the permeation enhancers 1-alkyl-2-pyrrolidones on permeant partitioning into the stratum corneum. J Pharm Sci, 87, 209–14.
- Seki T, Kawaguchi T, Juni K, Sugibayashi K, Morimoto Y. (1991). Sustained transdermal delivery of zidovudine via controlled release of penetration enhancer. J Contr Rel, 17, 41–8.
- Maurice DM, Riley MV. (1970). Ocular pharmacokinetics. In: Gragmore CN, ed. Biochemistry of the Eye. London: Academic Press, 6–16.